Introduction
Memory Problems in Major Depressive Disorder
Major depressive disorder (MDD) is the leading cause of disability in the United States for individuals between the ages of 15 and 44 (World Health Organization, 2008), and memory complaints are one of the most common symptoms reported by patients with MDD. A large corpus of research demonstrates that memory difficulties are present in MDD, unrelated to poor effort or lack of engagement (Ashendorf, Constantinou, & McCaffrey, Reference Ashendorf, Constantinou and McCaffrey2004; Bora, Harrison, Yücel, & Pantelis, Reference Bora, Harrison, Yücel and Pantelis2013; Considine et al., Reference Considine, Weisenbach, Walker, McFadden, Franti, Bieliauskas and Langenecker2011; Hermens, Naismith, Redoblado-Hodge, Scott, & Hickie, Reference Hermens, Naismith, Redoblado-Hodge, Scott and Hickie2010; Milne, MacQueen, & Hall, Reference Milne, MacQueen and Hall2012; Rees, Tombaugh, & Boulay, 2001; Roberson-Nay et al., Reference Roberson-Nay, McClure, Monk, Nelson, Guyer, Fromm and Pine2006). Particular types of memory, such as explicit memory, but not implicit memory, have been shown to be differentially affected in MDD (Elderkin-Thompson, Moody, Knowlton, Hellemann, & Kumar, Reference Elderkin-Thompson, Moody, Knowlton, Hellemann and Kumar2011; Mulligan, Reference Mulligan2011). Meta-analyses suggest that episodic memory may be most affected for individuals with MDD (Liu, Li, Xiao, Yang, & Jiang, Reference Liu, Li, Xiao, Yang and Jiang2013; Riggs, Carr, Bogue, & Dooley, Reference Riggs, Carr, Bogue and Dooley2005) revealing moderate effect sizes on verbal list learning, recall, and working memory tasks (Bora et al., Reference Bora, Harrison, Yücel and Pantelis2013). Individuals with MDD have also been shown to exhibit relatively preserved encoding and retention, although these studies are typically in comparison to individuals with mild cognitive impairment and dementia (Langenecker, Lee, & Bieliauskas, Reference Langenecker, Lee and Bieliauskas2009; Snyder, 2013).
Despite substantial literature documenting memory impairment in depression, mechanisms for these disruptions have not converged on clearly defined neural networks. Some studies suggest that memory dysfunction is linked to abnormalities in the limbic hypothalamic–pituitary–adrenal axis resulting from chronic elevated cortisol levels potentially causing cell death or dendritic atrophy, primarily in hippocampal regions (Lee, Ogle, & Sapolsky, Reference Lee, Ogle and Sapolsky2002; McEwen, Magariños, & Reagan, Reference McEwen, Magariños and Reagan2002; Sapolsky, Reference Sapolsky2000). Other evidence attributes memory dysfunction in MDD to greater duration of untreated MDD, as repeated untreated episodes are associated with greater hippocampal volume loss (Sheline, Gado, & Kraemer, Reference Sheline, Gado and Kraemer2003). Others suggest that MDD poses a constant distractor, diminishing attentional resources that might otherwise be available to assist with memory encoding and retrieval (Langenecker et al., Reference Langenecker, Lee and Bieliauskas2009; Watkins & Brown, Reference Watkins and Brown2002). In theory, attentional distraction reduces the capacity for attentional focus on soon-to-be encoded information, and is thought to be mediated by fronto-parietal cognitive control networks. Additionally, some studies posit that effortful memory processes are disrupted during MDD, whereas automatic processes remain relatively intact, although there are conflicting results supporting that hypothesis (Rohling & Scogin, 1993). These disparate theories suggest that careful research is still needed to understand the neural structures and networks associated with memory problems in MDD. This is further complicated by the fact that memory may involve multiple processes, including executive functioning (EF), and is easily degraded by distraction, poor sustained attention, slow processing speed, and disrupted organizational skills (Rogers et al., Reference Rogers, Kasai, Koji, Fukuda, Iwanami, Nakagome and Kato2004).
The neural structures and circuits supporting memory are well known and have been extensively studied outside of MDD (Kim, Reference Kim2011; Squire, Reference Squire1992), including the Papez circuit, comprised of limbic structures such as the hippocampus, mammillary bodies and cingulate gyrus (Papez, Reference Papez1937), as well as fronto-parietal networks involved in EF and short-term memory (STM). Structural imaging studies of memory in MDD have demonstrated perturbation of frontal lobes and hippocampi, regions that facilitate memory encoding and recall. Smaller hippocampal volume in MDD has also been widely reported (Bremner et al., Reference Bremner, Narayan, Anderson, Staib, Miller and Charney2000; Cole, Costafreda, McGuffin, & Fu, 2011; Kempton et al., Reference Kempton, Salvador, Munafò, Geddes, Simmons, Frangou and Williams2011; MacQueen, Yucel, Taylor, Macdonald, & Joffe, Reference MacQueen, Yucel, Taylor, Macdonald and Joffe2008; MacQueen & Frodl, Reference MacQueen and Frodl2011), as well as smaller thalamus, overall frontal lobe, and orbitofrontal cortex (Kempton et al., Reference Kempton, Salvador, Munafò, Geddes, Simmons, Frangou and Williams2011). Similar findings in MDD were reported in another meta-analysis, indicating abnormalities in cortico-striatal-pallidal-thalamic circuits, critical for attention, memory encoding, and retention (Bora, Harrison, Davey, Yücel, & Pantelis, Reference Bora, Harrison, Davey, Yücel and Pantelis2012).
Although fewer in number, functional imaging studies have started to examine whether the aforementioned structural differences might be extended to decreased functional capacity and efficiency in MDD. Decreased hippocampal, amygdala, and anterior cingulate cortex activation during verbal memory tasks have been observed in MDD (Bremner, Reference Bremner2004; Milne et al., Reference Milne, MacQueen and Hall2012). Specifically, healthy individuals undergoing PET showed greater increases in blood flow in bilateral hippocampi during a word and paragraph memory task, compared to MDD, with no behavioral differences between the groups, nor a correlation between activation and memory (Bremner, Reference Bremner2004). This suggests that individuals with MDD may engage in activities of learning and memory differently, or to a lesser extent than those without MDD.
A more recent study reported that hippocampal activation was related to better performance on a paired semantic word matching task, but only in the never-depressed comparisons (NDC; Milne, et al., Reference Milne, MacQueen and Hall2012), suggesting low coupling between activation and performance in the context of MDD. MDD patients also displayed different patterns of activation relative to NDC, in frontal and parietal regions in tasks requiring learning and memory. These and other reports led to the conclusion that integrated and perhaps complimentary fronto-parietal and limbic networks support memory function and may be disrupted in MDD, yet the relationship between disrupted network function and memory deficits remains unclear (Dietsche et al., Reference Dietsche, Backes, Stratmann, Konrad, Kircher and Krug2014; Werner et al., Reference Werner, Meindl, Materne, Engel, Huber, Riedel and Hennig-Fast2009).
It is difficult to translate the rather specific way that memory is measured in most neuropsychological probes to the neuroimaging environment. Recall, and not recognition, is often considered the more prototypic pattern of impairment in MDD, yet verbal recall is avoided in neuroimaging environments to reduce the likelihood of head motion. Furthermore, memory probes are often multifaceted with a heavy EF load for organization and encoding strategies during learning and retrieval. This traditional task design makes memory tasks more ecologically valid, but also, unfortunately, results in a more balanced contribution of these multifactorial processes. To better understand the memory challenges that individuals with MDD may face, it is valuable to use a memory task that targets memory systems more heavily, retains external validity, and has sensitive performance characteristics to allow translation to clinical settings.
We designed a Semantic List Learning Task (SLLT) as a more targeted probe of memory processes in depression using two distinct strategies to reduce the influence of individual variability in STM and EF processes (Langenecker, Caveney, Persad, & Giordani, Reference Langenecker, Caveney, Persad and Giordani2004; Schallmo, Kassel, & Weisenbach, Reference Schallmo, Kassel, Weisenbach, Walker, Guidotti-Breting, Rao and Langenecker2015; Weisenbach, Kassel, et al., Reference Weisenbach, Kassel, Rao, Weldon, Avery, Briceño and Langenecker2014). First, the task uses a Brown-Peterson paradigm to minimize rehearsal of recent words, and thereby diminish the contributions of individual variability in STM processes to overall memory (long-term memory, LTM) performance. In the absence of distraction, STM processes can aid in LTM behavioral performance, potentially compensating for LTM weakness (Brown, Reference Brown1958; Peterson & Peterson, Reference Peterson and Peterson1959). Adding a distractor component immediately following the encoding stage of the task requires the participant to shift attention to a separate task, thereby preventing rehearsal of the most recently displayed items, theoretically diminishing STM impact on LTM performance.
Second, we provided semantic organizational cues for both encoding and recall to minimize individual differences in EF support (e.g., clustering strategies) in encoding. Variance attributable to individual differences in both EF and STM is minimized in the SLLT design; hence, subsequent individual differences in memory performance will have a relatively large share of individual variability attributable to networks involved in encoding and consolidation of memory. We hypothesized that use of the SLLT would clarify the extent and underlying neural basis of performance difficulties in MDD, including in relation to other traditional neuropsychological measures of attention, EF, and memory. Hypothesis one predicted that individuals with MDD would display poorer memory recall performance compared to NDC. Hypothesis two proposed that the MDD group would have decreased activation in fronto-parietal and limbic regions relative to NDC. Hypothesis three investigated whether specific brain activation patterns were predictors of SLLT performance, or potential converging findings between behavioral and activation abnormalities in MDD.
Methods
Participants
All participants were between 18 and 65 years of age. Forty (21 males, 19 females) participants were NDC, and 42 (19 males, 23 females) participants had received a diagnosis of MDD, as confirmed by the diagnostic criteria outlined in the Structured Clinical Interview for DSM-IV (SCID-I; First, Spitzer, & Gibbon, Reference First, Spitzer and Gibbon1995). Participants were also administered the Hamilton Depression Rating Scale (HDRS; Hamilton, Reference Hamilton1960) and Beck Depression Inventory (BDI) as depression symptom severity measures. See Table 1 for sample demographic and clinical information.
Table 1 Demographic and clinical information
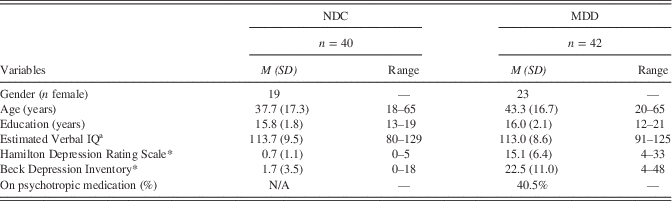
Note. Supplemental Table 3 compares MDD with and without medications. Those taking medications are significantly older than those not taking medications.
a Estimated from the Shipley-2, Verbal subtest, administered in Superlab 2.
*p<.05.
NDC=never-depressed comparison; MDD=major depressive disorder.
MDD participants with comorbid anxiety disorder (n=12) were eligible for participation, but could have no other previous or current psychiatric comorbidities. Participants were recruited through printed advertisements in the community and a university website designed for recruitment purposes. The Institutional Review Board at the University of Michigan approved the study. All participants provided written informed consent before study participation and were compensated up to $60. Consistent with our prior work (Langenecker, Kennedy, et al., Reference Langenecker, Kennedy, Guidotti, Briceño, Own, Hooven and Zubieta2007, Weisenbach, Kassel, et al., Reference Weisenbach, Kassel, Rao, Weldon, Avery, Briceño and Langenecker2014), all participants were screened to ensure they were free of any medical or neurological disorder, contraindications for magnetic resonance imaging (MRI), uncontrolled hypertension or diabetes, head injury with loss of consciousness greater than five minutes, psychotic symptoms, and current substance use or history of substance dependence within 5 years of the MRI. All NDC participants had no personal history of psychiatric illness.
Measures
Participants were administered the California Verbal Learning Test – Second edition (CVLT-II; Delis, Kramer, Kaplan, & Ober, Reference Delis, Kramer, Kaplan and Ober2000) as part of a neuropsychological battery completed during the study. Other neuropsychological tests were included to evaluate divergent validity, including the Parametric Go/No-go Test (Votruba & Langenecker, 2013), Wisconsin Card Sort Test (Grant & Berg, 1948), and Trail Making Test (Army Test Battery, 1944).
The SLLT is a learning and memory task conducted during functional MRI (fMRI) and designed for translation for clinical applications (Schallmo et al., Reference Schallmo, Kassel, Weisenbach, Walker, Guidotti-Breting, Rao and Langenecker2015, Weisenbach, Kassel, et al., Reference Weisenbach, Kassel, Rao, Weldon, Avery, Briceño and Langenecker2014). As such, the focus is on defining a robust performance signature to capture differences between NDC and MDD (or other patient groups). The SLLT is comprised of three blocks: Encoding, Distraction, and Silent Rehearsal (Figure 1). The task consists of 15 lists of 14 semantically related words, for a total of 210 words. A semantic category cue is visually provided at the beginning of each list, displayed for 3.5 s. Following the category cue, 14 words are displayed on a screen one at a time, for an average display time of 3.5 s. The inter-stimulus interval is a 1- to 4-s jittered range, where a fixation cross is displayed. The total time for one Encoding block is typically 58.25 s.

Fig. 1 Diagram of Semantic List Learning Test. Participants are presented with 15 lists of 14 semantically related words (210 words total). Each list is preceded by its semantic category cue. A period of instructed silent rehearsal follows a distractor task after each list in the fMRI scanner, whereas free recall is conduced upon completion of the scanning session, outside of the scanner.
A distractor task immediately follows the last word of each list. This is the “Go” portion of the Parametric Go/No-go (PGNG) task during which “x,” “y,” and “z” are presented (Langenecker, Zubieta, Young, Akil, & Nielson, Reference Langenecker, Zubieta, Young, Akil and Nielson2007). Each Distraction block lasts for 14 s. The Silent Rehearsal block presents participants with the category cue of the previous list, displayed for 14 s. The SLLT then repeats the Encoding, Distractor, and Silent Rehearsal blocks for a new category and list of words. There are three lists presented in each run, with five runs total. Lists are randomized within runs, and words and randomized within lists to avoid any order by position by frequency confounds (Schallmo et al., Reference Schallmo, Kassel, Weisenbach, Walker, Guidotti-Breting, Rao and Langenecker2015). The SLLT includes a post-scan cued recall task with semantic category cues provided for all 15 lists, as well as a recognition trial.
Procedure
After informed consent was obtained and the SCID-I completed, all participants were administered a brief neuropsychological battery. On a separate day participants underwent the fMRI session employing the SLLT. Prior to entering the scanner, participants were verbally introduced to the SLLT. Participants were told they would see a category cue followed by words within that category, shown one at a time on the screen. They were instructed to read each word silently without moving their lips and to try to remember each word. Participants were then informed that they would complete a reaction time task (Distraction) where they should respond by pressing their index finger any time they see the letter “x,” “y,” or “z” on the screen, regardless of order of presentation. It was explained that a category cue prompt would appear on the screen instructing the participant to silently recall the words from the current word list. There was a 30-min delay between the final SLLT run in fMRI and the recall session. Participants were given a recall packet that provided the semantic category cues only, and asked to record any words that they could remember from each of the categories.
MRI Acquisition
Whole brain imaging was performed using a GE Signa 3 Tesla scanner (release VH3). The fMRI series consisted of 36 contiguous oblique-axial sections acquired using a forward–reverse spiral sequence, which provides excellent fMRI sensitivity (Glover & Thomason, Reference Glover and Thomason2004). The image matrix was 64×64 over a 24-cm field of view for a 3.75×3.75×4 mm voxel. The 36-slice volume was acquired serially at 1750 ms temporal resolution (TR) for 154 time points in each of five SLLT runs for a total of 770 time points. Anatomical images were also collected using 104–124 high-resolution Fast SPGR IR axial images [slice thickness; 1–1.5 mm, FOV (field of view); 24 cm, matrix size; 256×256] for each participant, used for co-registration and normalization purposes.
Statistical Analyses
To test Hypothesis one (performance), a series of repeated-measures multivariate analyses of variance (rmMANCOVAs) were conducted. Because there are known sex (and age) differences in verbal memory, sex and age were used as covariates in all analyses. Where relevant, false positives were also used as covariates to account for performance variability potentially attributable to low engagement or inattention. A MANCOVA was also computed for CVLT-II long-delay free recall hits, as this is a frequently reported metric from the CVLT-II (Considine et al., Reference Considine, Weisenbach, Walker, McFadden, Franti, Bieliauskas and Langenecker2011). MANCOVAs were computed for false positives from the CVLT-II (long-delay free recall, trial 1) and SLLT, covarying recall hits. Serial position curve effects were evaluated using rmMANCOVAs computed for SLLT and CVLT-II trial 1, with group as the independent variable (Cohen’s d also reported for comparison across tests and with existing literature) and recall in primacy (first four words), middle (six words in SLLT, eight words in CVLT-II), and recency phases (last four words).
To test hypothesis two (activation), fMRI data were processed and analyzed using MATLAB (The MathWorks, Natick, MA) with SPM8 and FSL (Friston et al., Reference Friston, Ashburner, Frith, Poline, Heather and Frackowiak1995). Slice timing, coregistration, and realignment were conducted at the individual subject level. Functional images were then normalized to fit a Montreal Neurological Institute (MNI) canonical template using DARTEL and were smoothed with a 5-mm FWHM kernel. The Encoding, Silent Rehearsal, Distraction, and Rest blocks were entered into first level models to determine global activation patterns for list learning, and contrast images were used in second level analyses (i.e., Encoding – Silent Rehearsal), with sex as a covariate. A second set of contrast images was also derived from behavioral data for memory hits to estimate the hemodynamic response function for the recall models in an event-related analysis (Words Recalled). Contrasts generated from Encoding – Silent Rehearsal and Words Recalled were analyzed to compare activation patterns between MDD and NDC.
Furthermore, as a follow-up, targeted test of hypothesis two, we used the MarsBaR toolbox (Brett, Anton, Valabregue, & Poline, Reference Brett, Anton, Valabregue and Poline2002) to extract mean signal change during all blocks in regions of interest (ROIs) to evaluate Papez circuit specific ROIs in relationship to condition. These regions were based upon prior literature and a meta-analysis of explicit memory tasks (Kim, Reference Kim2011; Schallmo et al., Reference Schallmo, Kassel, Weisenbach, Walker, Guidotti-Breting, Rao and Langenecker2015). Mean activation in each region (10), by side (2), and by condition (4), was analyzed in a rmANOVA in SPSS. A total of 10 spherical ROIs were created bilaterally in MarsBaR and mean signal was extracted for each of four conditions (Encoding, Distraction, Silent Rehearsal, Rest). Three spherical ROIs were created on the long axis of the hippocampus in 12-mm intervals, using MNI coordinates (30, −12, −18; 32, −24, −11; and 31, −36, −4).
To determine ROI positions, we used activation foci from a recent meta-analysis (Kim, Reference Kim2011) or those identified in two other independent studies of memory imaging (Beason-Held, Golski, Kraut, Esposito, & Resnick, Reference Beason-Held, Golski, Kraut, Esposito and Resnick2005; Braskie, Small, & Bookheimer, Reference Braskie, Small and Bookheimer2009), and the Wake Forest PickAtlas (Maldjian, Laurienti, Burdette, & Kraft, Reference Maldjian, Laurienti, Burdette and Kraft2003; Maldjian, Laurienti, & Burdette, Reference Maldjian, Laurienti and Burdette2004). A 5-mm radius spherical ROI was used for most regions except two that were smaller (mammillary bodies and fornix=3 mm radius), and one that was larger (anterior insula=7 mm radius). We note that the mammillary bodies are very close to air sinuses and may be less reliable. The fornix is primarily a white matter tract thus BOLD signal there may be less relevant to gray matter function and memory performance. All of these analyses used hits, false positives, sex and age as covariates.
To test Hypothesis three (activation to performance relationships), the mean ROI activation values were correlated with SLLT performance, separately in each group, to evaluate activation to performance relationships. Areas of significant effects (between group differences) were extracted from SPM maps using MarsBaR, consistent with our prior work (Langenecker et al., Reference Langenecker, Weisenbach, Giordani, Briceño, Guidotti-Breting, Schallmo and Starkman2012). This strategy can help elucidate whether group differences are more specifically related to performance or disease group, or both. For the imaging analyses, two contrasts of primary interest were used: (1) Activation during the Encoding block minus the Silent Rehearsal block, and (2) Event-related analyses using the hemodynamic response function for Words Recalled were used for comparison as a second strategy to control for performance (only activation related to successfully recalled items are used, otherwise known as the subsequent memory effect, Kim, Reference Kim2011).
AlphaSim correction (1000 Monte Carlo iterations) was used for all whole brain analyses, balancing height (p<.005) and extent (440 mm3) thresholds to achieve a whole brain correction of p<.05. Behavioral performance and extracted ROI/Papez thresholds were set at p<.05 for theoretically based tests, uncorrected for multiple comparisons.
Results
Memory Performance Data
Hypothesis 1 predicted that MDD would display poorer memory performance for the CVLT-II and SLLT, compared to NDC. First, the hypothesis was tested for CVLT-II performance long-delay free recall, as that is typically reported in memory studies. There was no difference in performance between the groups for the CVLT-II long-delay free recall hits [F(1,60)=1.41; p=.24; d=.25; Figure 2A] or for CVLT-II long-delay free recall false positives [F(1,60)=0.64; p=.43].

Fig. 2 (a) Percent recall of the CVLT-II long-delay free recall (CVLT-LDFR) by Group; (b) Percent recall along the Serial Position Curve by Task (CVLT-II Trial 1, SLLT mean performance) and by Group. (NDC=Never-Depressed Comparisons; MDD=Major Depressive Disorder).
The second test of hypothesis 1 was for CVLT-II trial 1 to match to the subsequent SLLT analysis. In a rmANOVA testing group and group by serial position curve effects (three serial positions: mean primacy, middle, recency values), NDC showed a trend toward greater recall hits on CVLT-II trial 1 compared to MDD [F(1,61)=3.11; p=.08; d=.55; Figure 2B]. The interaction of serial position curve with group was not significant [F(2,122)=0.41; p=.67]. There were no group differences in false positives for CVLT-II trial 1 [F(1,61)=0.01; p=.92].
The third test of hypothesis 1 examined overall SLLT performance. During the SLLT, NDC exhibited greater recall overall [F(1,78)=5.09; p=.03]. The NDC and MDD groups did not differ on the number of false positives for the SLLT [F(1,78)=0.91; p=.34]. An additional, planned rmANOVA of three serial positions (mean primacy, middle, recency values) by two Group (MDD, NDC) revealed that NDC participants performed better than MDD participants [F(1,78)=4.63; p=.04; d=.42, Figure 2B]. The interaction of serial position curve with group was not significant [F(2,156)=0.12; p=.89].
Whole Brain Activation Differences between Groups
Hypothesis 2 proposed that MDD would have decreased activation in fronto-parietal and limbic regions relative to NDC. For comparison, the positive effect of Encoding-Silent Rehearsal condition for all participants, both NDC and MDD together, revealed activation in a broad fronto-temporal network (Supplementary Table 1; Figure 4A). The main contrast of interest was the Encoding block minus the Silent Rehearsal block, as our goal was to examine memory network(s) during the encoding phase as a foundation to establish memory circuitry differences in MDD compared to NDC. All of these analyses used hits, false positives, sex, and age as covariates. NDC displayed greater activation than MDD in several regions including primarily bilateral middle frontal gyrus, dorsal anterior cingulate, insula, precuneus, superior parietal lobule, thalamus, and various cerebellar regions (Table 2; Figure 3A). MDD did not exhibit greater activation in any regions at the same threshold for the Encoding-Silent Rehearsal comparison. Z values of the following factors are also reported in Table 2, though, in this data, the majority of clusters were not affected by these factors: if individuals with MDD taking medications were excluded; effects based upon differences between MDD and MDD plus anxiety; ROI specific gray matter VBM differences.

Fig. 3 Brain regions of significantly greater activation for Never-Depressed Comparisons than Major Depressive Disorder during the (a) Encoding minus Silent Rehearsal condition and the (b) Event Related Words Recalled condition.
Table 2 Brain regions of significantly greater activation for NDC relative to MDD during the SLLT

Note. No areas of greater activation for MDD were found in the above contrasts.
a Z value when excluding medicated MDD participants (i.e. NDC greater than unmedicated MDD). Note the medicated MDD participants typically have greater severity on many illness metrics, also observed in this sample. Note all results remain in the same direction if equivalent or diminished extent.
b Regions where a significant grey matter volumetric subcluster difference was found between groups (NDC > MDD).
c Region where a subcluster of reduced gray matter volume was inversely correlated with activation in the MDD group (r=−.32, p=.04).
d Region where a subcluster of reduced gray matter volume was positively correlated with activation in the MDD group (r=.31, p=.05).
Blk=block design; ER=event-related.
Event Related (ER) analyses of Words Recalled were also conducted as a parallel analysis. For comparison, the positive effect of condition for all participants, both NDC and MDD in an overlapping analysis, revealed activation in fronto-temporal regions (Supplementary Table 1; Figure 4B). NDC displayed greater activation in the following gyri: primarily bilateral middle and inferior frontal, postcentral, parahippocampal, superior temporal, inferior occipital, anterior cingulate, and posterior cingulate cortices (Table 2; Figure 3B). NDC also exhibited greater activation in primarily bilateral precuneus, inferior parietal lobule, and claustrum (Table 2; Figure 3B). MDD did not exhibit greater activation in any regions for the ER analysis of Words Recalled.

Fig. 4 Brain regions of significant greater activation in the (a) Encoding minus Silent Rehearsal condition and the (b) Event Related Words Recalled condition for both groups (NDC and MDD).
Papez Circuit Specific ROI Activation Differences between Groups
An rmANOVA was computed in SPSS including 10 Papez regions, 2 sides, 4 conditions, and 2 groups extracted using MarsBaR. Interactions between ROI and group [F(9,720)=10.76; p<.01], side, ROI, and group [F(9,720)=2.31; p=.01], and condition, ROI, and group [F(27, 2160)=1.89; p<.01] were found. We focused on the interaction between condition, ROI, and group to test Hypothesis two, regarding condition and region-specific differences in MDD versus NDC. For purposes of illustration, we display activation for these regions in the two key conditions of interest (Encoding and Silent Rehearsal), and activation for the ER Words Recalled analysis (Figure 5). In the extracted activation averaged across hemispheres, MDD exhibited less activation than NDC during Encoding in the following regions: subgenual cingulate, mammillary bodies, posterior cingulate, and anterior, middle, and posterior hippocampi. MDD demonstrated less activation than NDC during Silent Rehearsal in the following regions: mammillary bodies, anterior insula, fornix, and anterior and middle hippocampi. The ER Words Recalled analysis revealed lower activation for MDD compared to NDC in nine of the 10 Papez regions extracted: subgenual and rostral anterior cingulate, retrosplenial and posterior cingulate, mammillary bodies, anterior insula, fornix, and middle and posterior hippocampi (Figure 5).

Fig. 5 Illustrates the significant interaction between condition, regions of interest (ROI), and group. Displays activation for 10 Papez ROIs in the two key conditions of interest (Encoding and Silent Rehearsal), as well as activation for the Event Related Words Recalled analysis.
Subsequent Memory Prediction in MDD
Hypothesis three investigated whether specific brain activation patterns were predictors of SLLT performance. Multiple regression models were conducted to establish a relationship between activation and performance in SPM8 using whole brain analyses, independently for each group. Multiple regressions of Encoding-Silent Rehearsal and recall hits revealed 13 fronto-temporal regions of greater activation related to better performance on the SLLT (Table 3). Greater activation for 3 (precentral, parahippocampal gyri, and uncus) out of the 13 regions was related to enhanced recall on the SLLT in both NDC and MDD. Greater activation in the superior frontal and inferior frontal gyri as well as the insula and pulvinar of the thalamus were related to greater recall hits and lower false positives in NDC. In MDD, only greater activation in the lingual gyrus was related to lower false positives. For both NDC and MDD, greater activation in anterior cingulate was related to poorer performance (lower recall hits, Table 4).
Table 3 Whole brain activation correlated with better SLLT performance for Encoding minus Silent Rehearsal, by Group.

Blk=block design; ER=event-related.
Table 4 Whole brain activation correlated with poorer SLLT performance for Encoding minus Silent Rehearsal, by Group.

Note. No areas of greater activation significantly associated with high false positives were found for either NDC or MDD groups.
Blk=block design.
Convergent and divergent validity for the SLLT and CVLT-II were evaluated with post hoc analyses assessing their relationships to each other and with different EF tasks (SupplementaryTable 2). Of 42 correlations, 5 (11.9%) of SLLT serial position curve measures with EF measures were significant, whereas 9 of 42 (21.4%) correlations from CVLT-II serial position curve measures were significant.
Discussion
The present study found decreased memory performance and robustly decreased activation in fronto-limbic regions, including those that are key regions in the Papez circuit, STM, and EF networks, in young and middle-aged adults with MDD, using a task designed to specifically challenge memory pathways and diminish variability in how EF and STM may contribute to memory performance. Surprisingly, there were also decreases in somatosensory areas in MDD. Within the Papez circuit, the mammillary bodies and middle hippocampi consistently displayed decreased activation in MDD in all three of the analysis conditions (Encoding, Silent Rehearsal, ER Words Recalled). When looking at all three conditions between groups, MDD showed decreased activation in many overlapping Papez regions across conditions, with each of the 10 Papez ROIs investigated showing decreased activation in MDD in at least one of the three conditions (Encoding, Silent Rehearsal, ER Words Recalled).
Variability in performance explained activation differences between groups at a minimal level. Performance was a contributing factor for a substantial minority of regions—the majority of functional aberrations of memory networks in MDD are independent of performance variability. In contrast to the SLLT, there was no performance difference between groups on the CVLT-II long-delay free recall, one of the most widely used memory measures in neuropsychology practice (Camara, Nathan, & Puente, Reference Camara, Nathan and Puente2000; Rabin, Barr, & Burton, Reference Rabin, Barr and Burton2005).
The discrepancy between CVLT-II and SLLT performance results is interesting for several reasons. First, other studies report memory difficulties in MDD with the CVLT-II, typically for long-delay free recall, and effect sizes are moderate (Bora et al., Reference Bora, Harrison, Yücel and Pantelis2013). The effect size for long-delay free recall for this study was small, suggesting that our sample may be a more conservative test of memory difficulties. As participants recruited for fMRI experiments must meet more stringent exclusion criteria (e.g., medical comorbidities, BMI, metal in the body), it is not surprising that the extent of memory difficulties observed would be less than in non-imaging studies.
In older adult samples, these exclusion criteria might lead to a comparison of super-select MDD individuals without cognitive difficulties, especially due to worries about confounding depression- and dementia-related cognitive difficulties in older adults (Weisenbach, Kassel, et al., Reference Weisenbach, Kassel, Rao, Weldon, Avery, Briceño and Langenecker2014). Even in younger samples, exclusions related to tolerability, comorbidity, body mass index (BMI), etc., could lead to a more restricted range of less ill MDD participants. Yet we still found differences between MDD and NDC in SLLT recall performance, suggesting that the SLLT is more sensitive to memory difficulties observed in MDD.
Furthermore, the present study investigates individuals with MDD ranging from only one episode to those with more chronic disease, possibly weakening the ability to show CVLT-II performance decrements in MDD; many other studies have reported that duration, severity, and chronicity of illness is related to memory difficulties in MDD (Snyder, 2013). Furthermore, the CVLT-II allows for multiple chances at encoding the same information, which can inform about the process of learning, but may underestimate real-world, one-trial learning realities often reported by individuals with MDD.
Our results reveal that both groups use similar networks during encoding of semantically cued and grouped words, but NDC activate the networks to a greater extent. Some of these activation foci are related to performance in both groups, yet group differences remain when performance values are used as covariates, suggesting they are driven by disease or disease-by-network efficiency/utilization processes. It is notable, nonetheless, that for regions where performance to activation relationships are present, there is a challenge in interpreting whether these differences are due to overlap of performance and/or disease differences (e.g., see Miller & Chapman, Reference Miller and Chapman2001, for discussion of challenges to covariation). These results suggest decreased usage of distributed networks involved in memory for adults with MDD.
We also note that frontal and parietal regions show decreased activation in MDD, suggesting lesser involvement of STM and EF network(s) in semantically cued explicit memory. It is possible that even when given an explicit strategy and cue as in the SLLT, MDD are less able to marshal EF resources to assist primary memory resources. It is also evident that implicit semantic cuing strategy (semantic clustering from CVLT-II) was related to SLLT performance in MDD and not NDC. Thus, semantic clustering, whether explicit or implicit, may play a larger role in individual differences in MDD than in NDC. Hence decreased activation in Papez circuit as well as STM and EF network regions co-occurs with decreased memory performance in some instances, reflecting decreased efficiency of these networks in the active disease state. In contrast, in some other regions, such as dorsal anterior cingulate, increased activation was associated with worse performance in MDD, suggesting that either conflict or mediation of interference might be reflected in activation within this region.
Follow-up studies to further parse the processes involved in deficient memory performance and strategies that can be used to augment these weaknesses are needed. Investigating the specific strategies that individuals report when completing the SLLT may also prove useful. Memory performance declines with age in non-depressed individuals, and is negatively affected in individuals with MDD; therefore, treatment for individuals with MDD might involve memory exercises to strengthen functioning and performance of memory network circuitry early on to ward off potential harmful effects of burden of illness. Effective and early treatment may stave off chronic burden and cognitive difficulties in mental illness (Weisenbach, Marshall, et al., Reference Weisenbach, Marshall, Weldon, Ryan, Vederman, Kamali and Langenecker2014).
Notably, previous work has shown a bias toward negative affect in MDD (Hsu, Langenecker, Kennedy, Zubieta, & Heitzeg, Reference Hsu, Langenecker, Kennedy, Zubieta and Heitzeg2010), which may influence memory, possibly related to the amygdala activation observed in MDD during memory challenge (Ai et al., Reference Ai, Opmeer, Veltman, van der Wee, van Buchem, Aleman and van Tol2015; Roberson-Nay et al., Reference Roberson-Nay, McClure, Monk, Nelson, Guyer, Fromm and Pine2006). Consideration of arousal and valence associated with words may be beneficial, as these can enhance memory processes (Bradley & Lang, Reference Bradley and Lang1999). In such studies, it would be possible to test the negativity bias in memory and links to neural correlates by examining whether increased or decreased MDD memory performance is linked to decreased or increased activation in these regions, even with distraction paradigms.
Our study design does not allow disambiguation of several other factors that may be attributable to differences in memory performance. For example, state of illness effects are unconstrained, potentially distorting or combining trait and state symptoms. In addition, burden of illness or potential scarring effects of MDD over time are uncontrolled. Future research could examine memory and other cognitive domains across a longitudinal spectrum to elucidate burden of illness effects. Although we provided encoding strategy cues by presenting the semantic category of each list, we did not ask participants whether they used other strategies for encoding.
The significant correlations of SLLT values with semantic clustering, if only for MDD in primacy and middle phases of the serial position curve, suggest that those strategies were being used by some of the participants. Furthermore, although participants were matched on age in both groups, the large age range overall could have reduced the potential task effects observed, or masked age by disease interactions. Additionally, our sample was highly educated, which may impact performance and/or activation patterns. CVLT-II, other neuropsychological measures and the HDRS and BDI were measured days to weeks before fMRI, potentially weakening relationships of these measures with SLLT and fMRI measures. Finally, our sample was not particularly ethnically diverse, and thus may not best represent the general population of individuals with MDD.
It remains imperative to pinpoint the underlying neural circuitry related to disrupted memory in MDD, such that effective targets for treatment and/or accommodation are pursued. As MDD is the largest disability cost in the 15–44 age range (World Health Organization, 2008), understanding and treating memory difficulties in addition to depressive symptoms is critical. Through refined neuroimaging techniques and with tasks like the SLLT, including a more ecologically valid presentation (e.g., single trial, with distraction), it may be possible to tease apart the neural network(s) dysfunction that contribute to impaired memory function in MDD.
Acknowledgments
This research was supported by funding from the National Institute of Mental Health (K-23 MH 074459, S.A.L.), National Alliance for Research on Schizophrenia and Depression (S.A.L.), the University of Michigan Depression Center (S.A.L., S.L.W.), the University of Michigan fMRI Laboratory (S.A.L., S.L.W.), and the University of Michigan Department of Psychiatry. We also thank Lawrence Own, Michael-Paul Schallmo, Kathleen E. Hazlett, Rachel E. Kay, Hadia Leon, Monique Bowles, Justin B. Miller, and Leslie Guidotti-Breting for their contributions to this project in data collection and processing. This work was presented in part at the 2013 Annual Meeting of Organization for Human Brain Mapping. The authors report no conflicts for this work.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1355617716000023