Introduction
Impulsivity is broadly defined as action without foresight (Winstanley, Eagle, & Robbins, Reference Winstanley, Eagle and Robbins2006); a definition that encompasses processes ranging from myopia for the future, to seeking potential rewards despite potential hazards (Bechara, Damasio, Damasio, & Anderson, Reference Bechara, Damasio, Damasio and Anderson1994; Lejuez et al., Reference Lejuez, Read, Kahler, Richards, Ramsey, Stuart and Brown2002). While impulsivity may be adaptive in some situations, overly impulsive choices can threaten personal health and safety, as in clinical conditions like addiction, pathological gambling, and dopamine dysregulation syndrome in Parkinson's disease (PD) (Dagher & Robbins, Reference Dagher and Robbins2009; Kirby & Petry, Reference Kirby and Petry2004; Voon & Fox, Reference Voon and Fox2007).
While the neurobiology of impulsivity is not fully established, dopamine seems to play a central role. PD, a neurodegenerative disorder characterized by a loss of nigrostriatal dopamine neurons (Bruck et al., Reference Bruck, Aalto, Nurmi, Vahlberg, Bergman and Rinne2006; Kish, Shannak, & Hornykiewicz, Reference Kish, Shannak and Hornykiewicz1988), provides an important model for studying these issues in humans. The loss of dopaminergic neurons in PD results in aberrant functioning of fronto-striatal circuitry (Albin, Young, & Penney, Reference Albin, Young and Penney1989; Cools, Reference Cools2006; Frank, Reference Frank2005) and has been associated with risk aversion and reduced reward seeking behavior (Evans et al., Reference Evans, Lawrence, Potts, MacGregor, Katzenschlager, Shaw and Lees2006; Menza, Golbe, Cody, & Forman, Reference Menza, Golbe, Cody and Forman1993; Ondo & Lai, Reference Ondo and Lai2008; Ragonese et al., Reference Ragonese, Salemi, Morgante, Aridon, Epifanio, Buffa and Savettieri2003; Tomer & Aharon-Peretz, Reference Tomer and Aharon-Peretz2004). In contrast, in a small subset of PD patients, dopamine replacement therapy (DRT) can lead to impulse control disorders (Dagher & Robbins, Reference Dagher and Robbins2009; Driver-Dunckley, Samanta, & Stacy, Reference Driver-Dunckley, Samanta and Stacy2003; Seedat, Kesler, Niehaus, & Stein, Reference Seedat, Kesler, Niehaus and Stein2000; Voon & Fox, Reference Voon and Fox2007; Weintraub et al., Reference Weintraub, Koester, Potenza, Siderowf, Stacy, Voon and Lang2010). Although causality has yet to be unequivocally established, it is thought these disorders result from interactions between DRT and intrinsic vulnerabilities to such behaviors in individual patients (van Eimeren, Monchi, Ballanger, & Strafella, Reference van Eimeren, Monchi, Ballanger and Strafella2009; Voon et al., Reference Voon, Reynolds, Brezing, Gallea, Skaljic, Ekanayake and Hallett2010). A separate line of work has shown that PD and DRT influence feedback-driven learning (Cools, Altamirano, & D'Esposito, Reference Cools, Altamirano and D'Esposito2006; Frank, Samanta, Moustafa, & Sherman, Reference Frank, Samanta, Moustafa and Sherman2007; Frank, Seeberger, & O'Reilly, Reference Frank, Seeberger and O'Reilly2004; Shohamy, Myers, Kalanithi, & Gluck, Reference Shohamy, Myers, Kalanithi and Gluck2008; van Wouwe, Ridderinkhof, Band, van den Wildenberg, & Wylie, Reference van Wouwe, Ridderinkhof, Band, van den Wildenberg and Wylie2012). In principle, learning mechanisms could moderate maladaptive impulsivity in situations where choices can be repeated.
Research on the risk-taking aspect of impulsivity in Parkinson's patients has focused on lab-based gambling tasks. The most widely used task, the Iowa gambling task (Bechara et al., Reference Bechara, Damasio, Damasio and Anderson1994) is complex (Dunn, Dalgleish, & Lawrence, Reference Dunn, Dalgleish and Lawrence2006), and results to date are variable. Both impaired (Kobayakawa, Koyama, Mimura, & Kawamura, Reference Kobayakawa, Koyama, Mimura and Kawamura2008; Mimura, Oeda, & Kawamura, Reference Mimura, Oeda and Kawamura2006; Pagonabarraga et al., Reference Pagonabarraga, Garcia-Sanchez, Llebaria, Pascual-Sedano, Gironell and Kulisevsky2007; Perretta, Pari, & Beninger, Reference Perretta, Pari and Beninger2005) and intact (Euteneuer et al., Reference Euteneuer, Schaefer, Stuermer, Boucsein, Timmermann, Barbe and Kalbe2009; Stout, Rodawalt, & Siemers, Reference Stout, Rodawalt and Siemers2001; Thiel et al., Reference Thiel, Hilker, Kessler, Habedank, Herholz and Heiss2003) Iowa gambling task performance have been reported in PD. Small samples and between-group designs likely contribute to this variability. PD patients on DRT have shown greater risk-taking in a more specific risk-taking task, the Game of Dice, compared to healthy control subjects (Brand et al., Reference Brand, Labudda, Kalbe, Hilker, Emmans, Fuchs and Markowitsch2004; Euteneuer et al., Reference Euteneuer, Schaefer, Stuermer, Boucsein, Timmermann, Barbe and Kalbe2009), but this does not establish whether the effect is due to PD or its treatment.
Here, we used the Balloon Analog Risk Task (BART) to examine the effects of DRT and PD on baseline risk-taking and on risk-taking over repeated gambles, with feedback. The BART is a widely used measure of risk-taking with ecological validity, showing associations with real-world risk-taking such as alcohol and drug use, cigarette smoking, risky-sexual behaviors, and self-reported impulsivity (Aklin, Lejuez, Zvolensky, Kahler, & Gwadz, Reference Aklin, Lejuez, Zvolensky, Kahler and Gwadz2005; Bornovalova et al., Reference Bornovalova, Cashman-Rolls, O'Donnell, Ettinger, Richards, deWit and Lejuez2009; Crowley et al., Reference Crowley, Wu, Crutcher, Bailey, Lejuez and Mayes2009; Hopko et al., Reference Hopko, Lejuez, Daughters, Aklin, Osborne, Simmons and Strong2006; Hunt, Hopko, Bare, Lejuez, & Robinson, Reference Hunt, Hopko, Bare, Lejuez and Robinson2005; Lejuez et al., Reference Lejuez, Read, Kahler, Richards, Ramsey, Stuart and Brown2002, Reference Lejuez, Aklin, Jones, Richards, Strong, Kahler and Read2003; Lejuez, Simmons, Aklin, Daughters, & Dvir, Reference Lejuez, Simmons, Aklin, Daughters and Dvir2004). The task requires participants to pump up virtual balloons on a computer screen, gaining more money with each pump. Participants can collect their earnings at any time, but they also risk “losing it all,” as each pump is associated with a monotonically increasing chance of balloon explosion with loss of the money earned up to that point on that trial. Thus, as the balloon inflates and potential winnings accumulate, the risk of explosion (and so monetary loss) also increases. The task provides feedback in the form of wins or explosions (losses), providing an opportunity for participants to learn about the riskiness of subsequent choices (Bishara et al., Reference Bishara, Pleskac, Fridberg, Yechiam, Lucas, Busemeyer and Stout2009). In principle, the task can thus distinguish initial risk-taking propensity from how risk-taking is influenced by feedback across repeated trials (Lejuez et al., Reference Lejuez, Aklin, Jones, Richards, Strong, Kahler and Read2003). Two recent studies using variations of this task in PD focused only on overall risk-taking: one found that DRT does not affect overall BART performance (van Eimeren, Ballanger, et al., Reference van Eimeren, Ballanger, Pellecchia, Miyasaki, Lang and Strafella2009), and another suggested DRT increases overall risk-taking only in PD patients with impulse control disorders (Claassen et al., Reference Claassen, van den Wildenberg, Ridderinkhof, Jessup, Harrison, Wooten and Wylie2011). We pursue this issue in more detail, in patients without impulse control disorders, aiming to disentangle initial risk-taking from the effects of experience on repeated risk-taking.
Temporal discounting, the preference for smaller immediate rewards over larger delayed rewards, is a second impulsivity-related phenomenon (Ainslie, Reference Ainslie2001). The rate at which a reward loses its subjective value across delay can be described by a hyperbolic or exponential function, the steepness of which varies across individuals (Ainslie, Reference Ainslie2001; Kirby & Herrnstein, Reference Kirby and Herrnstein1995; Madden, Begotka, Raiff, & Kastern, Reference Madden, Begotka, Raiff and Kastern2003), and is relatively stable over time, at least in healthy subjects (Kirby, Reference Kirby2009). Populations with impulse control problems, such as substance abusers, pathological gamblers and those with attention deficit/hyperactivity disorder, reliably show steeper discounting functions than healthy controls (Bickel & Marsch, Reference Bickel and Marsch2001; Coffey, Gudleski, Saladin, & Brady, Reference Coffey, Gudleski, Saladin and Brady2003; Housden, O'Sullivan, Joyce, Lees, & Roiser, Reference Housden, O'Sullivan, Joyce, Lees and Roiser2010; Petry, Reference Petry2002; Reynolds, Reference Reynolds2006; Voon et al., Reference Voon, Reynolds, Brezing, Gallea, Skaljic, Ekanayake and Hallett2010; Vuchinich & Simpson, Reference Vuchinich and Simpson1998; Winstanley et al., Reference Winstanley, Eagle and Robbins2006).
The dopamine-rich ventral striatum is among the brain regions implicated in temporal discounting (Cardinal, Winstanley, Robbins, & Everitt, Reference Cardinal, Winstanley, Robbins and Everitt2004; Kable & Glimcher, Reference Kable and Glimcher2007; McClure, Ericson, Laibson, Loewenstein, & Cohen, Reference McClure, Ericson, Laibson, Loewenstein and Cohen2007; McClure, Laibson, Loewenstein, & Cohen, Reference McClure, Laibson, Loewenstein and Cohen2004; Peters & Buchel, Reference Peters and Buchel2009; Pine et al., Reference Pine, Seymour, Roiser, Bossaerts, Friston, Curran and Dolan2009; Weber & Huettel, Reference Weber and Huettel2008; Xu, Liang, Wang, Li, & Jiang, Reference Xu, Liang, Wang, Li and Jiang2009). Amphetamine and methylphenidate decrease discounting rates in healthy subjects (de Wit, Enggasser, & Richards, Reference de Wit, Enggasser and Richards2002; Pietras, Cherek, Lane, Tcheremissine, & Steinberg, Reference Pietras, Cherek, Lane, Tcheremissine and Steinberg2003). The picture in PD is less clear, with medicated PD patients having discounting rates either similar to (Housden et al., Reference Housden, O'Sullivan, Joyce, Lees and Roiser2010), or steeper than (Milenkova et al., Reference Milenkova, Mohammadi, Kollewe, Schrader, Fellbrich, Wittfoth and Munte2011) those of healthy controls. DRT may increase discounting only in PD patients with impulse control disorders (Voon et al., Reference Voon, Reynolds, Brezing, Gallea, Skaljic, Ekanayake and Hallett2010).
Heterogeneity of PD and baseline individual differences in impulsivity may explain the conflicting findings. Here, we used within-subject designs to examine the effects of DRT on risk-taking and temporal discounting in mild-moderate PD patients. The effects of PD progression were explored in a subset of PD patients and controls tested again 1.5–3 years later. To avoid complex interactions between pre-existing risk-taking tendencies, disease and treatment, we excluded patients with impulse control disorders.
Materials and Methods
Subjects
Twenty-three patients with mild-moderate idiopathic Parkinson's disease (mean age, 65 years; SD 8.6) and 20 demographically matched healthy control subjects (HCTL) (mean age, 63 years; SD 7.4) participated in this study. Patients were recruited from the McGill University Health Centre Movement Disorders clinic. Healthy controls were recruited from the local community. Demographic and clinical data are shown in Tables 1 and 2. Experienced movement disorder neurologists identified PD patients without dementia or impulse control disorders for this study, based on comprehensive clinical assessment. All patients scored ≥24/30 on the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., Reference Nasreddine, Phillips, Bedirian, Charbonneau, Whitehead, Collin and Chertkow2005), a screening test for mild cognitive impairment, and were free of pathological gambling, confirmed by an average score of 0.04 (SD = 0.2) (range, 0–1) on the South Oaks Gambling Screen (SOGS; scores >5 suggest pathological gambling) (Lesieur & Blume, Reference Lesieur and Blume1987). Patients with other neurological diagnoses that might affect cognition or overt clinical depression were excluded. Controls were excluded if they had a history of neurologic or psychiatric disease, closed head injury, or were taking psychoactive medication. They scored at least 28/30 on the Folstein Mini-Mental State Examination (Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975). There was no significant difference between patients and controls with regard to age or education (t test, p's > .4). PD patients had higher BDI scores (t(44) = −3.07, p < .01) compared to controls, although patient's scores were still well below the usual thresholds for depression on this scale.
Table 1 Characteristics of subjects (mean (SD))
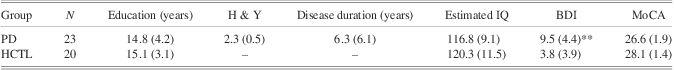
BDI, Beck Depression Inventory; MoCa, Montreal Cognitive Assessment.
**p < .01, PD-HCTL.
Table 2 Motor symptom and treatment characteristics of Parkinson's patients (mean (SD))

RH, Right hand; LH, Left hand; LEDD, Levodopa equivalent daily dose; DA, Dopamine agonist.
***p < .001, On-Off; *p < .05, RH On-Off.
The severity of motor signs was rated using Part III of the unified Parkinson's disease rating scale, UPDRS (Fahn & Elton, Reference Fahn and Elton1987), and motor function was assessed with the Purdue Pegboard (Lafayette Instrument Co). The mean onset of PD symptoms, by patient report, was 7.0 (5.9) years, and from diagnosis was 6.3 (6.1) years before enrollment. Hoehn and Yahr ratings (Hoehn & Yahr, Reference Hoehn and Yahr1967) ranged from 1.5 to 3. Seventeen patients were taking L-dopa/carbidopa, and 10 were taking dopaminergic agonists (ropinirole or pramipexole) in isolation or in combination with L-dopa therapy. Six patients were taking a COMT inhibitor (entacapone), 3 were taking MAO B inhibitors (selegiline or rasagiline) and 3 were taking amantadine. Additional medications included venlaflaxine (in 3 patients, none of whom were depressed at the time of testing) and trihexyphenidyl in 1 patient. All patients were on stable medication for at least 3 months before the study. Levodopa Equivalent Daily Dosage (LEDD) and Dopamine Agonist Levodopa Equivalent Daily Dosage (DA LEDD) calculated according to Pahwa et al. (Reference Pahwa, Wilkinson, Smith, Lyons, Miyawaki and Koller1997) are shown in Table 2.
All participants completed two morning testing sessions, once while taking their usual medications, and once after an overnight (minimum 18 h) washout of their DRT, in a randomized order, at least 2 weeks apart. Participants continued all other medications as usual in the washout condition.
In the longitudinal portion of the study, all available PD subjects (N = 12) were tested again 1.5–3 years later. A demographically similar subset of the healthy controls (N = 8) was also re-tested longitudinally (L-HCTL), after the same interval. The longitudinal PD sub-group (L-PD) is likely biased toward subjects with slower disease progression; common reasons for non-participation in this portion of the study included emergence of mild dementia (as assessed by their neurologist) or severe motor disability (i.e., Hoehn and Yahr [H&Y] score ≥4).
At follow-up, patients showed evidence of disease progression with increasing DRT doses and H&Y staging (Table 3). BDI scores remained unchanged. At re-testing, 9 patients were taking L-dopa, and 5 were taking a dopaminergic agonist (pramipexole) in isolation or in combination with L-dopa therapy. Six were taking a COMT inhibitor (entacapone), 6 were taking an MAO B inhibitor (rasagiline) and 2 were taking amantadine. One was taking an antidepressant (venlafaxine) but was not depressed at time of testing. All participants gave written informed consent and were compensated for their time, in addition to receiving their winnings from the BART. The local research ethics committee approved the study.
Table 3 Clinical characteristics of longitudinal Parkinson's patients at initial testing and at follow-up (1.5–3 years later) (mean (S.D))

RH, Right hand; LH, Left hand; LEDD, Levodopa equivalent daily dose; DA, Dopamine agonist.
**p < .01; *p < .05, Initial-Follow-up.
Tasks
The BART involves pumping up a virtual balloon on a computer screen. Participants were given the following verbal instructions. “Throughout the task you will see 30 balloons, one at a time. For each balloon, you can press this button to increase the size of the balloon. You will get 1 cent in a temporary bank for each pump. You will not be shown the amount of money in your temporary bank. At any point, you can stop pumping up the balloon and press this button to collect your earnings. Pressing this button will start you on the next balloon and will transfer the money you have won from your temporary bank to your permanent bank. It is your choice to determine how much to pump up the balloon, but be aware at some point the balloon will explode. The explosion point varies across balloons, ranging from the first pump to enough pumps to make the balloon fill the entire computer screen. If the balloon explodes before you collect your earnings, then you move on to your next balloon and all the money in your temporary bank is lost. Exploded balloons do not affect the money accumulated in your permanent bank. At the end of the task, you will receive the money you earned in your permanent bank.”
Participants completed a one-balloon practice trial (with no explosion) to ensure they understood the task. During the task, the probability that a balloon would explode was 1/128 for the first pump, 1/127 for the second and so on. Thus, after 64 pumps, the risk of loss begins to outweigh the chance of gains. The task involved 30 trials grouped into 3 blocks of 10 for the analysis, in keeping with prior work (Fecteau et al., Reference Fecteau, Pascual-Leone, Zald, Liguori, Theoret, Boggio and Fregni2007; Lejuez et al., Reference Lejuez, Aklin, Jones, Richards, Strong, Kahler and Read2003). The dependent measure was the average number of adjusted pumps for each block (the average number of pumps on trials when the balloon did not explode). Higher values reflect greater risk-taking. The average number of exploded balloons and earnings per block were also examined, as was the effect of a monetary loss (exploded balloon) on adjusted pumps in the subsequent trial, similar to recent work (Claassen et al., Reference Claassen, van den Wildenberg, Ridderinkhof, Jessup, Harrison, Wooten and Wylie2011). At the end of the session, participants received their earnings (≤$11.00 CDN).
Temporal discounting rate was estimated with a widely used, computerized temporal discounting task (Fellows & Farah, Reference Fellows and Farah2005; Kirby & Marakovic, Reference Kirby and Marakovic1996; Kirby, Petry, & Bickel, Reference Kirby, Petry and Bickel1999). In this task, subjects are asked to make hypothetical choices between various amounts of money now, and larger amounts delayed by 7–180 days. The task assumes a hyperbolic discounting function (V = v/(1 + kD), where V is the current (subjective) value, v the absolute value, and D the delay), offering 27 choices across 9 values of k (k = 0.00016; 0.00040; 0.0010; 0.0025; 0.0060; 0.016; 0.041; 0.10; 0.25). By definition, choices should consistently favor the “now” option for k values less than the indifference curve, and the “later” option for k values larger than the indifference curve. Participants’ choices are not always perfectly consistent; the best fitting k value was determined for each participant following published methods (Kirby et al., Reference Kirby, Petry and Bickel1999). When more than one k value provided an equally good fit, the geometric mean of these values was used. We also report the number of inconsistent choices as a metric of “goodness of fit.”
Statistical Analysis
Raw data from the BART were normally distributed. The k values and temporal discounting inconsistencies approximated normal distributions following logarithmic or square root transformations, respectively. All data were analyzed using analysis of variance (ANOVA) followed by post hoc pair-wise t tests where appropriate. Effect size, Cohen's d (Cohen, Reference Cohen1988), was calculated for contrasts of interest, using the Morris and DeShon (Reference Morris and DeShon2002) equation to account for within-subject measures.
Results
Effects of DRT and Parkinson's disease on Risk-Taking as Captured by the BART
Figure 1 shows the BART performance of the PD group both on and off DRT, as well as that of the HCTL group tested twice. There was a significant treatment by block interaction in the PD group (F(2,44) = 8.75; p < .01). Simple main effects analysis comparing performance in each block in on versus off DRT conditions revealed patients pumped significantly more on the last block of trials on DRT compared to off (p < .05; d = 0.63). There was a similar trend in the second block (p = .07). Similarly, simple main effects analysis comparing performance in each block, within treatment conditions, showed only medicated patients pumped significantly more across blocks. Tukey's HSD test revealed medicated patients pumped more on the second and third blocks, relative to their own performance on the first block (all p's < .01; d's = 1.1; 1.5, respectively). Patients off DRT showed stable performance across blocks. When medicated PD patients were compared to HCTL, there was again a significant interaction of group by block (F(2,82) = 6.0; p < .01), driven by the increasing number of pumps made by the PD group across blocks, compared to the stable performance of HCTL. There was no significant interaction or main effects when PD patients off DRT were compared to HCTL. The total number of adjusted pumps was similar in all groups (t tests, all p's > .05): [mean, (SD)], PD “on” 38.43 (13.0); PD “off” 35.31 (11.7), HCTL 35.67 (9.7)].

Fig. 1 Average number of adjusted pumps on the BART for Parkinson's patients (PD; on and off dopamine replacement therapy) and healthy control subjects (HCTL). Error bars indicate SEM. **p < .01, treatment by block interaction for Parkinson's patients.
As expected, the number of exploded balloons for each block was closely correlated with adjusted pumps (r = 0.91) and showed exactly the same pattern of significant effects when entered into the statistical analysis described above. The total number of explosions was similar across groups (t tests, all p's > .05): [mean, (SD)]: PD “on” 9.14 (3.9); PD “off” 8.48 (4.3); HCTL 8.34 (3.0). The money earned in the task is a coarser, more integrative measure of performance. All groups earned more money across blocks (effect of block, all p's < .05). There were no significant interactions of group or treatment with block, and overall, total earnings were similar in all groups (t tests, all p's > .05): [mean, (SD)], PD “on” $7.43 (1.50); PD “off” $7.27 (1.51); HCTL $7.35 (1.53).
Effects of Disease Progression on Risk-Taking as Captured by the BART
As shown in Figure 2, disease progression in the L-PD group was associated with a reduction in the average number of adjusted pumps (effect of time, F(1,11) = 6.09; p < .05; d = 0.8), nevertheless, the pattern of increasing pumps across blocks persisted (effect of block, F(2,22) = 32.23; p < .001). In contrast, the L-HCTL group showed stable performance across time points and across blocks. In line with these within-subject findings, there were significant interactions between group (L-PD vs. L-HCTL) and block, at initial testing (F(2,36) = 6.00; p < .01), and at follow-up (F(2,36) = 4.38; p < .05). The total number of adjusted pumps was similar between groups at initial testing and at follow-up (t tests, all p's > .05): [mean, (SD)], L-PD “initial” 40.8 (12.2); L-HCTL “initial” 31.0 (10.9); L-PD “follow-up” 32.7 (8.8); L-HCTL “follow-up” 35.6 (8.8).

Fig. 2 Average number of adjusted pumps on the Balloon Analog Risk Task (BART) for Parkinson's patients and healthy control subjects, at initial testing and at follow-up (longitudinally). Errors bars indicate SEM. *p < .05, main effect of time for Parkinson's patients; ***p < .001, main effect of block for Parkinson's patients. L-PD = longitudinal Parkinson's disease sub-group; L-HCTL = healthy controls re-tested longitudinally.
The explosion analysis again showed the same pattern of significant effects as for pumps. The total number of explosions was similar between groups at initial testing and at follow-up (t tests, all p's > .05): [mean, (SD)], L-PD “initial” 9.7 (3.4); L-HCTL “initial” 7.5 (3.7); L-PD “follow-up” 7.2 (2.8); L-HCTL “follow-up” 8.8 (4.2).
Finally, earnings also dropped with disease progression in the L-PD group (effect of time, F(1,11) = 4.91; p < .05), but continued to increase across blocks (effect of block F(2,22) = 8.66; p < .01). The L-HCTL group's overall earnings remained stable across time, and increased across blocks as well. Overall earnings were similar between groups at initial testing and at follow-up (t tests, all p's > .05): [mean, (SD)], L-PD “initial” $7.83 (1.17); L-HCTL “initial” $7.49 (1.21); L-PD “follow-up” $7.14 (1.29); L-HCTL “follow-up” $7.54 (1.13).
Effect of a Loss on Risk-Taking in the Subsequent Trial
When the average number of adjusted pumps on post-explosion trials was compared to that on all other trials, for each block, we found a significant effect of trial type within each group. PD patients “on” (F(1,19) = 12.11; p < .01, d = 3.2) and “off” DRT (F(1,17) = 6.29; p < .05; d = 2.9), as well as healthy controls (F(1,19) = 11.25; p < .01; d = 4.1), made significantly fewer pumps on trials immediately following a monetary loss (explosion). Only medicated PD patients showed an effect of block (F(2,38) = 18.44; p < .01), pumping more across blocks in both trial types. There was no effect of treatment in the PD patients (F(1,22) = 0.35; p > .05), nor effect of group in the PD (“on”; “off”) versus HCTL comparisons (all p's > .05).
A similar pattern of significantly reduced risk-taking on the trial immediately following an explosion was found in the patients and healthy controls tested longitudinally.
BART performance, measured by overall score and mean difference between the first and last block, was not significantly related to DRT dose (LEDD and DA LEDD), age, age at onset, BDI score, duration of illness at enrollment, or gender (all p's > .05).
Effects of DRT and Parkinson's Disease on Temporal Discounting
The mean discounting rates were similar in the “on” and “off” conditions (t(22) = 0.24; p > .05) and comparable to that of healthy controls in both conditions (all p's > .05). The (geometric) mean discounting coefficient, k (SD), for each group, was: PD “on” 0.009 (0.066); PD “off” 0.012 (0.059); HCTL 0.013 (0.023). In other words, $100 CDN in 6 months would be of subjectively equal value to $38 now for PD “on,” $31 for PD “off” and $30 for HCTL. Figure 3 shows prototypic temporal discounting curves for the 3 groups.

Fig. 3 Temporal discounting curves illustrating the average temporal discounting rate for Parkinson's disease (PD) patients on and off dopamine replacement therapy (DRT), and for healthy control subjects, for a hypothetical $100 CDN. The gray bar indicates the 95% confidence interval for the control group (HCTL). There was no significant difference between groups, or significant medication effect in the PD group.
We performed an additional analysis to explore the mean number of inconsistent responses. Choices that differed from those predicted by the overall best fitting discounting function for each subject, on each testing occasion, were counted as inconsistencies. These could be considered deviations from subjective value maximization (Camille, Griffiths, Vo, Fellows, & Kable, Reference Camille, Griffiths, Vo, Fellows and Kable2011; Fellows & Farah, Reference Fellows and Farah2007), or could result from systematic departures from the presumptive hyperbolic discounting function. The mean number of inconsistencies in each group was small [mean (SD)]: PD “on” 1.3 (1.2); PD “off” 0.6 (0.9); HCTL 1.0 (1.1). This value was significantly greater for PD patients on DRT relative to “off” (paired t test, t(22) = 2.24; p < .05; d = 1.2). There were no between group differences. All subjects were as likely to make inconsistent choices for “now” or “later” options, suggesting these inconsistencies reflected “noisy” choices rather than systematic changes in the discounting function.
Effects of Disease Progression on Temporal Discounting
Progression of PD was not associated with a change in temporal discounting rate, t(11) = 0.65; p > .05. L-HCTL subjects also showed stable discounting on follow-up. There were no significant differences between groups either initially or at follow-up. The (geometric) mean discounting constant, k (SD), for each group was: L-PD “initial” 0.010 (0.087); L-PD “follow-up” 0.013 (0.063); L-HCTL “initial” 0.011 (0.031); L-HCTL “follow-up” 0.015 (0.030).
Temporal discounting was not significantly related to DRT dose (LEDD and DA LEDD), age, age at onset, BDI score, duration of illness at enrollment, or gender (p's > .05).
Discussion
We used laboratory measures of risk-taking and temporal discounting with established real-world validity to assess the effects of DRT on risky and impulsive decision-making, in a cohort of mild-moderate PD patients free of clinical impulse control disorders. We found DRT did not affect global measures of impulsivity: overall risk-taking as measured by the BART, or temporal discounting rate. Furthermore, mild-moderate PD did not substantially affect these measures compared to healthy controls. However, DRT did affect BART performance across blocks, arguing DRT affects how experience influences repeated risky choices.
A single prior study administered the BART to PD patients on and off DRT (van Eimeren, Ballanger, et al., Reference van Eimeren, Ballanger, Pellecchia, Miyasaki, Lang and Strafella2009), while another examined the effects of pramipexole, a D2/D3 agonist, on BART performance in healthy subjects (Hamidovic, Kang, & de Wit, Reference Hamidovic, Kang and de Wit2008). These studies agree with our findings of no significant effects of dopamine manipulation on overall task performance, but neither examined the dynamics of risky choice across trials. Here, we show risk-taking as measured by the BART can be decomposed into initial tendencies and block-wise effects, with DRT affecting only the latter.
People generally err on the side of caution in risky situations. This was evident here: the average number of adjusted pumps (overall and across blocks) for all groups was well below what would have yielded maximal earnings, as is typical for healthy subjects and even clinical populations in other studies using this task (Lejuez et al., Reference Lejuez, Aklin, Jones, Richards, Strong, Kahler and Read2003; van Eimeren, Ballanger, et al., Reference van Eimeren, Ballanger, Pellecchia, Miyasaki, Lang and Strafella2009). While the medicated PD group made increasingly risky choices over the course of the task, they approached more optimal performance. Thus DRT yielded adaptive changes in risk-taking in this particular context. Whether this tendency would lead to harmful consequences in other risk settings, or with more trials in the present task, remains an open question.
These observations provide a novel perspective on risk-taking related to PD, emphasizing the influence of experience and learning on risk-taking in situations where gambles are repeated, with feedback. We speculate this relates to the growing evidence that DRT has specific effects on reinforcement learning in PD (Cools et al., Reference Cools, Altamirano and D'Esposito2006; Frank et al., Reference Frank, Samanta, Moustafa and Sherman2007; Jahanshahi, Wilkinson, Gahir, Dharminda, & Lagnado, Reference Jahanshahi, Wilkinson, Gahir, Dharminda and Lagnado2010). In animals, rewards are associated with phasic increases, and punishment or reward omission is associated with dips in dopamine firing; these changes are thought to serve as teaching signals underlying reinforcement learning. Phasic dopamine increases are thought to act preferentially on D1 receptors, and via the direct basal ganglia pathway, supporting “go” learning. Dopamine dips may act more through a D2 receptor mechanism, via the indirect pathway, reducing the likelihood of subsequent responses (Frank, Reference Frank2005). Consistent with this model, PD patients show relatively enhanced learning from reward compared to punishment when on DRT (Frank et al., Reference Frank, Seeberger and O'Reilly2004, Reference Frank, Samanta, Moustafa and Sherman2007). In line with these findings, we propose here that DRT shifts the balance toward learning more from gains than losses in the BART, leading to the tendency to take increasing risks across trials when on DRT. At the trial-by-trial level, DRT did not detectably affect behaviour immediately following a loss: whether on or off medication, PD patients were more cautious on post-explosion trials, as were controls. However, this immediate reaction did not translate into more global tendencies toward caution, to the extent this can be detected in this task. Future work might fruitfully apply tasks better suited to measuring distinct effects of reward and punishment in risky contexts to follow-up on this idea.
At the least, this work suggests that models of risk-taking in PD would benefit from looking beyond “one shot” risk-taking contexts. DRT effects on learning, particularly from reward, may be a critical factor in the repeated, cumulative risk-taking that is often seen in everyday settings such as casinos or shopping centers.
The longitudinal portion of this study, while including only part of the original cohort, offered a unique opportunity to explore the effects of PD progression on impulsivity. The longitudinally followed control group showed no change in overall risk-taking or temporal discounting, but the PD patients were more risk-averse compared to their own performance 1.5–3 years earlier. Of interest, even though they were more risk-averse overall, these DRT-treated patients continued to take increasing risks across blocks of the BART, different from the stable performance of the control group.
In contrast to the clear effects of PD and DRT on these two different aspects of risk-taking, we found no consistent effect of either disease or treatment on temporal discounting. The widely used measure of temporal discounting we used has previously detected group differences in a variety of clinical populations. Only one prior study applied the same task we used to PD patients on and off DRT. Milenkova et al. (Reference Milenkova, Mohammadi, Kollewe, Schrader, Fellbrich, Wittfoth and Munte2011) reported no significant effect of dopamine manipulation on discounting rates, consistent with our finding, but found steeper discounting in PD patients relative to healthy controls, whether the patients were on or off DRT. Here, we show PD patients do not differ from healthy controls on this measure, consistent with findings from Housden et al. (Reference Housden, O'Sullivan, Joyce, Lees and Roiser2010) who reported steeper discounting only in PD patients with impulse control disorders but not in otherwise healthy medicated PD patients. Given the wide variability in temporal discounting in healthy subjects, any between group differences detected with such designs in PD likely reflect sampling biases or chance rather than disease or dopamine related influences.
This task has been successfully applied to measure changes in temporal discounting within-subject in other contexts: temporal discounting rates were reduced by d-amphetamine and methylphenidate in healthy subjects (de Wit et al., Reference de Wit, Enggasser and Richards2002; Pietras et al., Reference Pietras, Cherek, Lane, Tcheremissine and Steinberg2003). However, recent work by Hamidovic et al. (Reference Hamidovic, Kang and de Wit2008) failed to find an effect of acute low (0.25 mg) and medium (0.50 mg) doses of the D2/D3 agonist pramipexole in healthy subjects with this task. An effect of DRT in PD patients has been reported with a different temporal discounting task involving experienced (much briefer) delays, but again, only in those with clinically evident impulse control disorders (Voon et al., Reference Voon, Reynolds, Brezing, Gallea, Skaljic, Ekanayake and Hallett2010).
The findings we report suggest a distinction between risk-taking and temporal discounting, in terms of the effects of DRT and disease progression, arguing for different neurobiological (likely non-dopaminergic) contributions to these two aspects of behavior. As with any complex behavioral measures, it is difficult to fully exclude the alternative explanation that there are differences in the measurement properties of the tasks. We think this is less likely: neither task was at floor or ceiling, the measures were remarkably stable over time in the control group, and, as reviewed above, at least some prior work using these tasks in other conditions has shown detectable differences.
Although DRT and PD were not associated with changes in the steepness of temporal discounting, a secondary analysis revealed that DRT did affect decision-making in the temporal discounting task. Patients made more inconsistent choices while on DRT compared to off. One potential explanation for this observation is that DRT impaired the ability to consistently estimate the subjective value of relative reward. There was no bias in the direction of this inconsistency; patients were as likely to make inconsistent choices for “now” or “later” options. Damage to the orbitofrontal cortex has shown similar effects on other kinds of preference judgments (Camille et al., Reference Camille, Griffiths, Vo, Fellows and Kable2011; Fellows & Farah, Reference Fellows and Farah2007; Henri-Bhargava, Simioni, & Fellows, Reference Henri-Bhargava, Simioni and Fellows2012), raising the possibility that changes in dopamine modulation within orbitofrontal cortex may have mediated this effect.
In conclusion, PD patients on DRT showed increasing risk-taking across trials of the BART, which was not present when they were off DRT, or in healthy controls. This effect was distinct from initial risk-taking in this task, which resembled that seen in controls, but declined as the disease advanced. We speculate that the tendency of PD patients to learn more from reward on DRT, and more from punishment off DRT (Frank et al., Reference Frank, Seeberger and O'Reilly2004) may underpin this risk-taking momentum in conditions where gambles are repeated. That is, dopamine may have a particular role in “knowing when to walk away” from risky choices.
Acknowledgments
We thank the neurologists at the MUHC Movement Disorders clinic for their help with patient recruitment, and the patients for their generous participation. This work was supported by the Parkinson's Society of Canada. The authors report no conflicts of interest.








