INTRODUCTION
Executive dysfunction (ED) is a frequent and disabling consequence of acquired brain injury (ABI) which in most cases impairs the patients’ abilities to function independently in daily life.
Executive functions (EF) are those capacities that make persons effective in the real world, allowing them to adapt to new situations and to develop and pursue their life goals in a constructive and productive way (Burgess & Simons, Reference Burgess, Simons, Halligan and Wade2005). In fact, EF is an umbrella term which encompasses a broad range of higher order capacities for planning, initiation, regulation, and verification of complex, goal-directed behavior (Lezak, Reference Lezak1982). EF are subserved by prefrontally driven brain circuits in which other cerebral and cerebellar areas take part (Lichter & Cummings, Reference Lichter and Cummings2001; Sbordone, Reference Sbordone and Groth-Marnat2000). It is acknowledged that ED not only results from lesions directly affecting the prefrontal cortex, but also from (diffuse) injuries elsewhere in the brain affecting these circuits (Andres, Reference Andres2003; Eslinger & Grattan, Reference Eslinger and Grattan1993; Stuss & Alexander, Reference Stuss and Alexander2000; Stuss, Reference Stuss2006). ED has been extensively documented in traumatic brain injury (TBI) patients (Bamdad, Ryan, & Warden, Reference Bamdad, Ryan and Warden2003; Bennett, Ong, & Ponsford, Reference Bennett, Ong and Ponsford2005; Busch, McBride, Curtiss, & Vanderploeg, Reference Busch, McBride, Curtiss and Vanderploeg2005; Hart, Whyte, Kim, & Vaccaro, Reference Hart, Whyte, Kim and Vaccaro2005) with evidence for even more severe problems in the case of focal frontal damage (Fontaine, Azouvi, Remy, Bussel, & Samson, Reference Fontaine, Azouvi, Remy, Bussel and Samson1999; Spikman, Deelman, & van Zomeren, Reference Spikman, Deelman and van Zomeren2000). Ample evidence of ED has also been found in other ABI patients, for example stroke (Leskela et al., Reference Leskela, Hietanen, Kalska, Yliskoski, Pohjasvaara and Mantyla1999; Pohjasvaraa et al., Reference Pohjasvaraa, Leskela, Vataja, Kalska, Yliskoski and Hietanen2002; Sachdev et al., Reference Sachdev, Braodaty, Valenzuela, Lorentz, Looi and Wen2004), cerebral tumors (Goldstein, Obrzut, John, Ledakis, & Armstrong, Reference Goldstein, Obrzut, John, Ledakis and Armstrong2004; Tucha, Smely, Preier, & Lange, Reference Tucha, Smely, Preier and Lange2000), and post-anoxic encephalopathy (Armengol, Reference Armengol2000; Simo-Guerrero et al., Reference Simo-Guerrero, Chirivella-Garrido, Ferri-Campos, Ramirez, Caballero and Noe-Sebastian2004).
Many ABI patients with ED are referred for rehabilitation. As ED hampers the capacities for changing and adapting behavior to altered situations, it often constitutes a major obstacle to the acquisition of independent living skills and hence to successful community re-entry (Fasotti & Spikman, Reference Fasotti, Spikman, Brouwer, van Zomeren, Berg, Bouma and de Haan2002). Therefore, effective interventions aimed at improving EF in daily life are sorely needed. However, difficulties with learning and applying training principles are inherent to ED, and designing clinically relevant interventions requires the consideration of several factors that make EF such complex functions.
The first of these factors is the heterogeneous construct of EF, encompassing a range of different subfunctions. Ylvisaker (Reference Ylvisaker1998), for example, distinguishes the following EF aspects: self-awareness of strengths and needs, realistic and concrete goal-setting, planning the steps to these goals, self-initiating these plans, self-monitoring and evaluating performance according to plan and goal, self-inhibiting behavior not leading to the goals set, flexibility and problem solving when situations cannot be dealt with according to plan, and finally, strategic behavior, transfer of successful behaviors to other situations. These aspects can be differentially impaired, leading to different patterns of EF symptoms in patients. Ideally, clinically relevant treatments should be multifaceted and aimed at improving a comprehensive but finite range of EF. So far, such treatments are sparse (Cicerone et al., Reference Cicerone, Dahlberg, Kalmar, Langenbahn, Malec and Bergquist2000, Reference Cicerone, Dahlberg, Malec, Langenbahn, Felicetti and Kneipp2005). The majority of studies have been carried out addressing a limited set of EF aspects, like problem-solving (Foxx, Martella, & Marchand-Martella, Reference Foxx, Martella and Marchand-Martella1989; von Cramon & Matthes-Von Cramon, Reference von Cramon and Matthes-von Cramon1994), goal management (Levine et al., Reference Levine, Robertson, Clare, Carter, Hong and Wilson2000), or self-regulation (Medd & Tate, Reference Medd and Tate2000). Notable exceptions are the studies of Rath, Simon, Langenbahn, Sherr, and Diller (Reference Rath, Simon, Langenbahn, Sherr and Diller2003), Cicerone and Giacino (Reference Cicerone and Giacino1992), and Suzman, Morris, Morris, and Millan (Reference Suzman, Morris, Morris and Millan1997), in which several aspects of EF were addressed. In the study by Rath et al., for example, the effects of group therapy aimed at improving emotional self-regulation as well as reasoning in everyday problem-solving situations were investigated.
Another factor is the targeted level of functioning. Interventions should be ecologically valid and optimize behavior in the real world (Worthington, Reference Worthington, Halligan, Kischka and Marshall2005). This usually involves teaching compensatory strategies, that is, top-down approaches, that can be flexibly adapted and applied to the various executive problems that patients encounter in daily situations (Fasotti & Spikman, Reference Fasotti, Spikman, Brouwer, van Zomeren, Berg, Bouma and de Haan2002).
A third factor concerns the measurement of interventions targeted at several executive aspects in daily functioning. Conventional neuropsychological tests tapping single executive aspects are not likely to uncover these effects. Also, many EF tests [the WCST (Wisconsin Card Sorting Test), Stroop test, or Trail Making test] do not fully assess executive abilities required in daily life (Burgess, Alderman, Evans, Emslie, & Wilson Reference Burgess, Alderman, Evans, Emslie and Wilson1998; Burgess, Alderman, Forbes, Costello, Coates, & Dawson, Reference Burgess, Alderman, Forbes, Costello, Coates and Dawson2006; Mountain & Snow, Reference Mountain and Snow1993; Spooner & Pachana, Reference Spooner and Pachana2006). The BADS’s (Behavioral Assessment of the Dysexecutive Syndrome; Wilson, Alderman, Burgess, Emslie, & Evans, Reference Wilson, Alderman, Burgess, Emslie and Evans1996) ecological validity, for example, is still debated (Norris & Tate, Reference Norris and Tate2000; Wood & Liossi, Reference Wood and Liossi2006). A more general problem is that to tap EF, tests should be complex and new, which they can be only once. This makes them unsuitable for repeated assessment and thus as the only outcome measures for a treatment for ED. Hence, treatment effects should also be measured in terms of improvement on indications of daily life functioning, which so far are neglected outcome-measures (Cicerone, Reference Cicerone2004).
In this study, a newly developed multifaceted treatment of ED is presented and evaluated. Herein ABI patients are trained to cope with everyday executive problems in all eight EF aspects distinguished by Ylvisaker. Such a comprehensive treatment has not been applied before. The training was given to ABI patients who were expected to resume or had already resumed (part of) their previous daily life activities. To control for nonspecific effects of the experimental treatment, it was compared with a computerized cognitive function training (Marker, Reference Marker1987). Such training programs may have specific effects, especially on attention deficits (Sturm et al., Reference Sturm, Fimm, Cantagello, Cremel, North, Passadori, Leclerq and Zimmermann2002; Sturm, Willmes, Orgass, & Hartje, Reference Sturm, Willmes, Orgass and Hartje1997), but their effectiveness in improving daily life skills is still debated. Therefore, and given the general character of the control training, no specific effects on daily executive activities were expected.
Several outcome measures assessing daily life executive functioning were included. The primary outcome measure pertained to the resumption of social roles. Our hypothesis was that the experimental treatment would significantly improve executive functioning in daily life activities and increase social participation immediately after training. However, because patients were taught to apply compensatory strategies autonomously, we considered the presence of treatment effects at follow-up as even more important.
METHODS
Study Design and Procedure
The study was set-up as a prospective multicenter randomized control trial (RCT) with two patient groups receiving treatment in seven Dutch rehabilitation centers and two academic settings. Previously to the trial, several experienced neuropsychologists received extensive training in the use and application of the experimental treatment protocol. During treatment, these therapists were monitored and given feedback in central meetings taking place every 3 months. The same therapists were responsible for the administration of the control treatment.
Data were obtained according to the ethical regulations of the participating institutions, in compliance with the Helsinki Declaration. Participants eligible for the study had to suffer from Acquired Brain Injury (ABI) of nonprogressive nature (i.e., TBI, stroke, or cerebral tumors), with a minimal time post-onset of 3 months. Age had to be between 17 and 70 and participants had to live at home. Candidates had to be referred for outpatient rehabilitation with post-injury dysexecutive problems either reported by themselves or observed by proxies. The signaled problems regarding planning, initiation, and regulation of complex daily life tasks had to hamper the resumption of previous activities and roles. Patients who gave their informed consent underwent a neuropsychological examination.
Dysexecutive problems were measured by means of the Dysexecutive Questionnaire (DEX: Burgess, Wilson, Evans, & Emslie, Reference Burgess, Wilson, Evans, Emslie, Wilson, Alderman, Burgess, Emslie and Evans1996). Final inclusion was based on the following criteria: a BADS standard age score in the category “low average” or lower, or a discrepancy between BADS standard age score and IQ (Dutch GIT-short version; Luteijn & van der Ploeg, Reference Luteijn and van der Ploeg1983) of 15 points (1 SD) , or standard scores of 2 or lower on the BADS’s most complex subtasks, Six Elements Test and Zoo Map Test.
Exclusion criteria were severe cognitive comorbidity (i.e., aphasia, neglect, amnestic syndrome, indicated by deficient scores on relevant neuropsychological tests) interfering with treatment, severe psychiatric problems, neurodegenerative disorders, and substance abuse.
Suitable candidates were blindly and randomly assigned to either the experimental or the control condition per center. Balanced assignment (per four patients) took place by lot (two “control” and two “experimental”). Lots were drawn blindly by an employee not involved in the study. Excluded patients were offered standard rehabilitation.
In each treatment condition, patients underwent 20–24 one-hour treatment sessions, twice a week, during a 3-month period. At baseline (T0), immediately after treatment (T1), and 6 months post-treatment (T2), an extensive battery of tests and questionnaires was administered by independent assessors who were blind for treatment condition, except for the DEX-therapist version and the Executive Observation Scale (EOS), which were therapist-rated. During the interval between T1 and T2, patients underwent no other treatments. At follow-up, these therapists were allowed to talk with and observe the patients to complete DEX and EOS forms. Executive Secretarial Task (EST), BADS, and DEX data were also collected in a group of healthy controls, recruited by means of an advertisement in a local paper. DEX-therapist forms were filled in by the assessor.
Patients
Seventy-five patients were included, underwent the treatment (experimental group: 38; control group: 37) and post-training assessment. Five rehabilitation centers supplied the majority of the patients (24, 15, 15, 10, and 9, respectively). In two other centers after treatment of one patient, the therapist had to withdraw for reasons not related to the study, and could not be replaced.
Table 1 shows the characteristics of both patient groups. These were well-matched, as no differences were found after statistical testing (Mann-Whitney U and χ2 tests). At follow-up, three patients in the experimental group did not show up and one control patient dropped out due to logistical problems. Figure 1 shows a CONSORT-diagram in which the flow of participants and attrition after initial enrollment is displayed.

Fig. 1. The CONSORT diagram.
Table 1. Means (and SDs) of demographic variables (age, education) as well as executive and cognitive tests
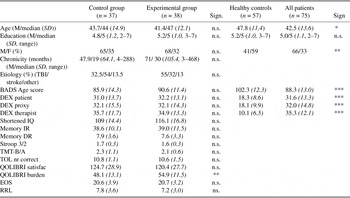
Note
Questionnaires and rating scales at T0 of the two patient groups separately. For the total patient group and healthy controls demographic data, BADS age score and DEX scores are included. Educational level was indicated on a 7-point scale, with 1 = < 6 years primary school and 7 = university education. Differences between groups were tested with two-tailed results of t tests or Mann-Whitney U tests. M/F = male/female; TBI = traumatic brain injury; BADS = Behavioral Assessment of the Dysexecutive Syndrome; DEX = Dysexecutive Questionnaire; IR = immediate recall; DR = delayed recall; TMT = Trail Making Test; TOL = Tower of London; EOS = Executive Observation Scale; RRL = The Role Resumption List; n.s., not significant.
*p < .05; ** p < .01; ***p < .001.
Experimental Treatment
The Multifaceted Treatment of Executive Dysfunction’s main objective is improvement of the eight EF aspects of Ylvisaker’s conceptual framework: self-awareness, goal-setting, planning, self-initiation, self-monitoring, self-inhibition, flexibility, and strategic behavior.
Improvement was fostered by teaching patients a comprehensive cognitive strategy, which allowed them to tackle daily life situations in a systematic and structured way. In several stages, this strategy forced patients to formulate intentions and actions explicitly in terms of goals and subgoals (planning) and effectively execute these plans, while monitoring their behavior. The treatment, described in a standard protocol, was given by an experienced rehabilitation- or neuropsychologist. It could be individually tailored to patients’ specific problems, needs, and goals, by varying content and number of sessions (up to a maximum of 24). Transfer of learning to the home situation was accomplished by using relevant exercises and home assignments. A diary was used as planning and memory aid.
The comprehensive multifaceted strategy relied heavily on Goal Management Training (GMT, Levine et al., Reference Levine, Robertson, Clare, Carter, Hong and Wilson2000) and Problem Solving Training (PST, von Cramon & Matthes von Cramon Reference von Cramon and Matthes-von Cramon1994). Its underlying idea was universal subgoaling, derived from cognitive architectures like SOAR (Newell, Reference Newell1991) and ACT-R (Anderson, Reference Anderson1993), and translated into a therapeutical approach, the General Planning Approach (GPA). Teaching and application of this strategy was administered step-by-step in three stages, namely (1) Information and Awareness, (2) Goal Setting and Planning, and (3) Initiation, Execution, and Regulation.
Stage 1, Information and Awareness, addressed Ylvisaker’s EF aspect of self-awareness. This preparatory stage consisted of four to six psycho-educative sessions aimed at improving awareness of executive deficits and enhancing motivation for treatment. Patients were extensively informed about executive problems and their negative consequences for daily life in general and their own lives in particular. This information, together with the results of the neuropsychological assessment, provided the basis for a strengths and weaknesses analysis, which formed the starting point for the treatment. Patients were continually stimulated to monitor and evaluate executive performance during training. Also, they were asked to systematically predict their performance in home assignments. Every next session, these predictions and their fulfillment, together with factors that did or did not help were extensively evaluated. These “awareness-exercises” were continued after stage 1 and incorporated in every subsequent training session.
Stage 2, Goal Setting and Planning, consisted of seven to nine sessions and was aimed at training Ylvisakers’ EF aspects of goal setting and planning in a systematic way. Patients were taught to apply the General Planning Approach (GPA) emphasizing the formulation of intended activities and tasks in terms of goals and steps leading to these goals. Daily life goals had to be verbalized explicitly and concretely in terms of when, where, with whom, with what and how long, on a fixed GPA worksheet. Patients learned to formulate concrete steps leading to previously set goals and put these steps in the right order. This was first practiced “in vitro,” using scripts of Sirigu (Sirigu, Zalla, Pillon Grafman, Agid, & Dubois, Reference Sirigu, Zalla, Pillon, Grafman, Agid and Dubois1996), and subsequently with goals brought forward by the patients. GPA was systematically shaped by asking critical questions (e.g., do you think it is practical to put step x before step y?, don’t you think that this step can be further split-up?, are you sure that you have allocated enough time to step x?) about goals and steps devised by the patient, urging him to reflect on his plans. When goal setting and planning were adequately mastered, patients were encouraged to anticipate on eventual problems by stimulating them to ask “what if…” questions (e.g., what if you need more time as a consequence of your slowness?, what if an ingredient in your meal is not readily available?) and to devise alternative steps or plans, following the same shaping procedure. Finally, without help of the therapist patients had to formulate three concrete goals they wanted to achieve by means of the treatment. These three goals had to originate from patients’ difficulties in daily life executive functioning, without other restrictions. Examples of goals suggested by patients were improving the organization of household chores, planning activities in advance, learning to use public transport facilities to increase mobility, organizing activities with family or friends to improve contacts, improving output and quality of activity in volunteer work, etc.
Not until the planning skills of stage 2 were mastered, was the last stage, Initiation, Execution and Regulation, commenced, tapping Ylvisakers’ aspects of self-initiation, self-monitoring, self-inhibition, flexibility/problem-solving, and strategic behavior. In 9–13 sessions, effective execution of plans “in vivo” was addressed. Initiative was facilitated by linking plans to an external device, such as a diary, alarm, PDA (personal digital assistant), or mobile phone, or to a routine activity, such as lunch time or the morning hygiene routine to prompt the first step. The next steps of execution and monitoring were taught according to GMT. Starting from session 17, PST was introduced to address problems that might arise during execution of plans. Examples of such problems were sudden situational changes, “open-ended” situations with multiple choices or overwhelming internal or external conditions (e.g., unexpected time pressure). PST was thus used as a method to cope with “what if…- questions” (see Stage 2) in reality. Patients used fixed worksheets to monitor, report, and discuss their performance in the therapy sessions. To stimulate generalization, the patients were repeatedly told they could use the proposed strategy to cope with all multi-step tasks in daily life and continually encouraged to do so.
A (Dutch) treatment manual can be freely obtained from the first author.
Control Treatment
Control treatment was Cogpack (Marker, Reference Marker1987), an individually administered computerized cognitive training package consisting of several repetitive exercises. It is aimed at improving general cognitive functioning (like reaction speed, attentional functioning, memory, and planning). The program is self-supporting; most tasks can be performed without assistance, but a therapist was present to provide support when needed. Task performance was followed by direct feedback from the computer program so that patients could gain insight into their strengths and weaknesses. Improvements over time could be monitored. However, no clues about strategic approaches to the proposed tasks were offered by the therapist. Just like in the experimental treatment, after 10 sessions patients were asked to formulate three goals they wanted to achieve by means of the training. During the remaining sessions, patients could freely select the exercises that they deemed useful for reaching these goals.
Measures
An extensive battery of tests and questionnaires was administered to the patients (see also Boelen, Spikman, Rietveld, & Fasotti, Reference Boelen, Spikman, Rietveld and Fasotti2009). For this study, the following instruments were relevant.
Primary outcome measure
To measure executive functioning at a social participation level, The Role Resumption List (RRL: Spikman, Brand, & Brouwer, Reference Spikman, Brand and Brouwer2003) was administered three times. Based on a structured interview with the patient, the RRL assesses changes in amount and quality of activities compared with premorbid levels in four daily life domains (vocational functioning; van Zomeren & van den Burg, Reference van Zomeren and van den Burg1985), social interaction with proxies, leisure activities, and mobility). These domains were rated by an assessor on a 5-point scale (0 = no change, 4 = severe loss of independence), with a total score ranging from 0 to 16. Based on the transcript of the interview, an independent rater also filled in the scale. The interrater agreement for the four scales (Cohen’s Kappa Scores) separately was .75, .43, .72, and .57, respectively. Because the total scores of both raters did not cover the same range, a Spearman correlation coefficient was calculated. This turned out to be high and significant (.97).
Adjunct outcome measures
Another measure for treatment effectiveness was derived from Goal Attainment Scaling, a method used to measure level of attainment of individual goals, originally introduced by Rockwood, Joyce, and Stolee (Reference Rockwood, Joyce and Stolee1997). In our version, Treatment Goal Attainment (TGA), patients in both groups had to determine three personal goals they wanted to accomplish by means of the training. This took place after ten training sessions, after patients had been given the opportunity to gain some insight into their strengths and weaknesses. After treatment, patients were asked to indicate on a 5-point scale (1 = not at all, 5 = entirely) to what extent they had attained each goal; the total score thus ranged from 3 to 15. At follow-up, patients were asked to rate this again.
To measure EF in a complex task, a newly designed test was administered at follow-up only. The Executive Secretarial Task (EST; Lamberts, Evans, & Spikman, Reference Lamberts, Evans and Spikman2009; Spikman, Hol-Steegstra, Rietberg, Vos, Boelen, & Lamberts, Reference Spikman, Hol-Steegstra, Rietberg, Vos, Boelen and Lamberts2007) is an ecological EF task, in which a job assessment procedure is simulated. This 3-hr task is comparable to the Multiple Errands Tasks (Shallice & Burgess, Reference Shallice and Burgess1991) or the Hotel Task (Manly, Hawkins, Evans, Woldt, & Robertson, Reference Manly, Hawkins, Evans, Woldt and Robertson2002) in that it requires the organization, initiation, and prioritization of multiple tasks over a longer time span than usual, while dealing with delayed intentions, interruptions, and deadlines. The task yields three scores, together forming a Total Score: Initiative; reflecting all the actions the subject has initiated without being told so, Prospective; reflecting all the actions that were correctly carried out in a later stage, and Executive, reflecting all the actions that were correctly carried out at all. The EST was administered only at follow-up. This warranted a “pure” indication of executive functioning without contamination of previous test knowledge or experience.
Questionnaires and observation lists
The presence of executive symptoms in everyday life was investigated by means of the Dysexecutive Questionnaire (DEX: Burgess et al., Reference Burgess, Wilson, Evans, Emslie, Wilson, Alderman, Burgess, Emslie and Evans1996), with a patient, proxy, and therapist version.
The Executive Observation Scale (EOS, based on Pollens, McBratnie, & Burton, Reference Pollens, McBratnie and Burton1988) consists of eight items covering the EF aspects distinguished by Ylvisaker. Items are rated by the therapist on a scale from 1 (complete inability) to 4 (complete independence) with a total score ranging from 8 to 32.
Quality of life was measured with the QOLIBRI (Quality of Life after Brain Injury (early version; VonSteinbüchel, Petersen, & Bullinger, Reference VonSteinbüchel, Petersen and Bullinger2005) filled in by the patient. The QOLIBRI consists of two parts; a satisfaction scale (higher score indicates more satisfaction) and a burden scale (higher score indicates higher burden).
At follow-up, patients were requested to rate their levels of satisfaction with treatment (results) on a 5-point Treatment Satisfaction Scale (TSS), ranging from score 1 (not satisfied) to score 5 (very satisfied).
Neuropsychological measures of executive and cognitive functioning
In addition to the mentioned behavioral measures, the following EF tests were administered. The Behavioural Assessment of the Dysexecutive Syndrome (BADS; Wilson et al., Reference Wilson, Alderman, Burgess, Emslie and Evans1996); all six subtests, resulting in a Standard Age Score. The Trail Making Test (ratio B vs. A, TMT B/A), Stroop Test (Stroop, Reference Stroop1935) (ratio time part three vs. time part two, Stroop 3/2); Tower of London (Shallice, Reference Shallice1982), (TOL, number correct).
To control for possible effects of the Cogpack training on memory, the 15 Words Test [Dutch version of RAVLT (Deelman, Brouwer, van Zomeren, & Saan, Reference Deelman, Brouwer, van Zomeren, Saan, Jennekens-Schinkel, Diamant, Diesfelt and Haaxma1980); immediate (Memory IR) and delayed recall score (Memory DR)] was administered at baseline and T1.
Statistical Analyses
T tests were used to compare patients’ scores on relevant measures at baseline with those of healthy controls, as well as to verify whether both patient groups differed at baseline.
Treatment effects were analyzed as follows. The primary outcome measure, the RRL, as well as those adjunct outcome measures for which a pre- and post-measurement was available, were analyzed using repeated measures analyses (GLM repeated measures, SPSS 16.0). Because data loss due to missing values was undesirable, all test-measures were analyzed separately in a univariate design, in three series. First, it was tested whether scores at T1 differed from baseline, visible in a time-effect, and whether improvement over time differed between experimental and control patients, reflected in an interaction effect. Similarly, scores at T2 were compared with baseline performance, as well as to performance on T1. To overcome the probability of spurious results, Bonferroni Holm corrections were applied.
Because there were no baseline measures for the TGA and the EST, results at T1 and T2 were compared using t tests. EST results of both patient groups were also compared with those of a healthy control group. Finally, the skewed scores on the TSS were compared using a nonparametric Mann-Whitney U test.
Because we considered executive performance at follow-up the most important indication of treatment success, a two-way multivariate analysis of variance (MANOVA) was performed on the measures that represented various relevant facets of executive functioning in daily life, namely the EST, TGA, and RRL.
RESULTS
To verify whether the patients had impaired EF and thus fulfilled inclusion criteria, their BADS and DEX scores were compared with those of a healthy control group. Table 1 shows that both groups were comparable with respect to educational level. However, healthy controls were slightly older, but as age is known to influence executive functioning negatively, this was not advantageous. On the BADS, patients showed significantly worse EF than healthy controls. Also, patients had higher scores on the three DEX- measures evidencing more executive problems in daily life.
After inclusion, patients were randomly assigned to one of the treatment conditions. Table 1 shows that at baseline no significant differences between the experimental and control patient groups were present on several relevant measures, such as indications of cognitive functioning (IQ, memory) and EF (Stroop 3/2, TMT B/A, TOL, BADS). Nor were there any differences on questionnaires measuring dysexecutive complaints (DEX), executive functioning observed during task performance by therapists (EOS), the extent to which previous roles were resumed (RRL) and the satisfaction part of the Quality of Life questionnaire (QOLIBRI). The only significant difference regarded the QOLIBRI Burden Score: before treatment experimental patients experienced a higher burden due to brain injury than control patients.
Effects of Treatment
Table 2 shows the results of repeated measures analyses on the primary outcome measure, the RRL. After treatment (T1), both groups had resumed their previous roles significantly more than before treatment, but the experimental patients to an even larger extent. The same result was found for T2, when compared with baseline. From post-treatment to follow-up, only the experimental group showed further improvement over time. Additional univariate analyses of variance were performed at T1 and T2 to find out whether there were differences between treatment centers; both analyses showed that this was not the case (T1: F(1,17); p = .34; T2: F(1,88); p = .124).
Table 2. Means (and SD) of the primary outcome measure Role Resumption List (RRL) at T0, T1, and T2

Note
Means of the individual difference scores (T1-T0, T2-T0, T2-T1) and repeated measures analyses on the test-scores at T1 compared to T0, T2 compared to T0 and T1 compared to T2. ANOVA = analysis of variance.
*p < .05; ** p < .01; ***p < .001.
Table 3 shows the results of repeated measures analyses on the adjunct outcome measures, on T1 compared with T0. Significant effects were found on several indications of daily life executive functioning. The DEX-patient and DEX-proxy showed that decrease of executive complaints was similar for both groups. On the DEX-therapist both groups also showed less executive problems after treatment, but the decrease was significantly larger for the experimental group. Executive abilities observed by professionals (EOS), had improved in both groups, but significantly more in the patients of the experimental group. On the standard EF tests (Stroop 3/2, TMT B/A, TOL, and BADS), no interaction was found, indicating differential treatment effects. The TOL and the BADS showed a time effect, but this was the same for both groups. With respect to quality of life, both the QOLIBRI Satisfaction and Burden scale showed improvement of satisfaction and reduction of burden after treatment to the same extent in both groups. Both groups also improved to the same extent on the Memory IR-score over time.
Table 3. Means and (SD) and means of individual difference scores (T1−T0) of the adjunct outcome measures at T1 for control and experimental group

Note
ANOVA = analysis of variance; IR = immediate recall; DR = delayed recall; TMT = Trail Making Test; TOL = Tower of London; BADS = Behavioral Assessment of the Dysexecutive Syndrome; DEX = Dysexecutive Questionnaire; EOS = Executive Observation Scale; n.s. = not significant.
* Significant p value < Bonferroni Holm corrected alpha.
Table 4 shows the results of repeated measures analyses at T2 compared with T0. Similar to T1, both groups showed improvement on the EOS but progress in the experimental group was significantly larger. For neuropsychological EF tests, including the BADS, once more no interaction effects were found. This time the Stroop and the BADS showed a time effect. This was also the case for the DEX-patient and -therapist. No effects were found on the QOLIBRI scales.
Table 4. Means (and SD) and means of individual difference scores (T2-T0) of the adjunct outcome measures at T2 for control and experimental groups

Note
Repeated measures analyses on the test scores at T2 compared to T0. ANOVA = analysis of variance; TMT = Trail Making Test; TOL = Tower of London; BADS = Behavioral Assessment of the Dysexecutive Syndrome; DEX = Dysexecutive Questionnaire; EOS = Executive Observation Scale; n.s., not significant.
* Significant p value < Bonferroni Holm corrected alpha.
In addition, repeated measures analyses were applied to the scores at T2 compared with T1. None of the adjunct measures showed further improvement over time for the experimental group.
Table 5 shows means and results of t tests for measures without baseline, the TGA and the TSS. At T1 as well as at T2, the patients of the experimental group had attained their previously set goals (TGA) to a significantly larger extent than the control group. The TSS showed no difference, indicating that both experimental and control groups were equally satisfied with the treatment (results).
Table 5. Means (and SD)of the TGA at T1 and T2, and for the TSS at T2 for the control and experimental group

Note
Two-tailed results of t tests on the TGA score and Mann-Whitney U test on the TSS score. TGA = Treatment Goal Attainment; TSS = Treatment Satisfaction Scale; Sign. = significance; n.s., not significant.
**p < .01; ***p < .001.
Table 6 shows the results of t tests between both patient groups on the EST Total Score and on EST subscores (Initiative, Prospective, and Executive). For this test, a few data were missing. Three patients had not been able to attend and the data of two other patients were lost. The experimental group performed significantly better on all scores, except for the Initiative score. Comparison of both groups with healthy controls showed that the control patients performed worse than the healthy reference group on all four EST scores, and the patients of the experimental group only on EST Total Score and the Executive score.
Table 6. Means (and SD) of control and experimental groups and healthy controls on the total score and the three subscores of the EST

Note
Two-tailed results of t tests. EST = Executive Secretarial Task; CG = control group; EG = experimental group; HC = healthy controls.
*p < .05; ** p < .01; ***p < .001.
Table 7 shows the results of a MANOVA on indications of daily life executive functioning (the EST, RRL, and TGA) at T2. A significant treatment effect on the combination of these three measures was found. Univariate analyses showed that this could be mainly attributed to the RRL and the GTA.
Table 7. Results of a multivariate analysis (MANOVA) at T2 on the EST, the TGA, and the RRL for the control and experimental groups

Note
EST = Executive Secretarial Task; TGA = Treatment Goal Attainment; RRL = The Role Resumption List; Sign. = significance; n.s., not significant.
DISCUSSION
The results of this study were two-fold. Those measures that were considered indications of daily life executive functioning showed that a multifaceted treatment for executive dysfunction after ABI led to a significant treatment effect for the experimental group compared with the control group, lasting at least 6 months post-treatment. However, other measures regarding well-being and subjective complaints showed similar improvements for both groups, whereas conventional cognitive and executive tests showed only time effects or no effects at all.
The improvement in daily life executive functioning was evidenced on the primary outcome measure, the RRL, indicating the ability to resume previous roles in work, social relations, leisure activities, and mobility and therefore of functioning at social participation level. Our expectation that these effects on daily life EF would be present after treatment and would subsequently remain stable for a substantial period was entirely met. The significant differences between both groups after treatment were even larger at follow-up, indicating that the experimental group had increased their wished-for roles in daily life even further, whereas the control group remained at the same activity level throughout. In addition, two other indications of daily life functioning showed significant differences between both groups after treatment; the TGA, reflecting the ability to set and accomplish realistic goals, and the EST, reflecting the ability to plan, organize, and regulate a series of real-life tasks. On the TGA, differences were evident after treatment as well as at follow-up, indicating that the patients of the experimental group had attained their goals to a larger extent than the control patients. In the EST, only administered at follow-up, the experimental group performed significantly better on all scores, except for the Initiative subscore.
The RRL was the only measure that was administered blindly at every measurement. For different reasons, both the TGA and the EST could not be administered at baseline. The TGA had to be an indication of the ability to set realistic treatment goals, for which participants had to gain insight into their strengths and weaknesses in the first part of the treatment. In both treatment conditions, goals were therefore set after the first 10 sessions. The EST is a complex problem-solving test, which is not appropriate for use in retest conditions. When EF tests are administered repeatedly, crucial aspects of novelty and problem-solving become biased by learning effects (for instance, retaining solutions and strategies in memory), which will influence performance a second time. Hence, these effects cannot be disentangled from the pure executive problem-solving components of test performance. Therefore, the BADS was not considered appropriate as a primary outcome measure in the present study. As expected, no treatment effects were found on the BADS; compared with baseline, both groups had improved to the same extent at T1 and T2, which we interpret as a test–retest effect. Previous evidence for a considerable test–retest effect on the BADS was found by Jelicic, Henquet, Derix, and Jolles (Reference Jelicic, Henquet, Derix and Jolles2001).
Although significant group differences were found on the TGA and EST, the lack of pretreatment measures suggests that these differences cannot be ascribed as strongly to treatment effects as in the case of the RRL. Nevertheless, there are robust indications that these differences are due to therapy. After all, both groups were carefully randomized (see Table 1) and they did not differ significantly with respect to their initial executive and cognitive capacities, nor with regard to biographical variables at baseline.
The significant difference found with a MANOVA performed on the combination of the three effect measures (RRL, TGA, and EST) at follow-up, indicated even more strongly that treatment effects were still present and clinically substantial at follow-up.
In our multifaceted treatment, several elements of proven treatment methods had been incorporated, namely Goal Management Training (Levine et al., Reference Levine, Robertson, Clare, Carter, Hong and Wilson2000) and Problem Solving Training (von Cramon et al., Reference von Cramon and Matthes-von Cramon1994). However, the additional value of our protocol is its multifaceted character: a comprehensive but finite range of dysexecutive symptoms is addressed, including problems with self-awareness and self-initiative. Another distinct feature is transfer to daily life situations as an integral treatment element. Training effects were measured and found on indications of EF at activity as well as social participation level. In our treatment protocol, Ylvisaker’s eight EF aspects, self-awareness, goal setting, planning, self-initiation, self-monitoring, self-inhibition, flexibility, and strategic behavior, were explicitly addressed and embedded in practical exercises and home assignments. Improvements on these aspects become obvious when the measures reflecting daily life functioning are analyzed. The ability to set and accomplish realistic goals in daily life, as reflected by the TGA, depends on the capacity to be aware of one’s needs, strengths and weaknesses. In the EST, patients have to plan and organize task execution toward preset goals. No cues or directions are provided, so patients must initiate these tasks, carry them out and simultaneously monitor their performance. Flexibility is necessary to adapt the execution of plans to changing circumstances and to solve potential problems, and this also requires the ability to self-inhibit irrelevant actions. The ability to apply all these skills at a strategic level is reflected in the RRL scores, which indicate the performance of relevant activities in daily life roles.
A more direct indication of improvement on Ylvisaker’s eight EF aspects was the score on the EOS which reflected each of these aspects. Compared with baseline both groups improved significantly at T1 as well as at T2, although on both occasions the experimental group’s improvement was significantly larger. Unfortunately, the EOS (and DEX-therapist) cannot be rated blindly, because they require knowledge of the subjects’ daily life executive functioning. Thus, despite this encouraging result, the scores on both measures should be interpreted with caution, as the therapists’ awareness of treatment condition might have influenced the score.
Our study demonstrated that it is actually possible to treat patients with dysexecutive problems, which is not always taken for granted (Alderman, Reference Alderman1991). After all, dysexecutive problems are known to hamper the acquisition of strategic behavior and the ability to benefit from treatment. Nevertheless, our experimental patient group was able to adhere to and remain motivated throughout an intensive, laborious treatment. Results on the TSS show that these patients appreciated the training and its effects on their lives. The lower scores on the DEX-patient and -proxy at T1 and T2 indicate that patients and proxies experienced and observed less dysexecutive problems after treatment. However, this was also true for the control patients. With respect to quality of life, both groups also showed the same pattern of results. This result was surprising, because we feared that patients’ motivation to perform the long and energy sapping Cogpack training would fade or that they would sense that this treatment would not be effective. On the contrary, the majority of patients were very enthusiastic about Cogpack as it provided immediate feedback on performance, so that patients could monitor their improvement over time. This suggests that Cogpack influenced patients’ sense of self-efficacy positively, exactly as the experimental training did. Apparently, this has led to higher levels of activity in control patients, reflected by increased social participation, although not to the same extent as in patients of the experimental group. In addition, the control training also ameliorated trainees’ executive abilities, rated by therapists on the DEX-therapist and the EOS.
As expected, there was no indication that either of the treatments had significant effects on cognitive or executive functioning as measured with neuropsychological tests, especially on the Stroop, Trail Making Test, and TOL as well as the memory test. This lack of effects on common executive tests indicates how difficult it remains to assess executive functions in daily life, and especially changes in daily executive functioning, with conventional neuropsychological tests. Additional research will be needed in the future to disentangle the relation between executive tests and daily life. The lack of effects on neuropsychological tests (including memory tests) also shows that Cogpack training did not live up to its promise of improving basic cognitive functions.
To summarize, control patients’ levels of satisfaction and subjective well-being were equal to those of the experimental group, although the latter group performed significantly better on measures that pertained to daily life executive functioning. This indicated that significant treatment effects can be accomplished by a multifaceted treatment, if it is aimed at improving activity and social participation and tailored to the individual patient. Moreover, these effects last for a substantial period after ending the treatment.
ACKNOWLEDGMENTS
This study was supported by The Dutch Organization of Health Research and Development (ZON-MW), Rehabilitation Research Program (Grant No. 1435.0009). The authors thank all the participants and their families, as well as the healthcare institutions and their neuropsychologists that participated in this study: Revalidatiecentrum Amsterdam (Amsterdam), Revalidatie Friesland (Beetsterzwaag and Leeuwarden), Academisch Revalidatiecentrum Beatrixoord (Haren), Revalidatiecentrum Heliomare (Wijk aan Zee), Revalidatiecentrum Groot Klimmendaal (Arnhem), Sint Maartenskliniek (Nijmegen), Revalidatiecentrum De Vogellanden (Zwolle), and University Medical Center Groningen (Groningen). We thank Anneke Hol, Sylvie Vos, and Hennerieke Rietberg for their assistance with the data collection.










