Published online by Cambridge University Press: 01 July 2004
The central executive component of working memory has been argued to play an important role in the performance of span tasks, particularly backward span. Age-related decline in central executive function has also been reported, and yet there have been inconsistent findings to indicate that with increasing age, the discrepancy between forward and backward span increases. A secondary analysis of the Wechsler Memory Scale–Third Edition standardization sample (N = 1030) was performed to investigate this relationship. It was hypothesized on the basis of past research indicating an age-related decline in central executive performance, that backward digit and spatial span performance would decrease at a greater rate than forward span performance. However, the results indicated that the rate of age-related performance decline was equivalent for both measures. It is proposed that both forward and backward span tasks recruit central executive resources for successful task performance. (JINS, 2004, 10, 475–481.)
Working memory (WM) has been described as the “desktop of the brain” (Logie, 1999), in an attempt to encapsulate the temporary storage and on-line, multi-component processing system first outlined by Baddeley and Hitch (1974). The model proposes storing new information in specialist, limited-capacity verbal (phonological loop) and visuospatial (visuospatial sketchpad) components, while processing incoming or recently accessed information using a central executive and episodic buffer (Baddeley, 2000). Age-related decline in WM has been reported in numerous studies (Robbins et al., 1998; Salthouse, 1994, 1996a, 1996b; Van der Linden et al., 1994, 1998), and has been linked to a decline in executive functioning with increasing age (Brennan et al., 1997; Daigneault & Braun, 1993; Keys & White, 2000); the central executive component of WM reported to be particularly vulnerable to aging (Fisk & Warr, 1996).
The Digit Span and Spatial Span subtests from the Wechsler Memory Scale–Third Edition (WMS–3; Wechsler, 1997) have been used extensively as simple clinical measures of WM (see Lezak, 1995). Both span tasks have a forward and a backward element, and require the stimulus (digits or spatial locations) to be recalled in the same order as given by the examiner (forward span), or in the reverse order (backward span). Forward digit span has been argued to represent a measure of the capacity of the phonological loop (Baddeley, 2000) and the forward spatial span may be considered an analogous measure of the visuospatial sketchpad (Wechsler, 1997). Successful performance on the backward element of these tasks has been argued to represent a measure of central executive function due to the additional requirement of manipulation of information within temporary storage (Groeger et al., 1999; Lezak, 1995). Furthermore, a number of studies have argued for central executive deficits in clinical and nonclinical samples on the basis of impaired backward span performance (Curtiss et al., 2001; Dobbs et al., 2001).
Age-related decrease in forward and backward digit span performances have been reported (Babcock & Salthouse, 1990; Gregoire & Van der Linden, 1997), and a meta-analysis of 14 studies by Babcock and Salthouse (1990) indicated that age-related decreases for backward digit span performance were greater than those for forward span (14% to 8% respectively). This analysis supported the clinical perspective that, with advancing age, forward digit span tends to remain stable, whereas backward digit span tends to decline (Lezak, 1995).
However, Gregoire and Van der Linden (1997) found that in the standardization of the French adaptation of the WAIS–R digit span task (a sample of 1000 participants aged between 16–79), increasing age did not increase the discrepancy between forward and backward digit span performance. Their findings indicated an age-related decline in performance of both forward and backward digit span, but the rate of decline for the two tasks did not significantly differ.
The expectation of a greater age-related decline in backward span performance appears to rely on two assumptions: (1) that the backward span tasks require central executive involvement; and (2) that central executive performance declines with normal aging. Support for the first assumption is derived primarily from the theoretical model of WM in which the central executive plays a crucial role in manipulation of information from the slave systems (phonological loop and visuospatial sketch pad; Baddeley, 1986, 1996, 2000, 2002). The active and conscious process of manipulating information to be recalled (Belleville et al., 1998) is argued to be required in the reversed reporting of either tapped blocks or spoken digits in the backward span tasks (Baddeley, 1986; Pearson et al., 1999).
Support for the second assumption is based on the consistent finding that executive skills decline in normal aging (Belleville et al., 1998; Brennan et al., 1997; Fisk & Warr, 1996; Keys & White, 2000; Schretlen et al., 2000; Van der Linden et al., 1998). However some authors have suggested that this decline is not generalized, with some specific components of executive skills being relatively resilient to the effects of aging (Baddeley et al., 1986, 2001; Greene et al., 1995).
In the present study, we expect that an age-related decline in digit and spatial spans will be observed in a large community sample. More specifically, given past evidence of an age-related decline in executive skills, we hypothesize that the backward span will decline at a greater rate than for the forward span because of the additional reliance on executive functioning in backward span tasks.
The study represents a secondary analysis of the standardization data compiled by the Psychological Corporation for the publication of the Wechsler Adult Intelligence Scale and Wechsler Memory Scale, Third Edition (WAIS–3 and WMS–3; Wechsler, 1997). The data comprised the results of the 1030 unique cases from the participants who completed both the WAIS–3 and WMS–3 standardization procedure for the Psychological Corporation during 1995, as reported in the WAIS–3/WMS–3 Technical Manual (Wechsler, 1997). The sample was recruited to be representative of the continental U.S. census data for 1995, on the basis of gender, race, ethnicity, education level and geographic distribution (see the Technical Manual (Wechsler, 1997) for further details). The age range for this sample was between 16 and 89 years of age, with a mean age of 49.60 years. The sample may be divided into 13 age groups: 16–17 years; 18–19 years; 20–24 years; 25–29 years; 30–34 years; 35–44 years; 45–54 years; 55–64 years; 65–69 years; 70–74 years; 75–79 years; 80–84 years; and 85–89 years. The gender and average level of education for each age group is presented in Table 1.
Number of participants in educationa and gender categories across age groups in the WMS–3 Standardization Sample (N = 1030)

The participants were administered the WAIS–3 and WMS–3 using the standardized instructions and order of testing. For each participant the results of the digit span and spatial span subtests (forward and backward) were recorded. In the forward digit span task the experimenter verbally presented an increasingly longer series of digits (spans of 2–9), at a rate of 1 digit/s. The score was the total number of correct trials, prior to failing two consecutive trials at any one span size. The backward digit span task was also verbally presented, and the participant was required to reproduce the same digits in the reverse order. The score for the backward task was again the total number of correct trials, prior to failing two consecutive trials at any one span size. The spatial span task was conducted using a 21 × 37 cm board containing 10 three-dimensional blocks. In the forward task the experimenter tapped an increasing number of the blocks in a predetermined order, with the participant required to tap the same sequence of blocks. The backward spatial span task required the participant to repeat the increasing sequence of tapped blocks in the reverse order. The score for either the forward or backward tasks was the total number of correct trials prior to failing two consecutive trials at any one span size.
The mean performances on both the forward and backward span measures were plotted against the participant's age group. It can be seen from Figure 1 that there is a substantial age-related decline in the forward and backward digit and spatial span measures.

Mean forward and backward span performances for the digit and spatial span tasks across age groups in the WMS–3 Standardization Sample (N = 1030)
Simple regression analyses indicate that age was a significant predictor of both forward and backward elements of the digit and spatial span tasks (see Table 2). In particular, the slope measures (B and β) show that the impact of age on spatial span measures was greater than for digit span measures.
Summary of simple regressions with age predicting each of the forward and backward Digit and Spatial Span measures (N = 1030)

The distributions of digit and spatial span measures did not reveal ceiling and floor effects. Indeed only 1 participant from the sample of 1030 could not repeat a minimum of two digits forward and two digits backward, and only 3 participants could not repeat two blocks forward and two blocks backward. Only 5 participants on the forward digit span and 3 on digits backwards reached ceiling.
The potential confound of education and gender on the age-related decline of span measures was considered since, as shown in Table 1, participants in the younger age groups tend to have more years of education than their older counterparts, and similarly gender varies as a function of age. The combined effect of age, education and gender on span performance may be dependent on each other (i.e., interactive) or independent (i.e., additive). To assess if the effects of the three variables on span performance were interactive, separate hierarchical regression analyses were conducted on each of the digit and spatial span measures. For example, education and age were entered together in the first step, since there was no good reason to assign causal precedence to either factor, followed by the interaction (product term) of education and age in the second step.
Results in Table 3 show that both education and age, adjusted for each other, were significant predictors of span performance. Furthermore, the statistically nonsignificant changes in R2 (ΔR2) of interaction terms suggest that the effects of education and age on span performance were additive, and independent of each other. Indeed, using alpha level set at .01, the combined effects of two-way and three-way interactions of age, education and gender were not significant, for forward digit span (ΔR2 = .003, p = .343), backward digit span (ΔR2 = .008, p = .029), forward spatial span (ΔR2 = .002, p = .484) and backward spatial span (ΔR2 = .002, p = .358)
Summary of hierarchical regressions with education and age predicting each of the forward and backward Digit and Spatial Span measures (N = 1030)

Next, structural equation modelling was used to test if the rates of decline (i.e., the regression slope) between forward and backward spans across the age range were different, after adjusting for the effect of education, and gender. Figure 2 shows the just-identified model of education and age predicting forward and backward digit spans.

Structural equation model of education and age predicting Digit Span Forward and Backward measures, with eForward and eBackward as correlated error terms (N = 1030)
To test if the effect of age on forward span was the same as that on backward span, the partial regression coefficients (i.e., a, b) were constrained to be equal in the model. The resulting model [χ2(1,N = 1030) = 0.009, p = .926] indicated that age-related declines in forward and backward digit spans were not different, after adjusting for individual differences in education. Likewise, the result for spatial span [χ2(1,N = 1030) = 0.002, p = .964], indicated that age-related declines in forward and backward spatial spans were not different, after adjusting for individual differences in education. Even after adjusting for both gender and education, the effect of age was not different between forward and backward spans for digit span [χ2(1, N = 1030) = 2.624, p = .269] and spatial span [χ2(1, N = 1030) = 2.603, p = .272].
The results of the present study confirm the general expectation that verbal and visuospatial span performance decline with increasing age, and support previous research indicating that working memory declines with increasing age (Baeckman et al., 2000; Botwinick & Storandt, 1974; Fisk & Warr, 1996; Gregoire & Van der Linden, 1997; Salthouse et al., 1995; Vecchi & Cornoldi, 1999). It was noted that spatial span in general demonstrated greater age-related decline than digit span. However, of specific interest to the focus of this study, there is no evidence of a differential rate of decline between forward and backward digit and spatial span.
Before addressing the differential forward and backward span performances, the lack of symmetry in general decline of digit and spatial span demands some comment. An explanation may relate to the argument that verbal materials such as digit spans, are relatively practiced and automatized, thereby requiring minimal demand on executive resources. In contrast, visuospatial material is typically less practiced, places more demand on executive resources and hence is more sensitive to age-related decline in central executive processing (Baddeley, 1996; Miyake et al., 2001).
The influence of education and gender on both forward and backward span tasks was also considered in our analyses. The results demonstrated that education provided a significant contribution to the explanation of variance in both spatial and digit span performance. However, the contribution of education was additive, rather than shared with the influence of aging, with the age-related decline in forward and backward span performance remaining after accounting for the variance explained by education. Furthermore, the rate of age-related decline in forward and backward span performance, for both digit and spatial tasks, remained equivalent after accounting for the variance explained by education. Gender, independently or via an interaction with education, age or both, did not account for significant variance in the performance of either span task.
In respect to the issue of the aging of the central executive, it should be acknowledged that much of the existing research documenting a central executive decline has failed to account for age-related change in information-processing speed. Salthouse (2000) and Verhaeghen and De Meersman (1998), amongst other researchers, argue that age differences in tests of central executive (the Stroop interference effect for example) may be explained by partialling out the contribution of variance provided by measures of processing speed. Nevertheless, even using a processing speed explanation of aging, it has been acknowledged that older adults have been found to be especially disadvantaged when required to perform complex or multiple tasks within a restricted time, because there are more cognitive components to be slowed (Salthouse, 1996a). Furthermore, much of the evidence for an age-related decline in central executive performance is based on dual task methodology (McDowd & Shaw, 2000; Salthouse et al., 1995). However, Della Sala and Logie (2001) argue that a number of these findings may have resulted from combining constituent tasks on which an age-related decrement was already evident. Therefore, simply combining single tasks in which an existing age-related decrement has been identified confounds the interpretation of dual-task performance. Indeed, several studies have been able to demonstrate that normal aging does not necessarily result in dual-task decrement when initial performance of the constituent tasks has been adjusted across age groups (Baddeley et al., 1986; Belleville et al., 1998; Greene et al., 1995).
An alternative assumption to explain the present results might therefore be that normal aging does not directly impact on the central executive, but that its efficiency can be reduced by age-related decline in the domain-specific stores in the slave systems. This alternative assumption can account for the results we have presented here, as we have found that forward span as well as backward span declines with age. An age-related limit in the amount of information available to the central executive through the passive storage of the slave systems will necessarily reduce the efficiency of the central executive. An age-related decline in passive storage capacity is sufficient to explain our results.
In considering the assumption as to whether backward span differentially demands central executive resources, our results concur with other recent research studies that both forward and backward span tasks recruit central executive resources for successful performance (Gregoire & Van der Linden, 1997; Miyake et al., 2001). While this alternative hypothesis is parsimonious with the present results, it initially appears to conflict with the theoretical foundations of WM (Baddeley, 1996, 2001).
Many researchers argue that forward span requires the relatively automatic processing of the slave systems in WM as required for immediate serial recall without reorganization of material (passive storage). Consequently, demand on central executive is minimal. In contrast, backward span, albeit in a limited fashion, requires transformation and manipulation of information while simultaneously storing information. Thus, demand on the central executive in this situation is expected to be significant. However, although Baddeley (1996) suggested that the level of performance on the digit span task, which was argued to involve relatively little complex processing, would be determined primarily by storage rather than executive function, he also cautioned (Baddeley, 1996, 2001) that maximal verbal memory span depended on both the phonological loop and central executive, “as the digit load increased, the demands made on the central executive will increase”(Baddeley, 1996, p. 11). Neuroimaging data also suggests that the DLPFC, implicated in a variety of central executive functions, is significantly activated during supra span tasks (Cabeza & Nyberg, 2000; Cohen et al., 1997; Klingberg et al., 1997; Rypma et al., 1999).
The lack of a clearly differentiated rate of span decline in forward and backward span provides an impetus for research to consider the specificity of the contribution of central executive function to performance of span tasks in younger and older adults. The role of the central executive in clinical measures of working memory has remained underspecified, and further experimentation into this area would clarify the contribution of central executive performance to commonly used measures of WM.
The authors would like to thank Drs J.J. Zhu, Larry Price, Larry Weiss, Agnes Stephenson, and Linda Murphy for their assistance with the acquisition and permission to use the data reported in this paper (Standardization data of the Wechsler Memory Scale: Third Edition. Copyright 1997 by the Psychological Corporation, a Harcourt Assessment Company. Used by permission. All rights reserved).

Number of participants in educationa and gender categories across age groups in the WMS–3 Standardization Sample (N = 1030)
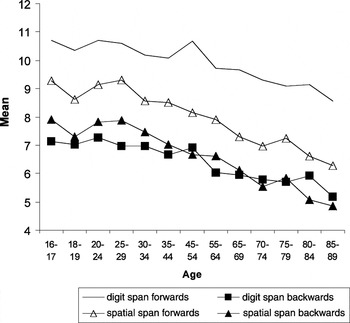
Mean forward and backward span performances for the digit and spatial span tasks across age groups in the WMS–3 Standardization Sample (N = 1030)
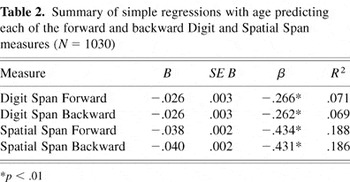
Summary of simple regressions with age predicting each of the forward and backward Digit and Spatial Span measures (N = 1030)

Summary of hierarchical regressions with education and age predicting each of the forward and backward Digit and Spatial Span measures (N = 1030)

Structural equation model of education and age predicting Digit Span Forward and Backward measures, with eForward and eBackward as correlated error terms (N = 1030)