INTRODUCTION
Human memory for temporal and spatial context information is naturally linked in daily life. Sequential events tend to coincide in time and space. Remembering a specific event you experienced (episodic memory) typically involves remembering not only the content (“what”), but also a spatiotemporal context: “where (location) and when (time and temporal order)” (see Healy, Cunningham, Gesi, Till, & Bourne, Reference Healy, Cunningham, Gesi, Till and Bourne1991). Recent studies have demonstrated the existence of dedicated neural mechanisms for integrating spatial and temporal information (Howard & Eichenbaum, Reference Howard and Eichenbaum2015). Despite their natural connection, there are several indications of separate processing mechanisms for spatial and temporal order information in episodic memory. Van Asselen, Van der Lubbe, and Postma (Reference Van Asselen, Van der Lubbe and Postma2006) showed that encoding of the location and temporal order is not obligatory.
In this study, we set out to dissociate these two highly relevant aspects of contextual episodic memory, directly comparing retrieval of very recent visual information for temporal order and spatial location in a parallel paradigm. One can assume that the “episodic buffer” is mainly recruited here, a system in the most well-known model of human working memory that is capable of binding contextual information (Baddeley, Reference Baddeley2000).
Previous neuroimaging and neuropsychological studies have globally sketched the neural circuitry of spatial and temporal order memory separately. Brain regions that are probably involved in spatial (working) memory are the dorsolateral prefrontal cortex, posterior parietal cortex and hippocampal formation (Constantinidis & Wang, Reference Constantinidis and Wang2004; Friedman & Goldman-Rakic, Reference Friedman and Goldman-Rakic1994; Glabus et al., Reference Glabus, Horwitz, Holt, Kohn, Gerton, Callicott and Berman2003; Inoue, Mikami, Ando, & Tskukada, 2004; Kessels, Postma, Wijnalda, & de Haan, Reference Kessels, Postma, Wijnalda and de Haan2000), specifically in the right hemisphere (Feigenbaum, Polkey, & Morris, Reference Feigenbaum, Polkey and Morris1996; Miotto, Bullock, Polkey, & Morris, Reference Miotto, Bullock, Polkey and Morris1996; Nelson et al., Reference Nelson, Monk, Lin, Carver, Thomas and Truwit2000).
Temporal order memory has been associated with activity in the prefrontal cortex and temporo-parietal junction (Zhang et al., Reference Zhang, Zhang, Sun, Li, Wang, He and Hu2004; Van Asselen et al., Reference Van Asselen, Kessels, Kappelle, Neggers, Frijns and Postma2006). Kessels, Hobbel, and Postma (Reference Kessels, Hobbel and Postma2007) investigated age-related decline in spatial and temporal order episodic memory. They speculate that the left hemisphere may be more involved in temporal order memory, and that the right hemisphere is more critical for spatial order memory. Binding contextual features has been related to prefrontal activation and a hippocampal-diencephalic “binding circuit.” Dissociations between these processes, however, have not been directly tested thus far.
The specific contextual features of location and temporal order are relevant to study as they are typically combined in many activities, such as route learning, and dysfunctioning of these processes due to brain damage can have significant impact on daily life and cause cognitive complaints (Van der Ham et al., Reference Van der Ham, van Zandvoort, Meilinger, Bosch, Kant and Postma2010). Importantly, the behavioral patterns observed in stroke patients can provide invaluable insights in the extent to which temporal order and spatial object location and the binding of these features in episodic memory are functionally dissociable. Whereas in Korsakoff patients binding problems seem most prominent (Postma, Van Asselen, Keuper, Wester, & Kessels, Reference Postma, Van Asselen, Keuper, Wester and Kessels2006), in the stroke population, with focal lesions and heterogeneous in terms of localization, differential patterns can be expected.
Stroke patients comprise an ideal population to study dissociations in cognitive processes because of their heterogeneity with respect to lesion location, which allows for broad exploration of patterns of cognitive functioning. There is evidence that different types of contextual features in episodic memory and binding of those features can be affected by stroke (Kessels, Kappelle, de Haan, & Postma, Reference Kessels, Kappelle, de Haan and Postma2002). Also, temporal order memory can be affected in stroke patients (Schoo et al., Reference Schoo, Van Zandvoort, Reijmer, Biessels, Kappelle and Postma2014). Moreover, Van Geldorp, Kessels, and Hendriks (Reference Van Geldorp, Kessels and Hendriks2013) studied target and location memory in stroke patients. They found problems in both types of memory, but not in binding context with content. In contrast, Postma et al. (Reference Postma, Van Asselen, Keuper, Wester and Kessels2006) found that Korsakoff patients showed problems particularly in binding contextual features. Importantly, a direct comparison between spatial and temporal order memory has not been performed in stroke patients.
The rationale for the current study was to investigate functional dissociations between different relevant dimensions of contextual memory, by directly comparing performance on temporal order versus spatial location and binding of those features in memory, and how these aspects can be differentially affected following focal neurological damage. Problems in separate contextual attributes might be expected, and/or specifically in binding those features together, depending on site of lesion.
In addition to the investigation of functional dissociations, observed patterns of lesion localization were used to search for convergent evidence of separate neural networks that underlie spatial and temporal order memory and binding of spatial and temporal features.
As an important global neural difference between spatial and temporal order memory is thought to involve hemispheric lateralization, dissociations in task performance of patients with left, right, and bilateral lesions were investigated. Moreover, individual patterns of performance were investigated and patients’ performance was related to localization of lesion. Patients with deficits in temporal order memory were expected to have lesions in frontal regions, possibly with left lateralization. Spatial location memory deficits were expected to be related to right hemisphere lesions, in frontal as well as temporal and parietal regions. Binding problems could be related to frontal and (medial) temporal damage.
By testing how performance on spatial and temporal order memory and binding of those features changes following stroke, we aimed to better understand these processes.
METHOD
Participants
From the University Medical Center Utrecht (UMCU), 36 patients were recruited with diagnosis of stroke based on clinical assessment and confirmed by imaging (CT, MRI), in accordance with inclusion regulations described in UMCU Medical Ethical Committee protocol 05–109. Exclusion criteria were diagnosed comorbid psychiatric or neurological diseases and conditions (such as dementia, as derived from the medical charts of the patients) and pre-existing impairments that would limit completion of the assessment (such as blindness). Practice conditions of the experimental procedure were used to detect whether there were any confounding (stroke-related) factors, such as neglect, that would hamper task performance to such an extent that a patient could not follow the experimental procedure.
Location of lesion was confirmed by an experienced neurologist (CJMF), using computed tomography (CT) or magnetic resonance imaging (MRI) scans that were obtained according to clinical practice. All available scans were used to determine the ultimate structural damage as exactly as possible, using a human brain atlas as reference (Duvernoy, Reference Duvernoy1999).
Forty-four age- and education-matched people without self-reported neurological or psychiatric disorders served as control participants. These participants were either spouses or family of patients or volunteers who came to our attention through word of mouth. Participants were treated in accordance with the Declaration of Helsinki.
MATERIALS
Educational level was coded according to Verhage and Van der Werff (1963), range 1 through 7 from less than primary school to university degree. Intelligence (premorbid) was estimated using the Dutch version of the National Adult Reading Test, scores corrected for age and gender (Schmand, Bakker, Saan, & Louman, Reference Schmand, Bakker, Saan and Louman1991). Dexterity was assessed using the Annett Handedness Inventory (Annett, Reference Annett2004).
Screening Tasks
The neuropsychological screening battery included standardized tests that measured reasoning ability, (working) memory, and attention/executive functioning.
Abstract reasoning was measured by the Raven Advanced Progressive Matrices Short Form (Arthur & Day, Reference Arthur and Day1994). The Dutch version of the Rey Auditory Verbal Learning Test (Saan & Deelman, Reference Saan and Deelman1986) was administered to measure verbal learning, delayed recall and recognition memory. To assess verbal working memory, the Letter Number Sequencing task was used (a subtest of the Wechsler Adult Intelligence Scale III, Wechsler, Reference Wechsler1997). Visuospatial working memory was measured with the Corsi-Block task (Kessels, Van Zandvoort, Postma, Kappelle & de Haan, Reference Kessels, van Zandvoort, Postma, Kappelle and de Haan2000). Attention/executive functioning was measured by the Trail Making Test parts A and B (Reitan & Wolfson, Reference Reitan and Wolfson1992).
Experimental Tasks
To assess spatial and temporal order memory and binding of these features, an object relocation paradigm was used that has shown to be able to differentiate between various memory aspects and is sensitive to differences between clinical groups (Van Asselen et al., Reference Van Asselen, Kessels, Kappelle, Neggers, Frijns and Postma2006; Postma et al., Reference Postma, Van Asselen, Keuper, Wester and Kessels2006). Experimental procedures and stimulus materials were largely adapted from Postma et al. (Reference Postma, Van Asselen, Keuper, Wester and Kessels2006). The paradigm allowed for comparison between recall for single features (spatial location vs. temporal order) as well as combined recall of both features together (spatiotemporal binding). Subjects were asked to either remember the location of objects presented visually on a computer screen, or the order of appearance, or both (Figure 1).
The tasks were designed to suit the specific demands that are involved in patient studies, keeping speed, information load and other general processing demands as low as possible. The experiment was designed using Object Relocation© software (Kessels, Postma, & De Haan, Reference Kessels, Postma and de Haan1999). Stimuli were colored pictures of highly familiar objects that were presented in a 19×19 cm square frame centered on a gray background screen on a 17” touch-sensitive LCD monitor (ELO Accutouch®, resolution=1024×768, viewing distance=65 cm). Average stimulus size was 1×1 cm. Stimuli were never reused in the experiment.
Two task conditions served as practice and allowed participants to familiarize themselves with the procedure. One practice run preceded each experimental condition (containing two trials). The recall phase posed no time restrictions.
In the first experimental condition (purely temporal), six objects were shown serially, each object for 3 s, always in the middle. Next, participants were instructed to place the objects in the correct temporal order on a one-dimensional horizontal array of six dots, that is, the leftmost dot should be assigned the object that was presented first and the rightmost dot should be assigned the last shown item.
In the second experimental condition (purely spatial), six objects were shown simultaneously for 18 s, at different locations. Next, participants were instructed to relocate the objects to their correct positions, which were marked by black dots in the square frame.
In the third experimental condition (spatiotemporal presentation—temporal order recall), six objects were shown serially for 3 s, each at a different location. Next, participants had to arrange the objects in their correct temporal order.
In the fourth experimental condition (spatiotemporal presentation—spatial order recall), presentation was identical to the previous condition, but participants relocated the objects to their correct position.
In the fifth experimental condition (fully combined), presentation was similar to that of the previous two conditions. In the recall phase, objects were to be placed at their correct location in the correct temporal order.
The conditions as described above were adapted from other studies used for different purposes (e.g., Van Asselen et al., Reference Van Asselen, Kessels, Kappelle, Neggers, Frijns and Postma2006). Of interest here was to contrast single feature recall (spatial location vs. temporal order) and combined spatiotemporal recall. To this aim, and to simplify statistical analysis by minimizing the amount of between group comparisons and thereby decreasing risks of chance capitalization, three main outcome measures were calculated: spatial location, temporal order, and binding. For each experimental condition, the percentage of incorrectly relocated objects was calculated. This “percentage incorrect” per condition was averaged across the two experimental conditions for each feature (i.e., temporal order was calculated by averaging performance on the first and third condition, spatial location by averaging the second and fourth condition). To obtain a measure of binding spatial and temporal features, the percentage incorrect was calculated for the fifth, fully combined, condition. A score of 100% incorrect in combined recall signified that both spatial and temporal relocation were 100% incorrect.
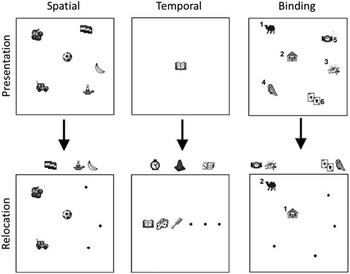
Fig. 1 Stimulus materials. Examples of spatial and temporal task conditions and the combined condition (binding). The digits were never shown but are used here to indicate the order of presentation and recall.
Data Analysis
Group differences
Group differences between patients and control participants’ demographical variables and neuropsychological task performance were tested using chi-square tests (gender, dexterity), Mann-Whitney U (educational level), two-tailed independent t tests (age, IQ), and multivariate analyses of (co)variances, controlling for gender and age (screening tasks). Bonferroni correction was applied for the screening tasks, resulting in adjusted criterion levels for significance of p<.005 and .0001.
Also, differences in demographical variables and stroke characteristics between the three patient subgroups (with left, right, and bilateral lesions) were tested, using analyses of variances for age, IQ, and Barthel Index scores (indicating stroke severity), and Fisher’s exact tests for educational level and lesion location (frontal, temporal/parietal, both or other).
To investigate overall differences between groups (control participants, and left, right, and bilateral stroke patients) on the experimental task conditions (spatial location memory, temporal order memory, and binding) a multivariate analysis of (co)variance was performed, with age and gender as covariates. Post hoc univariate analyses of variance (ANOVAs) and t tests were performed to investigate which groups differed on which variables.
Potential ceiling and floor effects were tested by comparing mean group performance on each condition to minimum and maximum level (0% and 100% incorrect) using one-tailed independent t tests.
Individual patterns
Because of heterogeneity of the patient group in terms of lesion location, a case study approach was used to investigate individual patterns of performance and dissociations with respect to lesion location. Statistical procedures by Crawford (Crawford, Howell, & Garthwaite, Reference Crawford, Howell and Garthwaite1998; Crawford & Howell, Reference Crawford and Howell1998; Crawford & Garthwaite, Reference Crawford and Garthwaite2002) were applied. Using these methods, single test scores were compared with norms derived from the control sample, to make an appropriate parametric statistical analysis of possible deficits. Essentially, this method is a modified independent samples t test in which the individual is treated as a sample of N=1. For the current study, the program “BTD_cov” was used: the Bayesian test for a deficit in a single case controlling for covariates, as described by Crawford, Garthwaite, and Ryan (Reference Crawford, Garthwaite and Ryan2011). Abnormalities of patients’ scores on spatial versus temporal order memory, and binding, were tested, correcting for age.
Patterns of deficits on the experimental tasks were explored in relation to standard neuropsychological performance (based on existing normative data) and site of lesion.
RESULTS
Demographical Data and Patient Characteristics
Table 1 shows that control participants did not differ from the patients with respect to level of education, (premorbid) intelligence, dexterity, and age, but were more often female than the stroke patients. Stroke is more prevalent in males (Wilson, Reference Wilson2013), and control participants typically included spouses and family members from the opposite sex. Potential influence of gender was, therefore, examined in the primary group analyses.
Table 1 Patient and control participant demographics and performance on screening tasks

Note. Group differences between patients and control participants were tested using chi-square tests (gender, dexterity), Mann-Whitney U (educational level), two-tailed independent t-tests (age, IQ), and multivariate analyses of variances, controlling for gender and age (screening tasks). RAVLT: Dutch version of the Rey Auditory Verbal Learning Test; TMT: Trail Making Test. °p<.05, *p<.005, **p<.0001 (Bonferroni correction for multiple comparisons was applied for the screening tasks).
Indications of stroke severity in terms of impact on activities in daily living (ADL) were derived from medical status at time of discharge. Barthel Index (Mahoney & Barthel, 1965) scores were estimated for each patient. For 21 of the 36 patients (58%), no significant ADL limitations were reported. The remaining 15 patients (42%) were mildly dependent in functional ability at time of discharge. Mean stay on the neurology unit was 12 days (SD=11). Destination of discharge was unknown for 1 patient, home for 58% of the patients (n=21), and a rehabilitation center for 10 patients (28%). Four patients (11%) were admitted elsewhere (to a hospital or residential care facility). Duration of hospital admission, discharge destination, and estimated stroke severity did not differ significantly across patient groups. Barthel Index scores were significantly correlated to the experimental variables. However, they did not explain group differences.
In the patient group, ischemic strokes predominated (28% hemorrhagic). Average interval between date of stroke and testing was 17 months (SD=8.2). Time between the index event and scanning varied, with a median of 5 days (interquartile range 1–18, when no infarction was visible on day 0 follow-up scans were used to confirm location of lesion). No influence of elapsed time between event and test date or between event and date of most recent scan was found on any variable. For 12 patients, only CT scans were available. Lesion locations in patients were widespread throughout the brain; 16 patients had strokes in the right hemisphere, 12 in the left, and 8 showed bilateral damage. Two patients had a history of a previous stroke.
Distribution of educational level, estimated premorbid IQ, lesion location (frontal, temporal/parietal, both or other) and Barthel Index scores (indicating stroke severity) did not differ significantly across patient subgroups with left, right or bilateral lesions (education p=.264, IQ p=.972, lesion location p=.442, stroke severity p=.563). As age was differently distributed between these subgroups (p=.041), it was controlled for in all further analyses.
Table 1 shows that patients on average scored worse than control participants on all neuropsychological measures, except the TMT index score (divided attention) and IQ.
Main Analyses
Group differences
Performance on spatial and temporal order memory and binding of spatial and temporal features was compared between groups In Table 2, performance of each group (control participants; bilateral, right, and left hemisphere stroke patients) on the experimental variables is shown.
Table 2 ANOVAs in object relocation task performance across groups

Note. * p<.05.
The assumption of homogeneity was met, as the test for equality of the covariance matrices was not significant (Box’s M=44.48; p>.05). However, error variances were not equal across groups for the variable “spatial location memory,” as shown by Levene’s test of equality, F(7,72)=4.00, p=.001. Error variances for “temporal order memory” and “binding” were not significantly different across groups (p>.05).
Using Pillai’s Trace, there was a significant overall group effect, V=.23, F(9,222)=2.05, p=.036. Effect size η p ²=.077, observed Power=.857. The covariate age significantly affected the outcome variables (F(3,72)=12.34, p<.001, effect size η p ²=.340, observed Power=1). The effect of gender was not significant (F(3,72=1.67, p=.178, effect size η p ²=.066, observed Power=.424).
In addition, using a general linear model with group and gender as between subjects factors, and experimental task condition as within subjects factor, no significant effects of gender were detected (spatial location p=.562, η p ²=.005; temporal order p=.828, η p ²=.001; binding p=.930, η p ²<.001), and no interaction effects between gender and group either (p>.05).
To investigate for which variables the groups differed, separate univariate ANOVAs on the outcome variables were performed. These revealed significant effects for temporal order memory (F(3,76)=4.02; p=.010; η²=.137), but not for spatial location memory (F(3,76)=0.82; p=.487; η²=0.031) and binding (F(3,76)=1.56; p=.206; η²=.058), see Table 2. Post hoc Bonferroni-corrected pairwise comparisons show significantly lower scores for temporal order memory in left-hemisphere stroke patients compared with control participants (p=.014; r=0.913) and bilateral stroke patients (p=.047; r=0.886), but not compared with right-hemisphere stroke patients (p=.633; r=0.760). Patients with bilateral or right hemispheric lesions did not perform worse than control participants (p>.05).
All participants were able to perform the tasks according to procedure, based on criteria qualitatively judged during administration of the practice conditions: they showed sufficient motor function for using the computer with touchscreen, and sufficient comprehension, attentional capacity, and perceptual ability to replace all objects following instructions. All participants were able to replace every object on the screen and did not incorrectly place more than one object on the extreme left or right side. Therefore, it was concluded that, in all included patients, the practice conditions were feasible and no signs of neglect or hemianopia were apparent to such extent that they interfered with the ability to comply with the task requirements.
No significant ceiling or floor effects were found on any condition, as mean performance was significantly different from 0% and 100% (p<.05) in both patients and control participants. However, the scores were not normally distributed (Kolmogorov-Smirnov and Shapiro-Wilk tests p<.001).
Crawford statistics
Each individual patient’s scores on spatial location, temporal order memory and binding were compared to the control participants group performance, controlling for age. Seven patients (18%) obtained abnormal scores (see Table 3, and Supplementary Material for test statistics).
Table 3 Performance for the seven individual patients who showed abnormal scores against the normative sample of control participants

Note. Abnormality of scores expressed in the (two-tailed) probability that the case’s score is an observation from the control population. Higher scores indicate worse performance (% incorrectly replaced objects).
a A check mark indicates no abnormal performance on this task; an x indicates a score below the 5th percentile or equivalent standardized score based on existing normative data; a dash indicates a missing data point. The neuropsychological assessment included measures of verbal learning and memory ((D)AVLT direct recall, delayed recall and delayed recognition), verbal working memory (WAIS CLN), visuospatial working memory (Corsi Block), attention/executive functioning (EF, Trail Making A/B index) and abstract reasoning (IQ, Raven Advanced Progressive Matrices short form).
b L=left hemispheric stroke, R=right hemispheric stroke, B=bilateral stroke.
* p<.05, ** p<.001, uncorrected.
Patterns of performance
Three patients showed deficits in spatial location memory, but not in temporal order memory (M.S., D.G., and L.S.), one of whom (D.G.) showed better performance on binding compared to the control participants. Two patients showed selective temporal order memory impairment (P.M. and P.S.), and two showed impaired performance on both spatial and temporal order memory (HB and JB), but not on binding. None of the patients performed significantly below the control mean for the binding condition.
To relate spatial and temporal order memory to traditional neuropsychological task performance, Table 3 shows patterns of deficits on standard measures of memory and executive functioning. Only one out of the five patients with impairment in spatial location memory showed decreased performance on a standard measure of visuospatial working memory. Decreased performance on the same measure was not found in any of the patients with temporal order memory deficits. In all patients with temporal order memory deficits, one or more aspects of standard measures of verbal (working) memory fell below normal performance, but which specific aspects were affected varied greatly. For example, in one patient (P.S.) all measures of verbal (working) memory were impaired, in another patient who showed deficits in both spatial and temporal order memory only one standard memory test value fell below normal performance (J.B., decreased performance on verbal memory delayed recognition). All patients with spatial location memory deficits also showed decreased performance on at least one measure of verbal (working) memory.
Decreased performance on a measure of attention/executive functioning did not occur in patients with selective spatial location memory deficits, but did occur in one patient with a selective problem in temporal order memory (P.M.) and in two patients with spatial as well as temporal order memory deficits (H.B. and J.B.). Only one of the seven patients showed problems in abstract reasoning, in this patient (M.S.) only spatial location memory was affected.
Additional Data Exploration
Lesion localization
In addition to the group analyses, individual patterns also showed laterality effects. Temporal order memory was associated with left lateralization: all four patients with abnormal scores on this measure had left hemispheric strokes. This group represents 25% of the patients with left hemispheric strokes in our sample. In comparison, none of the patients with right hemispheric strokes showed temporal order memory problems. Lateralization of spatial memory was less pronounced. Two of three patients with selective spatial location memory problems had right lateralized lesions, and one bilateral. The patients who showed both spatial and temporal order memory problems were left hemispheric stroke patients.
It was explored whether lesions in the stroke patients with problems in spatial and/or temporal order memory extended to the frontal, temporal and parietal lobes. Only one of these patients showed damage in the parietal lobe, and this patient displayed problems only with temporal order memory, and not with spatial location memory. To compare, 12 out of the 36 stroke patients (33%) did have parietal lesions, but did not perform abnormally. In two out of the four patients with temporal order memory deficits, the temporal lobe was affected. The frontal lobe was affected in one patient with selective temporal order memory deficits (P.S.), and in two of the patients with selective spatial location memory problems (M.S. and L.S.).
DISCUSSION
The aim of this study was to investigate functional dissociations between different dimensions of contextual memory in stroke patients, specifically comparing performance on temporal order memory, spatial location memory, and binding of temporal and spatial features. These processes are crucial for everyday activities such as navigation, where one needs to both keep track of the location of landmarks along the route as well as the order in which one encounters these. Pinpointing whether spatial and temporal memory processes can be differentially affected by stroke helps to better understand memory problems in patients.
The main outcome of the study was that functional dissociations between spatial and temporal order memory were found, and these were confirmed by lateralization effects.
First, a group effect was observed for temporal order memory, where patients with left-hemispheric lesions showed worse performance than control participants and bilateral stroke patients. Other studies also indicated left hemisphere involvement in temporal order memory (Hirose et al., Reference Hirose, Kimura, Jimura, Kunimatsu, Abe, Ohtomo and Konishi2013; Jacques, Rubin, LaBar, & Cabeza, Reference Jacques, Rubin, LaBar and Cabeza2008). In an extensive review, Nicholls (Reference Nicholls1996) described a left hemisphere advantage for temporal processes, among which memory for temporal sequences. The left hemisphere is specialized in a specific form of sequential processing: language (where processing of the sequential order of words in a sentence is necessary to distill its meaning). Nicholls states that language is one example of multiple functions of a left hemispheric “rapid sequential processor” in a broader sense. The current finding that patients with damage in the left hemisphere show impaired performance on the temporal order memory task is in line with this idea, especially since a visual task was used that required little linguistic processing.
No obvious laterality effect was found for spatial location memory on group level. This is slightly surprising, since spatial memory typically shows a right hemisphere bias. One possible explanation could be that, when the right hemisphere is damaged, the left hemisphere might be recruited to solve the spatial location task. A verbal encoding strategy might have been applied, since nameable objects were used in the current paradigm (see also Dent & Smyth, Reference Dent and Smyth2005; Kessels et al., Reference Kessels, Kappelle, de Haan and Postma2002). Also, the spatial task used here may be considered to involve categorical spatial relations, and no absolute spatial coordinates (i.e., the spatial locations were predefined and relocation could be solved by labeling of the relative position such as “the ball was shown below the church”).
Both verbalization and categorical memory for spatial locations are shown to be preferentially processed in the left hemisphere (Van Asselen et al., Reference Van Asselen, Kessels, Kappelle and Postma2008; Van der Ham et al., Reference Van der Ham, Van Wezel, Oleksiak, van Zandvoort, Frijns, Kappelle and Postma2012). This might explain why cerebral asymmetry for spatial location memory was not apparent on group level, and also the finding that some left hemisphere stroke patients fail on both temporal order and spatial location memory. Verbal comprehension problems are not likely to explain the difficulty that left hemispheric stroke patients show on this particular task, as they show no floor effect and were well capable of performing other (sub)tasks with similar instruction difficulty.
Notably, no group differences were observed for binding of temporal order and spatial location memory. Memory problems in different clinical populations do concern binding of different features (Kessels and Kopelman, Reference Kessels and Kopelman2012; Postma et al., Reference Postma, Van Asselen, Keuper, Wester and Kessels2006). Postma et al. (Reference Postma, Van Asselen, Keuper, Wester and Kessels2006) showed that Korsakoff patients display problems not merely in remembering contextual features separately, but particularly in binding spatial location and temporal order in memory: performance compared to control participants dropped significantly when both features needed to be remembered at the same time. A possible reason for the absence of group effects for binding in the current data is that performance on this test was also low in control participants and yielded high variances. Whether binding problems exist in stroke patients, therefore, remains inconclusive. However, it does become apparent that stroke patients show single-feature memory deficits, which is also in line with the work of Van Geldorp et al. (Reference Van Geldorp, Kessels and Hendriks2013).
Analysis at individual level further confirmed the presence of dissociations between spatial and temporal order memory. Impairment on temporal order memory was not necessarily accompanied by impairment on spatial location memory. In total, seven (18%) of the included stroke patients showed significantly lower performance on spatial and/or temporal order memory compared to control participants, corrected for age. No binding problems became apparent.
The object relocation tasks were shown to be suitable for patient populations, as well as for elderly people. For example, they did not require computer skills. Also, although scores were not normally distributed, no floor or ceiling effects were found. This is important, since tests that measure specific subcomponents of contextual memory are scarce. Moreover, the current results show that standard neuropsychological assessment falls short in predicting what problems in spatial location and/or temporal order memory may occur in (stroke) patients. Performance on well-known and commonly used tasks (of memory, executive functioning and abstract reasoning) was overall diminished in the patient group, but is insufficient for providing any reliable indication of what contextual memory aspects may be impaired in patients. Van Geldorp et al. (Reference Van Geldorp, Kessels and Hendriks2013) reached the same conclusion.
Possibly, the currently used paradigm can be applied as diagnostic instrument in clinical neuropsychological practice in the future. Using test material that is sensitive to subtle deficits in these specific functions can be valuable for diagnostic purposes, and can provide insights for patient-tailored cognitive rehabilitation and for development of programs aimed specifically at remembering the location and order in which events occur. To be able to implement the findings in this study to clinical practice, suitability of the application of the paradigm should be tested using additional internal and external validity and reliability analyses, including sensitivity/specificity analyses. Possibly, adaptions should be made in the operationalization of binding, as the test materials at hand showed little discriminability for binding problems in the current sample. Concerning the external validity, it would be relevant to study how the findings from the current study relate to other contextual memory tasks with spatial and temporal aspects, for example route learning and long term memory for locations and order.
A limitation of the current study is that it was not possible to thoroughly examine the neuro-anatomical underpinnings of the behavioral patterns of functioning that were found. Image acquisition was part of standard clinical care and not standardized. This also limited the available information on stroke severity in the patient sample. Also, between-group differences might have been influenced by differences in baseline characteristics. Specifically, the lack of gender matching was a weakness; however, gender was shown not to influence the results. Also, comorbidities and stroke-related confounding factors such as neglect and hemianopia were not formally assessed. Although patients were examined for comorbidities in the course of their rehabilitation process and no signs of dementia were reported at time of discharge or follow-up, development of neurological or psychiatric conditions could have occurred in the chronic phase after their stroke. By the time of testing, influence of possible symptoms of (latent) neglect or hemianopia interfering with the experimental tasks was checked in the practice conditions.
A tentative exploration of the available imaging data indicated a laterality effect, but no conclusive information on the specific involvement of parietal, temporal and frontal brain regions. No clear indication was observed for frontal involvement in temporal order memory or parietal involvement in spatial location memory. It is interesting that none of the patients who suffered problems on spatial location memory showed damage in the parietal lobe (and vice versa), an area commonly associated with (spatial) working memory (Constantinidis & Wang, Reference Constantinidis and Wang2004; Friedman & Goldman-Rakic, Reference Friedman and Goldman-Rakic1994; Glabus et al., Reference Glabus, Horwitz, Holt, Kohn, Gerton, Callicott and Berman2003; Inoue et al., Reference Inoue, Mikami, Ando and Tsukada2004; Zhang et al., Reference Zhang, Zhang, Sun, Li, Wang, He and Hu2004) and that those who showed both spatial and temporal order memory deficits showed unilateral left hemisphere damage. Future studies might focus particularly on the underlying neuro-anatomical architecture, to allow for meaningful interpretation of these observations. Specifically, characteristics of left hemispheric contributions to temporal order memory ask for in-depth investigation.
To summarize: co-occurrence of deficits in spatial location and temporal order memory is not obligatory. These results suggest that those types of memory processes are separable. They support the notion that spatial location and temporal order contain functionally separable processing mechanisms. A possible explanation for the co-existence of dissociations and strong associations between processing spatial and temporal order memory might be that memory systems can adapt their function to the task at hand. This is congruent with the ideas of Logie, Brockmole, and Jaswal (Reference Logie, Brockmole and Jaswal2011), who state that the visual working memory system is flexible in allowing formation of bindings between task-relevant features, in the face of major changes in task-irrelevant object properties. The existence of “hybrid” cells in addition to dedicated cells that react only to spatial or temporal information also shows the flexibility of the underlying neural systems (Howard & Eichenbaum, Reference Howard and Eichenbaum2015), and indicates functional plasticity. In this way, the interaction between spatial and temporal aspects of memory is functionally adaptive in the human brain. The results of this study add to our understanding of how contextual information is processed in human memory.
ACKNOWLEDGMENTS
All authors declare no conflicts of interest.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617717000212






