Article contents
Detailed assessment of activities of daily living in moderate to severe Alzheimer's disease
Published online by Cambridge University Press: 01 July 2005
Abstract
Patients with Alzheimer's Disease (AD) who have reached a stage of moderate to severe dementia are capable of completing a restricted range of cognitive tests and performing a limited range of activities of daily living (ADL). As part of an initiative to develop instruments to evaluate AD, we analyzed data describing the performance of a large number of ADL and scores on cognitive and global assessment measures in a cohort of patients with AD with moderate to severe cognitive impairment, defined as a Mini-Mental State Examination score ranging from 0–15 (out of 30). From the large pool of ADL, 19 met criteria of applicability, reliability, good scaling, concordant validity, and sensitivity to detect change in performance over 6–12 months. A total score derived from these 19 ADL ratings, comprising a scale termed the Alzheimer Disease Cooperative Study ADL-sev, correlated strongly with measures of cognition and of global dementia severity. Patients with moderate to severe AD showed a decline on the ADL-sev and cognitive measures over 6 and 12 months, consistent with the progression of AD. Detailed evaluation of ADL may provide a useful index to evaluate patients with moderate to severe AD and may complement cognitive assessment, especially for characterizing change in interventional or therapeutic studies. (JINS, 2005, 11, 446–453.)
Keywords
- Type
- Research Article
- Information
- Journal of the International Neuropsychological Society , Volume 11 , Issue 4 , July 2005 , pp. 446 - 453
- Copyright
- © 2005 The International Neuropsychological Society
BACKGROUND
Alzheimer's disease (AD) is clinically defined by the progressive impairment of memory and other cognitive abilities. As a result of cognitive decline, patients lose the ability to perform complex (instrumental) activities of daily living (ADL), and eventually basic ADL. Clinical assessment of cognition and functional abilities provides important indices of dementia severity. There are many instruments designed to assess these abilities in AD, several of which are used routinely in clinical trials. At a stage of moderate to severe dementia, patients with AD have marked impairment of recent memory and usually have lost the ability to carry out most instrumental ADL (IADL). They have a high risk of institutionalization. At this stage of dementia, patients are difficult to evaluate clinically and have received relatively little study in clinical trials. Typical measures, such as the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) or Alzheimer's Disease Assessment Scale-cognitive (ADAS-cog) (Rosen et al., 1984), are suboptimal in moderate to severe AD, because patients' performance has reached a floor on many components. Functional abilities in these patients are usually measured by performance of basic ADL such as dressing, grooming, and bathing. Most basic ADL scales assess a limited range of activities. A clinical trial of vitamin E and selegiline in moderately severe AD (mean entry MMSE = 11) used clinical milestones such as death, institutionalization, and loss of basic ADL as primary outcome measures (Sano et al., 1997). Significant treatment effects were found regarding time to reach these milestones. However, there was no significant treatment-placebo difference regarding rates of cognitive change. It is possible that cognitive measures may not provide information under certain circumstances where ADL measures may do so. This question should be explored using instruments appropriate for patients with moderate to severe dementia.
For clinical trials in AD, outcome measures need to be reliable, comprehensive, reflect the abilities of the targeted patient population, have sensitivity to detect change over time, and be clinically meaningful. Because there are few detailed measures developed for research studies of moderate to severe dementia, we examined the performance and properties of an instrument specifically developed to assess ADL in this patient population, termed the Alzheimer's Disease Cooperative Study Activities of Daily Living scale for Severe impairment (ADCS-ADL-sev). In developing this instrument, we tried to expand on traditional basic ADL by including items covering IADL if they were relevant to moderate to severe AD, as well as activities involving simple, overlearned manual skills that need to be carried out in an appropriate context.
METHODS
Research Participants
The recruitment, evaluation, and follow-up of subjects have been described in detail (Ferris et al., 1997). In summary, 242 patients who met research criteria for probable AD were enrolled in a multicenter longitudinal study aimed at evaluating new assessment instruments for AD and followed over 12 months. All were evaluated at baseline, 6 months, and 12 months, and half of the participants were evaluated at 1 and 2 months. A battery of instruments that evaluated cognition, global function, ADL performance, and behavior was administered at each visit. To obtain anchoring scores, the Global Deterioration Scale (GDS) (Reisberg et al., 1988) and Clinical Dementia Rating (CDR) (Berg, 1988) were administered to informants at baseline and 12 months. All participants were community dwelling at the time of baseline evaluation. Recruitment was stratified according to baseline MMSE scores. The analyses we present are restricted to a subset of 145 patients whose MMSE scores ranged from 0–15 at entry, representing moderate to severe AD. Because this cohort of patients was studied prior to the introduction of cholinesterase inhibitor therapy on a large scale in the United States, the data describe the natural history of progression of AD.
ADL Ratings
The details of ADL performance were obtained from an informant for each patient. Informants who spent at least 2 days per week in direct contact with the patient were identified and were interviewed by a psychometrist or an experienced clinician. The interviews covered details of performance on an inventory of 45 ADL, including both basic and instrumental ADL (Galasko et al., 1997). For each ADL, the informant was offered a series of specific descriptions of levels of performance and asked to identify the one that most accurately described the patient's performance during the past 4 weeks. Interviewers attended a training session on the ADL inventory before the start of the study and had access to a procedural manual. From the pool of 45 ADL, we selected those that were most appropriate for moderate to severe AD according to prespecified criteria of applicability, reliability, scaling, validity, and sensitivity to detect change (defined later). Nineteen ADL were retained and combined to form the ADCS-ADL-sev.
Cognitive and Other Measures Used in This Cohort
The Severe Impairment Battery (SIB) was administered because it was designed to assess cognitive abilities in more detail than traditional cognitive tests in patients with moderate to severe AD (Saxton et al., 1990; Panisset et al., 1994). This scale covers social interaction, memory, orientation, language, attention, praxis, visuospatial ability, construction, and orientation to name. Nonverbal responses are allowed, thus decreasing the need for language output. There are 40 questions and the SIB score ranges from 0–100. The MMSE is a brief cognitive screening test, widely used in dementia research. The SIB and MMSE were administered by psychometrists who received training on administration and scoring procedures. To examine the relationship between a global rating and detailed ADL assessment, the CDR, a global assessment based on a structured interview of the patient and an informant (Berg, 1988), was used. The interview covers performance in six areas: memory, orientation, judgment, home and hobbies, community interactions, personal care. Each of these is rated from 0 (normal), 0.5 (inconsistently or questionably impaired), through 1, 2, or 3 (descriptions of increasing levels of impairment) by completing a grid. From this grid, a single global CDR score is obtained, ranging from 0–3. As a related index, the scores for all six CDR domains can be summed to provide a total score called the Sum of Boxes (CDR-SB). The GDS is a global staging score derived from a semistructured interview of an informant (Reisberg et al., 1988). Scores range from 0 (unimpaired), then 1 (very mild impairment) through 6 (severe impairment).
Data Analysis
Data were analyzed using SAS software. We used the following criteria, based on recommendations concerning scale development and ADL measurement (Feinstein et al., 1986; Streiner & Norman, 1995), to determine which ADL items were best suited for evaluating this patient population. Applicability of each ADL item was defined by the number (and percentage) of participants who performed that ADL meaningfully at baseline, that is, scored >0. A score of 0 meant that the subject either needed complete assistance to perform the ADL or did not attempt it at all. Note also that a patient could obtain a score of 0 for an ADL if he or she had not performed the activity habitually for many years. ADL items were retained provided that 50% or more of the patients attempted them meaningfully at entry to the study. Test-retest reliability was studied among those participants who received assessments at 1 and 2 months after baseline, using a kappa statistic (Cohen, 1960). Items were retained if reliability was acceptable or better (kappa ≥ .4). The ability of each ADL item to show stepwise scaling was analyzed by constructing item characteristic curves using logistic regression models, with ADL performance as the outcome variable and MMSE score as an independent variable. ADL performance was dichotomized in two ways: first as independent versus not independent (optimal score for item vs. lower scores), and second as complete impairment of ADL versus any degree of performance (0 vs. >0). The two curves for each ADL item were superimposed and visually inspected for the extent of separation to determine whether the descriptions of different levels of performance for each item showed differential (and hierarchical) degrees of difficulty across the MMSE range of 0–15. Sensitivity to detect longitudinal change was evaluated by calculating the extent of change of scores for each ADL at 6 and 12 months relative to baseline. For each ADL, significant change was arbitrarily defined as a mean change score of 0.2 points or more at 12 months (which roughly corresponds to 20% or more of subjects showing decline by 1 level on the item). Nineteen ADL items fit the above criteria. A factor analysis was carried out to further examine interrelationships between these ADL.
The scores of the 19 retained items were summed to yield a total, which ranged from 0–54. The relationships among cognitive impairment, functional abilities, and global dementia severity were examined in the patients with moderate-severe AD. Change scores were calculated for the ADL-sev and SIB over 6 and 12 months and for the MMSE, CDR, and GDS over 12 months. Correlations between scores on different instruments were assessed by examining scatter plots and calculating Pearson or Spearman correlation coefficients as appropriate.
RESULTS
There were 145 participants (49 men and 96 women) whose MMSE scores ranged from 0–15 at baseline. Their mean (± SD) age was 72.3 ± 9.2 years, and the mean MMSE score at entry was 7.3 ± 4.7. After we applied the criteria for item reduction, 19 ADL items were retained, as shown in Table 1. The retained items included typical basic ADL, such as bathing, grooming, and feeding, and a number of additional ADL that were relevant to this patient population. As shown in Table 1, each item had high applicability, provided a scaled measure of ADL performance, and tracked change over time.
Performance of ADL by patients with moderate to severe Alzheimer's disease (MMSE range 0–15)

A number of ADL were excluded from the final group. For example, ADL such as discussing recent events, making a snack or meal, writing a note or letter, using a household appliance, shopping, reading, selecting clothing, cleaning a room, setting a table, putting away belongings, and using a key were attempted by fewer than 50% of subjects with moderate to severe dementia at baseline. The ADL “carries out simple tasks” was performed by 83% of subjects at baseline, and listening to music by 66%, but there was insufficient change in levels of performance over 12 months to make these useful in detecting change. Making a bed was attempted by slightly less than 50% of subjects at baseline and had a strong gender bias. “Follows current events” was carried out by 70% of subjects at baseline (although at a low level of function) but showed insufficient change over 12 months.
“Don't know” responses are problematic in informant reports regarding specific ADL, because they lead to uncertainty in deriving total scores. The frequencies of “don't know” responses by informants regarding each ADL in Table 1 were very low, accounting for fewer than 2% of patients for each item. The rarity of “don't know” responses was most likely due to the requirement for frequent contact with patients by informants, which is a key factor in obtaining ADL ratings.
The scoring of each ADL item is ordinal, rated as different levels or steps. Examination of the item characteristic curves (Fig. 1a and 1b) showed that these intermediate ratings had hierarchical properties in relation to MMSE scores. For example, Figure 1a shows that walking and eating are relatively easy ADL, that is, performed at some level by virtually all patients with MMSE 0–15, whereas grooming, bathing, and getting dressed are more difficult and are progressively lost in association with decreasing MMSE scores. Figure 1b shows the more stringent requirement of optimal ADL performance versus MMSE. Walking remains a relatively easy ADL, while grooming, bathing, eating, and getting dressed have low likelihood of independent (optimal) performance as MMSE scores decline.
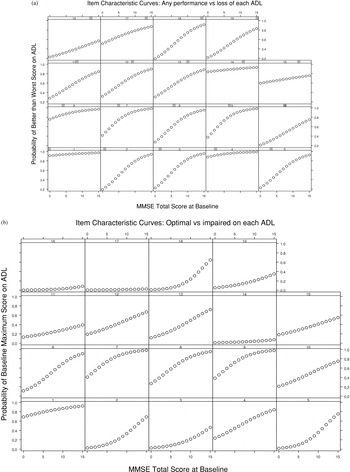
(a, b) Item characteristic curves for the 19 ADL items. In Fig. 1a, the probability of any meaningful performance versus total loss of each ADL is plotted, from a logistic regression, based on MMSE. The ADL, from left to right and bottom to top, are graphed in the same order as they are presented in Table 1: (1) walks, (2) grooms, (3) bathes, (4) eats, (5) gets dressed, (6) toilets, (7) turns a faucet on, (8) turns a faucet off, (9) turns a light on, (10) turns a light off, (11) finds belongings, (12) disposes of litter, (13) clears a table, (14) travels, (15) conversation, (16) watches TV, (17) uses a telephone, (18) prepares a beverage, (19) can be left alone. In Fig. 1b, the probability of performing the ADL at the highest (most independent) level versus any lower level is plotted similarly.
The interrelationship between ADL items was evaluated in several ways. To assess whether ADL items measured unrelated activities (and therefore could contribute independently to a composite scale), we had initially examined a Spearman correlation matrix of scores on each ADL item in all patients with AD across a full spectrum of severity on the MMSE. Most of the correlations ranged from .2 to .6. The highest correlations included the following (R values are in parentheses; all were significant at p < .01): bathing and dressing (.73); bathing and grooming (.77); dressing and grooming (.64); turning a faucet on and turning a faucet off (.64); using a telephone and bathing (.63); using a telephone and grooming (.60); turning lights on and lights out (.60). The lowest correlations were for the ratings of walking and eating with other ADL. Based on this analysis, there were no clear reasons to discard any the 19 items. We decided to retain separate items for turning lights on and off, and turning a faucet on and off because they captured different contexts of related activities, which combined praxis with decision making, and did not correlate exceptionally strongly with each other. Turning lights out appeared to be slightly more difficult than turning them on, and turning a faucet off is similarly slightly more difficult than turning the faucet on.
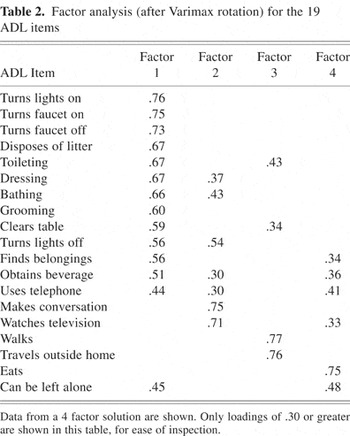
A principal components analysis, with varimax rotation, was carried out using the 19 ADL items, using data from the baseline visit. The principal components analysis yielded 19 factors, of which 4 were retained by the NFACTOR criterion (Table 2). After varimax rotation, the eigenvalues for the factors were: 5.49 for factor 1, 2.16 for factor 2, 1.94 for factor 3, and 1.69 for factor 4. Most but not all items loaded onto the first factor; the types of ADL with high loadings seem to involve praxis, sequencing, and decision making (e.g., turning lights on and off, turning a faucet on and off, bathing, grooming, finding belongings). The second factor had the highest loading for ADL that require verbal skills and reasoning: making conversation and talking about programs seen when watching television. Turning lights off, obtaining a beverage, and using a telephone loaded less strongly onto this factor for unclear reasons. Factor 3 appears to relate to mobility and sense of direction, because walking and travel loaded most strongly, while toileting and clearing a table loaded moderately. Eating loaded most strongly onto factor 4, with a few other items loading less strongly, and no obvious common elements between these ADL were evident.
Factor analysis (after Varimax rotation) for the 19 ADL items
The scores for the performance of all 19 ADL items were summed, yielding total scores for a scale that we have named the ADCS-ADL-sev. Test-retest reliability for the ADL-sev total score was calculated from data at the baseline, 1 month, and 2 month visits. The correlation from baseline to 1 month was .93, from baseline to 2 months was .89, and from month 1 to month 2 was .94; all were significant (Spearman's Rho, p < .001). Baseline ADL scores varied among patients, with a mean of 25.4 points out of a maximum obtainable score of 54, and an SD of 12.7. As shown in Table 3, total ADL scores declined on average by 5.6 points at 6 months and by 10.3 points at 12 months. On the SIB, the mean baseline score was 57.5 (out of a maximum of 100), with an SD of 27.9. Patients declined on average by 17.1 points at 6 months and by 27.2 points at 12 months. The other anchoring measures were obtained at baseline and 12 months, and the mean change at 12 months is shown.
Changes on ADL, cognitive, and global assessments at 6 and 12 months: (mean ± SD)

Concurrent validity of the ADL-sev was assessed by correlating scores with indices of cognitive ability (MMSE and SIB) and global function (the CDR and CDR-SB, and the GDS). There were strong cross-sectional correlations between the ADL and cognitive indices at baseline (ADL-sev vs. MMSE, Spearman Rho = .64, p < .001; ADL-sev vs. SIB, Rho = .71, p < .001). Correlations between the ADL-sev and global ratings were .63 (vs. CDR), .77 (. CDR-SB) and .71 (vs. GDS). The correlation was higher with the CDR-SB than with the overall CDR, because the CDR-SB has more sensitive scaling. The extent of change on the ADL over 12 months correlated moderately well with the extent of change on the other scales over the same interval, ranging from Rho = .37 versus the CDR to .54 versus the SIB. To compare responsiveness to detect change across the different scales, the extent of change on each scale over 12 months (Table 3) was converted to a percentage of the total available points on that scale. The mean percentage of change on the ADL-sev from baseline to 12 months (18%) compared favorably to change on the MMSE (10.3%) and was similar to that on the CDR-SB (15%). The SIB showed the highest mean decline by 27%.
DISCUSSION
As dementia progresses to a severe level, cognitive and functional abilities become increasingly difficult to evaluate using standard tests. Specialized measures such as the SIB (Saxton et al., 1990; Panisset et al., 1994) were therefore developed to target a range of cognitive tasks whose level of difficulty is appropriate for severely demented patients. We have applied the same concept of specialized scale development to ADL assessment.
There are many instruments for ADL assessment, including widely used scales for ratings of basic ADL (Katz et al., 1963) and instrumental ADL (Lawton & Brody, 1969), originally developed for general geriatric assessment. Several scales were later developed for AD. These include the Disability Assessment for Dementia (DAD) (Gelinas et al., 1999), Progressive Deterioration Scale (PDS) (DeJong et al., 1989), and Alzheimer's Disease Activities of Daily Living International Scale (ADL-IS) (Reisberg et al., 2001), all of which have now been used in typical AD clinical trials that enrolled patients with mild to moderately severe dementia. In research studies and clinical trials involving more severely impaired patients, scales such as the Sandoz Clinical Assessment in Geriatrics (SCAG) (Spiegel et al., 1991) and Gottfries-Brain-Skeen (GBS) (Brane et al., 2001) have been used. These scales are brief and combine ADL and behavioral symptoms. Because none of the above scales rates ADL in detail in patients with severe dementia, we developed the 19 item ADL-sev. The items under consideration included those typical of basic ADL scales (dressing, grooming, bathing, eating, walking, and toileting). We also considered more diverse items in the original larger item pool, with the aim of capturing ADL that could still be relevant to severely impaired patients, for example, items requiring skills such as communication and conversation (e.g., using a telephone and watching TV), simple manual praxis (e.g., using light switches and a faucet), visuospatial abilities and sequencing (finding belongings, clearing a table), and the ability to be left alone safely. The procedure to select this subset of ADL items followed guidelines that have been proposed for scale development (Feinstein et al., 1986; Streiner & Norman, 1995), and included the elements of applicability, short-term reliability, internal consistency, scaling of severity, sensitivity to detect change, and concurrent validity. The ADL-sev appears to have appropriate metric properties to make it suitable for research studies.
The factor analysis suggested that there was one major factor on which many ADL loaded, as well as three other factors that carried significant loading. ADL performance depends on several types of cognitive skills, including attention, planning, manual praxis, and sequencing. Different types of memory may be called into play for specific ADL; for example, procedural memory is essential for overlearned motor skills in activities involving personal care, while semantic memory is needed for communication (e.g., making conversation and watching and discussing television). The correlation between overall ADL-sev scores and SIB or MMSE scores was fairly high, indicating that performance on these general indices of cognitive function predicts functional abilities.
The patients in this study were all community dwelling at the time of their first evaluation. In spite of their limited cognitive performance, the majority of these patients attempted the selected ADL meaningfully. Decline or loss of ADL ability, measured as change on the overall ADL score, was clearly seen at both the 6 and 12 month rating periods. This indicates that the 19 items are sensitive enough to track decline over the short-term natural history of AD. Decline in ADL ability over time occurred in concordance with cognitive and overall decline, although ADL assessment evaluates a functional dimension of dementia that overlaps only moderately with cognitive performance. In research studies, it is necessary to measure both cognitive and functional abilities to estimate the “real world” meaning of cognitive change. Further, the data from this patient cohort suggest that the ADL-sev does not suffer from ceiling or floor effects in the range of patients studied given that items selected at baseline required that 50% of the participants performed the ADL, and over the 1-year study interval, the 19 ADL items could still be evaluated.
Brief ADL scales, such as those that assess basic ADL, are widely used to evaluate how much help a patient requires for basic function. They may be administered by unskilled personnel and are useful in evaluating the needs of frail elderly individuals or institutionalized demented patients. Both the ADL-sev and the SIB are too long to be routinely administered in clinical practice, however. The ADL-sev can be administered to an informant in about 15 minutes and provides a broader picture of ADL performance. This more detailed index of ADL abilities would be useful in longitudinal studies of pharmacologic or other interventions. In intervention studies in patients with moderate to severe dementia, cognitive instruments such as the MMSE may show floor effects. In this patient population, careful assessment of cognitive and ADL abilities should provide valuable complementary information about potential treatment effects on those skills and abilities that remain. In this regard, two recent multicenter clinical trials in AD found significant differences in favor of treatment versus placebo on the SIB and ADL-sev over 24–28 weeks of treatment (Reisberg et al., 2003; Tariot et al., 2004). A subsequent analysis of data from both trials showed that relative to patients on placebo, those who received active drug showed trends in favor of maintained abilities on all items rated in the ADL-sev (Doody et al., 2004).
ACKNOWLEDGMENTS
The study was supported by Grant NIA 10483 to UCSD.
References
REFERENCES

Performance of ADL by patients with moderate to severe Alzheimer's disease (MMSE range 0–15)

(a, b) Item characteristic curves for the 19 ADL items. In Fig. 1a, the probability of any meaningful performance versus total loss of each ADL is plotted, from a logistic regression, based on MMSE. The ADL, from left to right and bottom to top, are graphed in the same order as they are presented in Table 1: (1) walks, (2) grooms, (3) bathes, (4) eats, (5) gets dressed, (6) toilets, (7) turns a faucet on, (8) turns a faucet off, (9) turns a light on, (10) turns a light off, (11) finds belongings, (12) disposes of litter, (13) clears a table, (14) travels, (15) conversation, (16) watches TV, (17) uses a telephone, (18) prepares a beverage, (19) can be left alone. In Fig. 1b, the probability of performing the ADL at the highest (most independent) level versus any lower level is plotted similarly.

Factor analysis (after Varimax rotation) for the 19 ADL items

Changes on ADL, cognitive, and global assessments at 6 and 12 months: (mean ± SD)
- 122
- Cited by


