Introduction
Schizotypy usually is referred to as a “liability” to schizophrenia (Lenzenweger & Korfine, Reference Lenzenweger and Korfine1994), but it could also be referred to as non-specific “psychosis-proneness” (Claridge et al., Reference Claridge, McCreery, Mason, Bentall, Boyle, Slade and Popplewell1996). There are two different perspectives on schizotypy: a psychological one with schizotypy representing deviant personality traits [i.e., individuals in the general population exhibit schizotypal traits on a continuum (Kendler et al., Reference Kendler, Ochs, Gorman, Hewitt, Ross and Mirsky1991) and psychosis represents extremes of normal variation in the healthy personality (Claridge, Reference Claridge1985; Eysenck & Eysenck, Reference Eysenck and Eysenck1975)], and a psychiatric one with schizotypy representing attenuated psychotic symptoms (i.e., symptoms represent degrees of expression of the disease that are different from normal). The latter approach has given rise to the diagnostic criteria of Schizotypal Personality Disorder (SPD) (APA, 1980).
Apart from the close relationship of SPD clinical characteristics to the clinical profile of schizophrenia, the two disorders also share common genetic (Siever & Davis, Reference Siever and Davis2004), and neurobiological substrates (Dickey, McCarley, & Shenton, Reference Dickey, McCarley and Shenton2002; Siever & Davis, Reference Siever and Davis2004) as well as cognitive impairments (Siever & Davis, Reference Siever and Davis2004; Spaulding, Garbin, & Dras, Reference Spaulding, Garbin and Dras1989), which impact on patients’ daily living (Green, Reference Green1996). Longitudinal studies also suggest that schizotypy may be a forerunner of schizophrenia (Chapman, Chapman, Kwapil, Eckblad, & Zinser, Reference Chapman, Chapman, Kwapil, Eckblad and Zinser1994). Accordingly, unaffected first degree relatives of schizophrenia patients present with high schizotypal traits (Laurent et al., Reference Laurent, Duly, Murry, Foussard, Boccara, Mingat and d'Amato2001; Diwadkar, Montrose, Dworakowski, Sweeney, & Keshavan, Reference Diwadkar, Montrose, Dworakowski, Sweeney and Keshavan2006) and widespread cognitive impairments (Heydebrand, Reference Heydebrand2006; Hill, Harris, Herbener, Pavuluri, & Sweeney, Reference Hill, Harris, Herbener, Pavuluri and Sweeney2008) and while the incidence of the illness in the general population is approximately 1%, adolescent offspring of schizophrenia patients are up to 15 times more likely to develop a psychotic disorder compared to the general population (Erlenmeyer-Kimling et al., Reference Erlenmeyer-Kimling, Squires-Wheeler, Adamo, Bassett, Cornblatt, Kestenbaum and Gottesman1995; Gottesman & Shields, Reference Gottesman and Shields1982), with predicted conversion rates approximating 40% (Diwadkar et al., Reference Diwadkar, Montrose, Dworakowski, Sweeney and Keshavan2006). Among the cognitive deficits observed in schizophrenia and SPD, impairments in executive functions, attention, and memory appear to be the most prevalent (Dickinson, Ramsey, & Gold, Reference Dickinson, Ramsey and Gold2007; Fioravanti, Carlone, Vitale, Cinti, & Clare, Reference Fioravanti, Carlone, Vitale, Cinti and Clare2005; Heinrichs & Zakzanis, Reference Heinrichs and Zakzanis1998; Heydebrand, Reference Heydebrand2006; Laws, Reference Laws1999; Matsui, Sumoyoshi, Kato, Yoneyama, & Kurachi, Reference Matsui, Sumiyoshi, Kato, Yoneyama and Kurachi2004; Matsui et al., Reference Matsui, Yuuki, Kato, Takeuchi, Nishiyama, Bilker and Kurachi2007) and are, therefore, suggested as useful endophenotypes (Hill et al., Reference Hill, Harris, Herbener, Pavuluri and Sweeney2008).
Schizophrenia and SPD have also been associated with deficits in information processing (Bleuler, Reference Bleuler1911; Kraepelin, Reference Kraepelin1913). Prepulse Inhibition (PPI) of the startle reflex, a cross-species phenomenon, provides a valuable translational tool to study information processing abnormalities (Braff, Reference Braff1993). PPI refers to a reduction in startle amplitude in response to a strong startling stimulus (pulse), if this is preceded shortly by a pre-stimulus (prepulse) too weak to elicit a measurable startle response itself. PPI is thought to reflect “sensorimotor gating,” a form of central nervous system inhibition wherein irrelevant sensory information is filtered out during the early stages of processing so that attention can be focused on more salient features of the environment (Braff, Geyer, & Swerdlow, Reference Braff, Geyer and Swerdlow2001). The sensory overload resulting from reduced sensorimotor gating is thought to give rise to cognitive fragmentation and some of the complex clinical symptoms associated with schizophrenia spectrum disorders (Braff, Grillon, & Geyer, Reference Braff, Grillon and Geyer1992). It has been repeatedly demonstrated that patients with schizophrenia (Braff et al., Reference Braff, Geyer and Swerdlow2001), their unaffected relatives (Thaker, Reference Thaker2008), and patients with SPD (Hazlett, Buchsbaum, Zhang et al., Reference Hazlett, Levine, Buchsbaum, Silverman, New, Sevin and Siever2003, Reference Hazlett, Romero, Haznedar, New, Goldstein, Newmark and Buchsbaum2007, Reference Hazlett, Buchsbaum, Zhang, Newmark, Glanton, Zelmanova and Siever2008) show deficient gating as measured by PPI. PPI is also considered a valid endophenotypic marker of psychosis (Braff & Light, Reference Braff and Light2005; Calkins et al., Reference Calkins, Dobie, Cadenhead, Olincy, Freedman, Green and Braff2007).
Along with PPI, other neurophysiological endophenotypes, such as the P50 suppression and the P300 event-related potential, have undergone extensive review in the schizophrenia literature: schizophrenia patients and their unaffected relatives (for reviews, see Thaker, Reference Thaker2008; Turetsky et al., Reference Turetsky, Calkins, Light, Olincy, Radant and Swerdlow2007), SPD patients (Cadenhead, Light, Geyer, & Braff, Reference Cadenhead, Light, Geyer and Braff2000; Mannan, Hiramatsu, Hokama, & Ohta, Reference Mannan, Hiramatsu, Hokama and Ohta2001; Trestman et al., Reference Trestman, Horvath, Kalus, Peterson, Coccaro, Mitropoulou and Siever1996), and high schizotypal individuals in the general population (Arzy, Mohr, Michel, & Blanke, Reference Arzy, Mohr, Michel and Blanke2007; Evans, Gray, & Snowden, Reference Evans, Gray and Snowden2007; Sumich, Kumari, Gordon, Tunstall, & Brammer, Reference Sumich, Kumari, Gordon, Tunstall and Brammer2008) present with deficits compared with normal controls. However, although the gating mechanisms accessed via PPI and these paradigms are often conceptually linked, there is evidence that they actually diverge in healthy and psychiatric populations (Brenner, Edwards, Carroll, Kieffaber, & Hetrick, Reference Brenner, Edwards, Carroll, Kieffaber and Hetrick2004; Cadenhead, Light, Geyer, McDowell, & Braff, Reference Cadenhead, Light, Geyer, McDowell and Braff2002; Light & Braff, Reference Light and Braff2001; Schwarzkopf, Lamberti, & Smith, Reference Schwarzkopf, Lamberti and Smith1993). Thus, these seemingly closely related gating abnormalities may be characteristic of different subgroups of patients, with PPI impairments being more specific to the gating deficits characteristic of schizotypy and unrelated to other deficient gating processes observed in schizotypal individuals (Cadenhead et al., Reference Cadenhead, Light, Geyer, McDowell and Braff2002).
Apart from studying clinical populations or their biological relatives, another useful approach in endophenotypic research, is studying psychometrically defined schizotypal subjects in the general population [a review of general-population surveys indicated that schizophrenia-like experiences are observed in an attenuated form in 5–8% of healthy individuals (Van Os, Linscott, Myin-Germeys, Delespaul, & Krabbendam, Reference van Os, Linscott, Myin-Germeys, Delespaul and Krabbendam2009)]. This approach offers several advantages as it is devoid of several confounding variables such as medication and hospitalization effects, illness chronicity, psychosocial consequences of psychiatric diagnoses, and many others involved in the study of clinical populations (Gruzelier, Reference Gruzelier2003). It is worth noting that in accordance to schizophrenia (Bystritsky et al., Reference Bystritsky, Liberman, Hwang, Wallace, Vapnik, Maindment and Saxena2001; Dworkin, Lewis, Cornblatt, & Erlenmeyer-Kimling, Reference Dworkin, Lewis, Cornblatt and Erlenmeyer-Kimling1994; Harvey, Reference Harvey2011) and SPD patients (Dickey et al., Reference Dickey, McCarley, Niznikiewicz, Voglmaier, Seidman, Kim and Shenton2005; Skodol et al., Reference Skodol, Gunderson, McGlashan, Dyck, Stout, Bender and Oldham2002), high schizotypal individuals in the general population also present with poor behavioral outcomes, such as reduced social functioning (Addington et al., Reference Addington, Cornblatt, Cadenhead, Cannon, McGlashan, Perkins and Heinssen2011; Blanchard, Collins, Aghevli, Leung, & Cohen, Reference Blanchard, Collins, Aghevli, Leung and Cohen2011; Fonseca-Pedrero, Lemos-Giráldez, Paíno-Piñeiro, Villazón-García, & Muñiz, Reference Fonseca-Pedrero, Lemos-Giráldez, Paíno-Piñeiro, Villazón-García and Muñiz2010; Henry, Bailey, & Rendell, Reference Henry, Bailey and Rendell2008; Jahshan & Sergi, Reference Jahshan and Sergi2007; Seghers, McCleery, & Docherty, Reference Seghers, McCleery and Docherty2011), academic (Aguirre, Sergi, & Levy, Reference Aguirre, Sergi and Levy2008; Barrantes-Vidal, Lewandowski, & Kwapil, Reference Barrantes-Vidal, Lewandowski and Kwapil2010) and occupational (Thaker, Adami, & Gold, Reference Thaker, Adami and Gold2001) difficulties, deteriorated socioeconomic status (Thaker et al., Reference Thaker, Adami and Gold2001), interpersonal problems (Aguirre et al., Reference Aguirre, Sergi and Levy2008; Barrantes-Vidal et al., Reference Barrantes-Vidal, Lewandowski and Kwapil2010; Blanchard et al., Reference Blanchard, Collins, Aghevli, Leung and Cohen2011), and increased depressive and anxiety traits (Lewandowski et al., Reference Lewandowski, Barrantes-Vidal, Nelson-Gray, Clancy, Kepley and Kwapil2006; Seghers et al., Reference Seghers, McCleery and Docherty2011), resulting in poor quality of life (Cohen & Davis, Reference Cohen and Davis2009; Seghers et al., Reference Seghers, McCleery and Docherty2011). Also, from the standpoint of the psychological perspective described above, the study of schizotypal traits in the general population can yield findings that can be applied to clinical populations. As this perspective is proving to be a promising approach in the study of endophenotypes in schizophrenia spectrum disorders, studies of cognitive and PPI impairments in psychometrically high schizotypal subjects are increasing rapidly.
Schizotypy can be assessed either via interviews focusing on the detection of schizotypal traits [e.g., the Structured Interview for Schizotypy (Kendler, Lieberman, & Walsh, Reference Kendler, Lieberman and Walsh1989)] or via self-report scales. The latter allows for several advantages compared with other forms of assessment as it is non-invasive, rapidly applied, easy to administer, score and interpret, and thus highly cost-effective. This psychometric strategy is also suggested to help identify individuals at risk that might not be detected by other approaches [e.g., the genetic high-risk paradigm (Gooding, Tallent, & Matts, Reference Gooding, Tallent and Matts2007)]. Therefore, a variety of self-report assessment instruments have been developed [e.g., the Chapman Scales (Chapman, Chapman, & Raulin, Reference Chapman, Chapman and Raulin1976, Reference Chapman, Chapman and Raulin1978), the Schizotypal Traits Questionnaire (Claridge & Broks, Reference Claridge and Broks1984), the Schizotypal Personality Questionnaire (Raine, Reference Raine1991), the Oxford-Liverpool Inventory of Feeling and Experiences (Mason, Claridge, & Jackson, Reference Mason, Claridge and Jackson1995)] accompanied by adequate reliability and validity.
The aim of this critical review is to summarize findings on deficits in executive function, attention, memory, and PPI in psychometrically defined schizotypal, but otherwise healthy, subjects and to contrast these with findings in schizophrenia patients and their unaffected first degree relatives. For this reason, a PubMed search was conducted with combinations of the general search terms “schizophrenia,” “schizotypal,” “schizotypy,” “executive function,” “attention,” “memory,” “prepulse inhibition,” “genetic,” and “healthy.” A summary of the neuropsychological and PPI studies reviewed and their findings are presented in Tables 1 and 2, respectively.
Table 1 Neuropsychological impairments in psychometrically defined schizotypal subjects in the general population

Con = Controls; F = Females; COWAT = Controlled Oral Word Association Test; CPT = Continuous Performance Test; CVLT = California Verbal Learning Task; M = Males; NS = Non-Significant differences; SCT = Schizotypy; TMT = Trail-Making Test; WCST = Wisconsin Card Sorting Test.
1For a description of the task see Park et al. (Reference Park, Holzman and Lenzenweger1995).
2For a description of the task see Tallent and Gooding (Reference Tallent and Gooding1999).
3For a description of the task see Lenzenweger & Gold (Reference Lenzenweger and Gold2000).
4For a description of the task see Le Pelley et al. (Reference Le Pelley, Schmidt-Hansen, Harris, Lunter and Morris2010).
5For a description of the task see Breeze et al. (Reference Breeze, Kirkham and Mari-Beffa2011).
6For a description of the task see Kim et al. (Reference Kim, Oh, Hong and Choi2011).
Table 2 Prepulse Inhibition impairments in psychometrically defined schizotypal subjects in the general population

Con = Controls; F = Females; M = Males; PPI = Prepulse Inhibition; SCT = Schizotypy.
Executive functions’ deficits
Executive functions have long been synonymous with “frontal-lobe functions” but current definitions divide them into several interacting sub-processes (Miyake et al., Reference Miyake, Friedman, Emerson, Witzki, Howerter and Wager2000) mediated by a widely distributed brain network (Baddeley, Della Sala, Papagno, & Spinnler, Reference Baddeley, Della Sala, Papagno and Spinnler1997; Garavan, Ross, Li, & Stein, Reference Garavan, Ross, Li and Stein2000). Therefore, “executive control,” a broad term used to describe the neuropsychological processes incorporated in executive functions, refers to the interaction of a complex set of operations such as (a) initiation of behaviors and intentionality, (b) abstraction of patterns and concepts and giving meaning to stimuli based on prior experience, (c) prioritizing and assessing the emotional valence of stimuli, (d) holding information in working memory, (e) set shifting abilities and the ability to maintain set, (f) complex planning and problem solving, (g) response inhibition, and (h) strategy development, evaluation, and implementation (Frangou, Reference Frangou2010; Miller & Cummings, Reference Miller and Cummings2007).
Tasks that capture these different aspects of executive control have been developed, most of them being complex “multi-factorial” tasks (Stuss & Alexander, Reference Stuss and Alexander2000, page 290) making it difficult to delineate these interweaving processes. The most widely used executive function tasks include the Wisconsin Card Sorting Test (WCST), the Stroop Color-Word test, the Controlled Oral Word Association test (COWAT), and the Trail Making test (TMT) (Strauss, Sherman, & Spreen, Reference Strauss, Sherman and Spreen2006). Adequate performance in these tasks requires the activation of cognitive processes other than executive functions [e.g., COWAT performance also relies on the integrity of information retrieval and recall (Henry & Crawford, Reference Henry and Crawford2005)]; however, they are still considered as executive function tasks, as successful performance requires efficient executive control (Palmer & Heaton, Reference Palmer and Heaton2000). Meta-analytic studies in schizophrenia patients and their unaffected relatives indicate impaired performance in these tasks (Fioravanti et al., Reference Fioravanti, Carlone, Vitale, Cinti and Clare2005; Heinrichs & Zakzanis, Reference Heinrichs and Zakzanis1998; Henry & Crawford, Reference Henry and Crawford2005; Heydebrand, Reference Heydebrand2006; Stefanopoulou et al., Reference Stefanopoulou, Manoharan, Landau, Geddes, Goodwin and Frangou2009).
In studies examining schizotypy in the general population, high schizotypal subjects have been found to commit more total and perseverative errors, fail to maintain set and complete fewer categories (Gooding, Kwapil, & Tallent, Reference Gooding, Kwapil and Tallent1999; Kim, Oh, Hong, & Choi, Reference Kim, Oh, Hong and Choi2011; Lenzenweger & Korfine, Reference Lenzenweger and Korfine1994; Park, Holzman, & Lenzenweger, Reference Park, Holzman and Lenzenweger1995; Suhr, Reference Suhr1997; Suhr & Spitznagel, Reference Suhr and Spitznagel2001; Tallent & Gooding, Reference Tallent and Gooding1999) in the WCST, with effect sizes ranging between medium (0.34) to high (0.86), although negative findings have also been reported (Hori et al., Reference Hori, Teraishi, Sasayama, Matsuo, Kawamoto, Kinoshita and Kunugi2012; Noguchi, Hori, & Kunugi, Reference Noguchi, Hori and Kunugi2008; Spitznagel & Suhr, Reference Spitznagel and Suhr2002). The latter studies, however, are characterized by important methodological considerations, such as differences in sample characteristics compared with studies finding deficits (Hori et al., Reference Hori, Teraishi, Sasayama, Matsuo, Kawamoto, Kinoshita and Kunugi2012; Noguchi et al., Reference Noguchi, Hori and Kunugi2008), sampling biases and Axis II personality disorders not being examined at screening (Noguchi et al., Reference Noguchi, Hori and Kunugi2008), limited number of participants and a lengthy assessment (Spitznagel & Suhr, Reference Spitznagel and Suhr2002) or lack of corrections for multiple testing (Hori et al., Reference Hori, Teraishi, Sasayama, Matsuo, Kawamoto, Kinoshita and Kunugi2012).
In studies using the COWAT, TMT, Stroop, Hayling, and Zoo Map tasks, no significant differences between low and high schizotypy groups were found (Kim et al., Reference Kim, Oh, Hong and Choi2011; Laws, Patel, & Tyson, Reference Laws, Patel and Tyson2008; Suhr & Spitznagel, Reference Suhr and Spitznagel2001; Spitznagel & Suhr, Reference Spitznagel and Suhr2002). However, when further categorizing schizotypy into positive and negative, according to Crow's two syndrome concept of schizophrenia (Crow, Reference Crow1985) [Type I: positive psychotic-like symptoms (e.g., magical ideation, ideas of reference, unusual perceptual experiences) generated by hyperdopaminergia in subcortical mesolimbic structures; Type II: negative symptoms (e.g., affective flattening, avolition, apathy, asociality) generated by hypodopaminergia in the prefrontal cortex], negative schizotypy was associated with poorer performance in the TMT (longer reaction times) and the Divergent Thinking task (fewer alternate uses) (Dinn, Harris, Aycicegi, Greene, & Andover, Reference Dinn, Harris, Aycicegi, Greene and Andover2002). Also, in a study using cluster analysis that yielded three groups of schizotypal subjects (high negative dimension, high positive dimension, high on both dimensions), the cluster high on negative schizotypy performed worse on the WCST (Suhr & Spitznagel, Reference Suhr and Spitznagel2001). The latter finding has been replicated and extended by Chang et al. (Reference Chang, Lee, Chang, Yang, Huang, Chen and Chang2011), who found that schizotypal subjects with high either negative or positive schizotypy completed fewer categories in the WCST compared with low negative or positive schizotypal individuals.
Attention deficits
Attentional impairments have long being recognized as one of the core characteristics of schizophrenia patients (Bleuler, Reference Bleuler1911; Kraepelin, Reference Kraepelin1913) and are also reported in their unaffected first-degree relatives (Hill et al., Reference Hill, Harris, Herbener, Pavuluri and Sweeney2008; Kéri & Janka, Reference Kéri and Janka2004). It has long been recognized that attention is a non-unitary construct. William James (Reference James1890) remarked that “Everyone knows what attention is. It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought. Focalization, concentration of consciousness are of its essence. It implies withdrawal from some things in order to deal effectively with others, and is a condition which has a real opposite in the confused, dazed, scatterbrained state.” Acknowledging that attention comprises several sub-processes, Coull (Reference Coull1998) in a comprehensive review based on insights from electrophysiology, functional neuroimaging, and psychopharmacology, categorized the different ways one can attend to stimuli as attentional orientation (direction of attention to a specific stimulus), selective (or focused) attention (attentional priority to a stimulus over another), divided attention (alternating attention between more than two different stimuli), and sustained attention (attending to a stimulus for a long time).
Le Pelley, Schmidt-Hansen, Harris, Lunter, and Morris (Reference Le Pelley, Schmidt-Hansen, Harris, Lunter and Morris2010) found deficient attentional allocation in high schizotypal subjects; sustained attention, as measured with Continuous Performance tasks, has also been consistently reported as deficient (Bergida & Lenzenweger, Reference Bergida and Lenzenweger2006; Chen, Hsiao, Hsiao, & Hwu, Reference Chen, Hsiao, Hsiao and Hwu1998; Chen, Hsiao, & Lin, Reference Chen, Hsiao and Lin1997; Gooding, Matts, & Rollmann, Reference Gooding, Matts and Rollmann2006; Lenzenweger, Cornblatt, & Putnick, Reference Lenzenweger, Cornblatt and Putnick1991). Selective attention impairments have been found (Breeze, Kirkham, & Mari-Beffa, Reference Breeze, Kirkham and Mari-Beffa2011), although in one study using a Stroop test variation, selective attention was found to be intact in high schizotypal subjects, who, however, presented with lower switching capacity (Cimino & Haywood, Reference Cimino and Haywood2008), bringing onto surface the interplay between attentional and executive control processes [e.g., resolving conflict among responses (Fan, McCandliss, Sommer, Raz, & Posner, Reference Fan, McCandliss, Sommer, Raz and Posner2002)].
Memory deficits
Memory is another example of a cognitive process that can be further divided into several sub-processes. Thus, there is declarative memory (including episodic and semantic memory, memory for events and facts, respectively) and non-declarative memory (including procedural memory, non-associative learning, and classical conditioning) (Squire & Zola, Reference Squire and Zola1996). Declarative memory impairments are consistently found in schizophrenia patients and their non-psychotic relatives (Aleman, Hijman, de Haan, & Kahn, Reference Aleman, Hijman, de Haan and Kahn1999; Cirillo & Seidman, Reference Cirillo and Seidman2003; Dickinson et al., Reference Dickinson, Ramsey and Gold2007; Fioravanti et al., Reference Fioravanti, Carlone, Vitale, Cinti and Clare2005; Heinrichs & Zakzanis, Reference Heinrichs and Zakzanis1998; Whyte, McIntosh, Johnstone, & Lawrie, Reference Whyte, McIntosh, Johnstone and Lawrie2005). Although non-declarative memory has not been extensively studied in schizophrenia, there is evidence suggesting that patients present with either mild (Altshuler et al., Reference Altshuler, Ventura, van Gorp, Green, Theberge and Mintz2004) or no significant deficits (Goldberg, Saint-Cyr, & Weinberger, Reference Goldberg, Saint-Cyr and Weinberger1990; Schérer, Stip, Paquet, & Bédard, Reference Schérer, Stip, Paquet and Bédard2003) in procedural learning tasks. In healthy individuals, schizotypal traits are not consistently associated with either non-declarative (Ferraro & Okerlund, Reference Ferraro and Okerlund1995) or declarative memory (Kim et al., Reference Kim, Oh, Hong and Choi2011; LaPorte, Kirkpatrick, & Thaker, Reference LaPorte, Kirkpatrick and Thaker1994; Lenzenweger & Gold, Reference Lenzenweger and Gold2000).
Another categorization refers to the temporal characteristics of memory and distinguishes between short-term (or working) memory and long-term memory; working memory, also a component of executive function (Miller & Cummings, Reference Miller and Cummings2007), refers to temporary storage and manipulation of information while long-term memory refers to maintenance of information for longer time periods (Goldman-Rakic, Reference Goldman-Rakic1994). Working memory impairments have been extensively reported in schizophrenia patients and their relatives (Aleman et al., Reference Aleman, Hijman, de Haan and Kahn1999; Dickinson et al., Reference Dickinson, Ramsey and Gold2007; Giakoumaki, Roussos, Pallis, & Bitsios, Reference Giakoumaki, Roussos, Pallis and Bitsios2011; Hill et al., Reference Hill, Harris, Herbener, Pavuluri and Sweeney2008; Lee & Park, Reference Lee and Park2005), in accordance with the prefrontal cortex hypoactivation characteristic of the disorder (Andreasen et al., Reference Andreasen, O'Leary, Flaum, Nopoulos, Watkins, Boles Ponto and Hichwa1997; Carter et al., Reference Carter, Perlstein, Ganguli, Brar, Mintun and Cohen1998). Accordingly, studies in the general population indicate impaired working memory performance in individuals reporting high schizotypal traits (Kerns & Becker, Reference Kerns and Becker2008; Matheson & Langdon, Reference Matheson and Langdon2008; Park et al., Reference Park, Holzman and Lenzenweger1995; Park & McTigue, Reference Park and McTigue1997; Tallent & Gooding, Reference Tallent and Gooding1999).
Prepulse inhibition deficits
Several studies have reported that patients with schizophrenia (for review, see Braff et al., Reference Braff, Geyer and Swerdlow2001), even at first episode (Aggernaes et al., Reference Aggernaes, Glenthoj, Ebdrup, Rasmussen, Lublin and Oranje2010; Hammer, Oranje, Fagerlund, Bro, & Glenthøj, Reference Hammer, Oranje, Fagerlund, Bro and Glenthøj2011; Kumari, Fannon, Sumich, & Sharma, Reference Kumari, Fannon, Sumich and Sharma2007; Ludewig, Geyer, & Vollenweider, Reference Ludewig, Geyer and Vollenweider2003; Mackeprang, Kristiansen, & Glenthoj, Reference Mackeprang, Kristiansen and Glenthoj2002), have deficient PPI compared to controls; similarly, their unaffected relatives (for review see Thaker, Reference Thaker2008), and patients with SPD (Cadenhead, Geyer, & Braff, Reference Cadenhead, Geyer and Braff1993; Cadenhead, Swerdlow, Shafer, Diaz, & Braff, Reference Cadenhead, Swerdlow, Shafer, Diaz and Braff2000; Cadenhead et al., Reference Cadenhead, Light, Geyer, McDowell and Braff2002; Hazlett, Buchsbaum, Zhang et al., Reference Hazlett, Levine, Buchsbaum, Silverman, New, Sevin and Siever2003, Reference Hazlett, Romero, Haznedar, New, Goldstein, Newmark and Buchsbaum2007, Reference Hazlett, Buchsbaum, Zhang, Newmark, Glanton, Zelmanova and Siever2008) also present with deficient PPI. PPI impairments have also been reported in high schizotypal subjects in the general population (Simons & Giardina, Reference Simons and Giardina1992; Swerdlow, Filion, Geyer, & Braff, Reference Swerdlow, Filion, Geyer and Braff1995; Takahashi et al., Reference Takahashi, Iwase, Canuet, Yasuda, Ohi, Fukumoto and Takeda2010) accompanied by reduced activity in frontal, parietal and temporal brain regions (Kumari, Antonova, & Geyer, Reference Kumari, Antonova and Geyer2008). Smoking habits and attentional mechanisms have been suggested to modulate these effects, as significant negative associations between schizotypy and PPI were more notable in non-smoking subjects (Evans, Gray, & Snowden, Reference Evans, Gray and Snowden2005) and high-schizotypal subject failed to show the expected increase in PPI observed in instructed-PPI paradigms (Schell, Dawson, Hazlett, & Filion, Reference Schell, Dawson, Hazlett and Filion1995), when subjects are asked to attend to the stimuli.
Consistent with other research findings, negative results have also been reported (Abel, Jolley, Hemsley, & Geyer, Reference Abel, Jolley, Hemsley and Geyer2004; Blumenthal & Creps, Reference Blumenthal and Creps1994; Cadenhead, Kumar, & Braff, Reference Cadenhead, Kumar and Braff1996). The methodological limitations (e.g., small sample size, sample selection biases) of these studies are highlighted by the authors themselves making it more plausible that there is indeed an inverse relationship between schizotypal traits and PPI in the general population, as in schizophrenia and SPD.
Genetic effects
As mentioned earlier, schizotypy is usually used to refer to the vulnerability intrinsic in all disorders included in the schizophrenia spectrum (Meehl, Reference Meehl1989). According to this view, schizotypes are those with a schizophrenia predisposing genotype who lack the effects of other modulatory factors (e.g., environmental stress) associated with manifest psychosis (Cannon, van Erp, & Glahn, Reference Cannon, van Erp and Glahn2002). Twin studies have shown SPD to be genetically related to schizophrenia (Kendler, Gruenberg, & Strauss, Reference Kendler, Gruenberg and Strauss1981; Kendler et al., Reference Kendler, McGuire, Gruenberg, O'Hare, Spellman and Walsh1993) and genetic influences with heritability ranging from 29% to 67% (Linney et al., Reference Linney, Murray, Peters, MacDonald, Rijsdijk and Sham2003) have also been found in studies with twins from the general population. Genetic studies, however, indicate a polygenic mode of inheritance in schizophrenia (Karayiorgou & Gogos, Reference Karayiorgou and Gogos1997), making more plausible that only a subgroup of schizotypal subjects is genetically closer to schizophrenia patients (Faraone, Green, Seidman, & Tsuang, Reference Faraone, Green, Seidman and Tsuang2001). Even though this approach does not promise a comprehensive understanding of the disorder, early identification of the genetic architecture of this subgroup could have important early-intervention programme applications and therapeutic implications (Raine, Reference Raine2006).
To this end, the gene encoding catechol-O-methyltransferase (COMT), an enzyme regulating dopamine activity in the prefrontal cortex (Badner & Gershon, Reference Badner and Gershon2002) contains the val158met functional single nucleotide polymorphism (SNP), which explains part of the inter-individual variance in executive function and working memory task performance (Bertolino et al., Reference Bertolino, Caforio, Petruzzella, Latorre, Rubino, Dimalta and Scarabino2006; Egan et al., Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub and Weinberger2001) and predicts schizotypal personality characteristics (Avramopoulos et al., Reference Avramopoulos, Stefanis, Hantoumi, Smyrnis, Evdokimidis and Stefanis2002; Schürhoff et al., Reference Schürhoff, Szöke, Chevalier, Roy, Méary, Bellivier and Leboyer2007). In a study by Sheldrick et al. (Reference Sheldrick, Krug, Markov, Leube, Michel, Zerres and Kircher2008), the COMT val158met genotype was associated in an allele dose-response manner to performance in the TMT test and the disorganization factor of schizotypy; the met/met (high dopamine) carriers performed better in the test and scored higher in the disorganization schizotypy scale, replicating the findings of Ma et al. (Reference Ma, Sun, Yao, Wang, Hu, Deng and Li2007). However, an earlier study (Smyrnis et al., Reference Smyrnis, Avramopoulos, Evdokimidis, Stefanis, Tsekou and Stefanis2007) had found an opposite association, with the val/val (low dopamine) carriers scoring higher in disorganization and negative schizotypy dimensions, while no significant effects were found on cognitive performance.
Other candidate gene polymorphisms have also been examined, yielding conflicting results. Thus, the CGA carriers of the Proline Dehydrogenase (PRODH) gene haplotype that confers increased risk for schizophrenia (Li et al., Reference Li, Ma, Sham, Sun, Hu, Wang and Collier2004; Liu et al., Reference Liu, Heath, Sobin, Roos, Galke, Blundell and Karayiorgou2002) presented with impaired PPI, verbal memory performance, and increased schizotypy (Roussos, Giakoumaki, & Bitsios, Reference Roussos, Giakoumaki and Bitsios2009). In another study by the same group (Roussos, Giakoumaki, Georgakopoulos, Robakis, & Bitsios, Reference Roussos, Giakoumaki, Georgakopoulos, Robakis and Bitsios2011) assessing the effects of the rs1006737 CACNA1C genetic variant, which has also been implicated in schizophrenia (Green et al., Reference Green, Grozeva, Jones, Jones, Kirov, Caesar and Craddock2010), the risk allele was associated with increased schizotypal traits but no significant effects in cognitive task performance or PPI were found. Stefanis et al. (Reference Stefanis, Trikalinos, Avramopoulos, Smyrnis, Evdokimidis, Ntzani and Stefanis2007) found that none of four candidate genes (Neuregulin1 – NRG1; Dysbindin – DTNBP1; D-amino-acid oxidase activator – DAOA/G32 and D-amino-acid oxidase – DAAO) in schizophrenia pathology strongly modulated population variability in schizotypal features and cognitive functions. In a later study by the same group (Stefanis et al., Reference Stefanis, Trikalinos, Avramopoulos, Smyrnis, Evdokimidis, Ntzani and Stefanis2008), the common T allele of the SNP rs951436 of the regulator of the G-protein signaling 4 (RGS4) gene was associated with negative schizotypy while there were no significant associations with cognitive endophenotypes. Genetic variation in another risk gene associated with increased risk for schizophrenia, the Zinc Finger Protein 804A (ZNF804A) (Yasuda et al., Reference Yasuda, Hashimoto, Ohi, Fukumoto, Umeda-Yano, Yamamori and Takeda2011) gene, has also been found to be associated with schizotypal features, while its effects on schizotypal features in relation to cognitive function have not yet been examined.
Summary and conclusions
Understanding the relationship between schizotypal traits and cognitive function in the general population is a rapidly evolving field of research as it can significantly add to our understanding of schizophrenia. Executive function impairments are observed in high schizotypal subjects, in accordance with the schizophrenia literature; the most consistent findings seem to be with the WCST, a classical test of cognitive flexibility. Sustained attention Continuous Performance Tasks also provide consistent evidence for impaired performance in schizotypal subjects in analogy to schizophrenia. Regarding memory, the well-reported working memory deficits in schizophrenia are also found in high schizotypal subjects. Similarly, the PPI impairments observed in high schizotypal subjects suggest deficient sensorimotor gating, evident at the very early stages of the schizophrenia spectrum.
Studies in individuals from the general population (not stratified for schizotypy) have shown an association between PPI levels and performance on tasks assessing working memory, executive function and sustained attention (Bitsios & Giakoumaki, Reference Bitsios and Giakoumaki2005; Bitsios, Giakoumaki, Theou, & Frangou, Reference Bitsios, Giakoumaki, Theou and Frangou2006; Csomor et al., Reference Csomor, Stadler, Feldon, Yee, Geyer and Vollenweider2008; Giakoumaki, Bitsios, & Frangou, Reference Giakoumaki, Bitsios and Frangou2006). PPI in humans is modulated by the prefrontal cortex (Hazlett, Buchsbaum, Zhang et al., Reference Hazlett, Buchsbaum, Zhang, Newmark, Glanton, Zelmanova and Siever2008; Kumari et al., Reference Kumari, Gray, Geyer, ffytche, Soni and Sharma2003, Reference Kumari, Antonova, Geyer, ffytche, Williams and Sharma2007), working memory, executive function, and attention are mediated by prefrontal activation (for reviews, see Chudasama, Reference Chudasama2011; Kane & Engle, Reference Kane and Engle2002; Wager & Smith, Reference Wager and Smith2003) and high psychometric schizotypy is characterized by disturbed prefrontal function (Ettinger et al., Reference Ettinger, Williams, Meisenzahl, Möller, Kumari and Koutsouleris2011; Hori et al., Reference Hori, Nagamine, Soshi, Okabe, Kim and Kunugi2008). While PPI is a pro-cognitive measure distinctly different from performance in tasks measuring neurocognition, these findings suggest either a causal relationship and/or overlapping brain circuitry, also involved in schizotypal processes.
The critical link between reduced PPI and cognitive deficits in high schizotypal subjects seems to be prefrontal dysfunction: in analogy to schizotypal traits, prefrontal dysfunction is lying on a dynamic continuum, the extreme position being occupied by schizophrenia, psychotic symptoms, pronounced PPI, and generalized cognitive deficits, followed by SPD, high schizotypy, and so on (Figure 1). This is supported by neuroimaging findings, indicating reduced gray matter volume in the prefrontal cortex of schizophrenia patients compared with controls, while SPD patients are intermediate (Hazlett, Buchsbaum, Haznedar et al., Reference Hazlett, Buchsbaum, Haznedar, Newmark, Goldstein, Zelmanova and Siever2008; Matsui et al., Reference Matsui, Suzuki, Zhou, Takahashi, Kawasaki, Yuuki and Kurachi2008); accordingly, schizophrenia patients have been found to perform more poorly in sustained attention (Chan et al., Reference Chan, Wang, Cheung, Cui, Deng, Yuan and Gong2009; Obiols et al., Reference Obiols, Clos, Corberó, García-Domingo, de Trinchería and Doménech1992), working memory (Cadenhead, Perry, Shafer, & Braff, Reference Cadenhead, Perry, Shafer and Braff1999), and executive function (Cadenhead et al., Reference Cadenhead, Perry, Shafer and Braff1999; Matsui et al., Reference Matsui, Sumiyoshi, Kato, Yoneyama and Kurachi2004) tasks and present with lower PPI (Hazlett et al., Reference Hazlett, Romero, Haznedar, New, Goldstein, Newmark and Buchsbaum2007) compared with high schizotypal individuals or SPD patients. As Kirrane and Siever (Reference Kirrane and Siever2000, page 62) suggested, “Better frontal ‘buffering’ (in SPD) may prevent the more severe cognitive and social deterioration associated with schizophrenia” and could also serve as a “protective” mechanism in high schizotypal individuals from the general population, preventing them from reaching the thresholds for a formal SPD diagnosis.
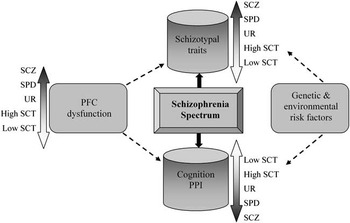
Fig. 1 Prefrontal dysfunction leads to schizotypal traits, cognitive and PPI deficits according to a continuum in the schizophrenia spectrum. Genetic and environmental risk effects act in concert and further modulate an individual's position in the continuum. PFC = Prefrontal; PPI = Prepulse Inhibition; SCT = Schizotypy; SCZ = Schizophrenia; SPD = Schizotypal Personality Disorder; UR = Unaffected Relatives.
Genetic studies assessing the effects of various candidate gene polymorphisms in schizotypy, cognitive function, and PPI are limited and their findings are inconsistent. The effects of single genetic variants seem to be attenuated when examined separately, while their additive effects are more likely to exert significant influences on phenotypic measures, further supporting the polygenic mode of inheritance also observed in schizophrenia. Although inconclusive, these findings are very promising, when considering the extension and future application they can have in schizophrenia spectrum research.
Longitudinal studies in the general population have shown that individuals who report high schizotypal traits have an increased risk of transition toward schizophrenia-spectrum disorders (Chapman et al., Reference Chapman, Chapman, Kwapil, Eckblad and Zinser1994; Dominguez, Wichers, Lieb, Wittchen, & van Os, Reference Dominguez, Wichers, Lieb, Wittchen and van Os2011; Gooding, Tallent, & Matts, Reference Gooding, Tallent and Matts2005; Poulton et al., Reference Poulton, Caspi, Moffitt, Cannon, Murray and Harrington2000; Welham et al., Reference Welham, Scott, Williams, Najman, Bor, O'Callaghan and McGrath2009). It is also well-established that as with schizophrenia, genetic and environmental influences act in concert and alter brain structure and function throughout development (Raine, Reference Raine2006) in schizotypy (Figure 1). Although the effects of environmental influences have been studied extensively in schizophrenia (for review, see Brown, Reference Brown2011), this is not the case with schizotypy. Thus, so far studies in high schizotypal individuals from the general population have focused on the effects of a limited number of environmental factors as risk indicators for the transition to psychosis, finding associations between high schizotypy and maternal exposure to influenza in pregnancy (Venables, Reference Venables1996), physical or sexual abuse during childhood (Steel, Marzillier, Fearon, & Ruddle, Reference Steel, Marzillier, Fearon and Ruddle2009; Startup, Reference Startup1999), migration (Linscott, Marie, Arnott, & Clarke, Reference Linscott, Marie, Arnott and Clarke2006), and season of birth (Cohen & Najolia, Reference Cohen and Najolia2011; Hori et al., Reference Hori, Teraishi, Sasayama, Matsuo, Kawamoto, Kinoshita and Kunugi2012). Therefore, based on the current findings we could hypothesize that the position one occupies in the schizophrenia spectrum is further modulated by the interaction of genetic and environmental risk factors.
Larger scale studies, including samples demographically and in general intellectual functioning closer to the patient samples reported in the literature, are needed to further elucidate similarities and/or differences in cognitive functions and sensorimotor gating between schizophrenia patients and high schizotypal subjects. Longitudinal studies should also examine the course of impairments in cognitive and sensorimotor gating functions as well as potential early and epigenetic effects and how they relate to the cognitive and PPI impairments and outcome of these individuals, as not all schizotypes convert into formally defined patients. This differential outcome pattern logically leads to another question. What kind of “advantage” protects a proportion of schizotypes from developing a psychotic disorder? Genetic, epigenetic, other personality factors, and most importantly their combined effects could serve as the underlying protective processes. Therefore, these protective factors are also very important to describe in future studies, as they can significantly aid not only the understanding of the etiopathogenesis of schizophrenia, but also the formulation and evaluation of intervention programs in schizophrenia-spectrum disorders.
Acknowledgments
The information in this manuscript and the manuscript itself has never been published either electronically or in print. There were no sources of financial support for the present work. The author has no conflict of interest to disclose.





