Introduction
Cerebellar brain tumors are the most common pediatric brain tumor (Gurney, Smith, and Bunin, Reference Gurney, Smith and Bunin1999; Ostrom et al., Reference Ostrom, de Blank, Kruchko, Petersen, Liao, Finlay and Barnholtz-Sloan2015). Tumors in the cerebellum account for 18–43% of brain tumors in children younger than 15 years old (Legler et al., 1999; Ostrom et al., Reference Ostrom, de Blank, Kruchko, Petersen, Liao, Finlay and Barnholtz-Sloan2015). Additionally, more than 75% of children survive 10 years post cerebellar tumor treatment (Ostrom et al., Reference Ostrom, de Blank, Kruchko, Petersen, Liao, Finlay and Barnholtz-Sloan2015). Approximately 40–43% of children treated for cerebellar tumors experience cerebellar atrophy 5 years post diagnosis (Dietrich et al., Reference Dietrich, Wanke, Mueller, Wieland, Moellers, Forsting and Stolke2001; Szathmari et al., Reference Szathmari, Thiesse, Galand-desmé, Mottolese, Bret, Jouanneau and Frappaz2010). Therefore, a better understanding of relationships among demographic and treatment factors, neuroanatomical underpinnings, and cognitive outcomes is important to improve long-term quality of life in this population.
The cerebellum is known for its role in supervised learning and cognitive efficiencies. Consequently, the cerebellum likely helps to automate learned processes and thus is indirectly related to the speed of processing (Koziol et al., Reference Koziol, Budding, Andreasen, D’Arrigo, Bulgheroni, Imamizu and Yamazaki2014). Investigating the impact of cerebellar atrophy and its associations with childhood cerebellar tumors is especially valuable. Spanos and colleagues (Reference Spanos, Wilde, Bigler, Cleavinger, Fearing, Levin and Hunter2007) inferred based on their volumetric results that the cerebellum and corresponding regions included in cerebellar loops, particularly the pons and the dorsolateral prefrontal cortex, may be vulnerable to a cascading impact of cerebellar atrophy following a moderate to severe traumatic brain injury. However, very little research examines cerebellar atrophy in brain tumor populations. The precise causes of cerebellar atrophy remain undetermined, but suggested etiologies include surgery, damage to the dentate, cranial radiation, seizures, or seizure medication (Poretti, Wolf, & Boltshauser, Reference Poretti, Wolf and Boltshauser2008).
While lesions and atrophy both fill with cerebrospinal fluid (CSF), they have different underlying etiologies and potential neurobehavioral correlates. In brain tumor survivors, a lesion is caused by the tumor growth and surgical resection. This type of lesion is considered a focal brain lesion. In contrast, acquired cerebellar atrophy corresponds with irreversible cellular death that results in enlarged cerebellar fissures (Poretti et al., Reference Poretti, Wolf and Boltshauser2008). Animal models of focal brain lesions suggest that the cells in the cerebellum are more likely to regenerate if there is a single lesion as opposed to repeated lesions or multifactorial trauma similar to atrophy-like diffuse damage (Rohkamm, Reference Rohkamm1977). Both cerebellar atrophy and cerebellar lesion size appear to be related to motor and cognitive difficulties (e.g., Schmahmann, Reference Schmahmann2004), but due to methodological limitations (e.g., Timmann et al., Reference Timmann, Brandauer, Hermsdörfer, Ilg, Konczak, Gerwig and Schoch2008) researchers have not been able to investigate the interaction between the lesion size and the amount of cerebellar atrophy in brain tumor populations.
Younger ages at diagnosis and radiation treatment are associated with reduced white matter volume and poorer neurocognitive functioning (Mulhern et al., Reference Mulhern, Reddick, Palmer, Glass, Elkin, Kun and Gajjar1999; Palmer et al., Reference Palmer, Gajjar, Reddick, Glass, Kun, Wu and Mulhern2003; Reddick et al., Reference Reddick, White, Glass, Wheeler, Thompson, Gajjar and Mullhern2003). Specifically, brain injury or disease commonly affects processing speed, and reductions in processing speed impact attention, working memory, intelligence, and academic achievement (Palmer, Reference Palmer2008). Furthermore, medulloblastoma tumors and radiation treatment at a young age are associated with processing speed declines relative to peers (<6 years; Palmer et al., Reference Palmer, Gajjar, Reddick, Glass, Kun, Wu and Mulhern2003, Reference Palmer, Armstrong, Onar-Thomas, Wu, Wallace, Bonner and Gajjar2013). Likewise, damage rating scales of atrophy that do not account for lesion size indicate that younger age (<5 years) at the time of radiation therapy is weakly associated with atrophy (Dietrich et al., Reference Dietrich, Wanke, Mueller, Wieland, Moellers, Forsting and Stolke2001). However, findings regarding the association between atrophy and cognitive performance are mixed; one study found that whole brain white matter volume and cortical thickness are not associated with executive functioning performance in survivors of childhood medulloblastoma on average 18 years post diagnosis (Brinkman et al., Reference Brinkman, Reddick, Luxton, Glass, Sabin, Srivastava and Krill2012), whereas another study reported that cerebellar atrophy relates to difficulty sustaining daily tasks (Szathmari et al., Reference Szathmari, Thiesse, Galand-desmé, Mottolese, Bret, Jouanneau and Frappaz2010). Mixed findings may be related to the measurement of atrophy, as Szathmari et al. (Reference Szathmari, Thiesse, Galand-desmé, Mottolese, Bret, Jouanneau and Frappaz2010) and Dietrich et al. (Reference Dietrich, Wanke, Mueller, Wieland, Moellers, Forsting and Stolke2001) measured atrophy using damage rating scales that did not account for lesion size, and Brinkman and colleagues (Reference Brinkman, Reddick, Luxton, Glass, Sabin, Srivastava and Krill2012) used volumetric measures that combine lesion and atrophy.
While prior literature has provided valuable information on brain tumor survivors, it is limited by a lack of differentiation between the effects of lesion versus atrophy. The primary aim of the current study was to address prior methodological limitations and develop and implement a measure of cerebellar atrophy in a cohort of survivors of childhood cerebellar tumors. Using the atrophy measure developed in this study, we investigated how cerebellar atrophy was related to radiation therapy and age at diagnosis in survivors of childhood cerebellar tumors. We hypothesized that an interaction between age at diagnosis and radiation therapy would be associated with cerebellar atrophy, such that survivors who were diagnosed and treated with radiation therapy at a younger age would have more atrophy. Furthermore, we hypothesized an interaction between cerebellar lesion size and cerebellar atrophy would be associated with written and oral processing speed, such that survivors with larger lesions and greater atrophy would have lower processing speed performance when compared to survivors with smaller lesions.
Methods
The study was reviewed and approved by the local Institutional Review Boards and informed consent was obtained from all participants. The participants were 25 healthy neurotypical controls recruited from a university community and 25 survivors of cerebellar brain tumors. Survivors and controls were matched with regard to gender, age, and level of education. Survivors of childhood brain tumors were recruited using mailings from three sources: (1) a previous longitudinal childhood brain tumor study, (2) a large hospital system database, and (3) an advertisement published in an annual newsletter circulated by the state Brain Tumor Foundation. Survivors were excluded if they had a diagnosis of neurofibromatosis or were <5 years from treatment. Information about survivors’ brain tumors and treatments were obtained from a retrospective medical records review. None of the survivors had history of progressions or recurrences of their brain tumor.
Thirteen survivors were diagnosed with medulloblastoma tumors, 10 astrocytomas, 1 ependymoma, and 1 pineoblastoma. Ethnicity in the sample was 80% Caucasian, 12% African American, 4% Hispanic, and 4% Asian. Treatment protocol numbers for radiation and chemotherapy included: CCG 9961 Arm A (n=3), POG 8695 (n=2), ACNS 0331 (n=1), ACNS 0332 (n=1), CCG 9961 and CHP 691 (n=1), CCG 9961 Reg B (n=1), POG 9331 Arm B (n=1), POG 9961 Arm A (n=1), and three participants did not have protocol numbers in their medical records (n=3). One survivor had a seizure disorder and was on medication, and one survivor had posterior fossa syndrome. All 25 tumor survivors had resections. Age at diagnosis and age at radiation treatment were highly collinear and not meaningfully different; therefore, age at diagnosis will be used in the analyses section to investigate how age at initial diagnosis and treatment impacts survivors who were treated both with and without radiation. Detailed treatment information is summarized in Table 1.
Table 1 Survivor and control demographic and descriptive comparisons

Note. Volumetric measures (e.g., whole brain gray matter) are reported in voxel units; Years of education are used because 100% of participants had a high school diploma.
* Indicates p<.05.
** Indicates trend at p<.10.
CSF=cerebrospinal fluid; ICV=intracranial vault; CB=cerebellar; inc=including.
Control participants were screened for past or current psychopathology with the Structured Clinical Interview for DSM-IV-TR Axis 1 (First, Spitzer, Gibbon, & Williams, Reference First, Spitzer, Gibbon and Williams2002). In addition, survivors and controls with a history of neurological or developmental conditions, for example, attention deficit hyperactivity disorder, learning disorders or brain injuries, were excluded.
Hollingshead Four-Factor Index of Social Status
Socioeconomic status was quantified using the Hollingshead Four-Factor Index of Social Status (SES; Hollingshead, 1957). For individuals who reported independent tax filing status, the participant occupation and education was used to calculate SES, whereas parents’ education and occupation was used for participants who reported dependency on their taxes. The Hollingshead (1957) estimates SES on a scale of 1–5 scale, where 5 represents the lowest SES bracket. SES was divided into two groups, high and low SES, based on the median SES (e.g., 1–2 high SES coded as 1 and 3–5 low SES coded as 0).
Symbol Digit Modality Test
Processing speed was measured using the Symbol Digit Modality Test (Smith, Reference Smith1982). In this 90-second speeded number-symbol task, participants were given a sheet of paper with a series of symbols, a blank box beneath each one. At the top of the page, there was a key where each symbol corresponds to a number. Participants were first asked to write the number that corresponds to the symbol (written processing speed). For oral processing speed, participants say the number that corresponds to the symbol in the box as fast as possible. The same form and the same order were used for all participants. The test–retest reliability was .76 for the Oral Symbol Digit Modality Test and .80 for the written portion (Smith, Reference Smith1982). Prior research suggests that performance on the Symbol Digit Modality Test was correlated to performance on the Digit Symbol subtest of the WAIS-R (Morgan & Wheelock, Reference Morgan and Wheelock1992).
Neurological Predictor Scale
The Neurological Predictor Scale (NPS; Micklewright, King, Morris, & Krawiecki, Reference Micklewright, King, Morris and Krawiecki2008; King & Na, Reference King and Na2015) is a cumulative measure that includes treatment complications such as hydrocephalus, hormone deficiency, seizures, amount of brain surgery, presence and type of radiation, and chemotherapy. The values range from 0 (no treatments or complications) to 9 (high degree of treatments and complications).
MRI Data Acquisition
All participants were scanned using a 3 Tesla Siemens Trio MRI scanner with a standard head coil for radiofrequency transmission. We acquired high-resolution (1.0 mm×1.0 mm×1.0 mm) T1-weighted structural images of the brain by collecting 176 contiguous sagittal slices. A three-dimensional (3D) magnetization prepared rapid gradient echo imaging (3D MPRAGE) sequence was used with the following parameters: field of view (FOV)=256 mm, acquisition matrix=256×256, slice thickness=1.0 mm, repetition time (TR)=2,250 ms, echo time (TE)=3.98 ms, and flip angle=9°.
Preprocessing Steps
Preprocessing steps were completed based on recommendations to prepare images for using the SUIT toolbox (Diedrichsen, Reference Diedrichsen2006; http://www.icn.ucl.ac.uk/motorcontrol/imaging/suit.htm). First, DICOM images were imported into dcm2nii and converted to SPM8 file format. Then, using SPM8, the origin of each image was set to the anterior commissure. All other functions (e.g., isolation, normalization, segmentation, reslice) were completed within the SUIT toolbox. Each cerebellar mask was visually inspected and corrected.
Lesion Size Measure
All images were converted into SUIT space to improve spatial resolution and normalization of the cerebellum. Using Matlab R2014a, all scripts were run using SPM8 with the SUIT toolbox, version 2.7 (Diedrichsen, Reference Diedrichsen2006). Lesions were identified based on the contrast observed on T1 weighted 3D MPRAGE images. The lesions were manually drawn on each slice of the T1-weighted image in the coronal view and checked in the axial and sagittal views using MRIcron software (Timmann et al., Reference Timmann, Brandauer, Hermsdörfer, Ilg, Konczak, Gerwig and Schoch2008; http://www.mricron.com) and saved as a region of interest for each survivor. Inspection bilaterally and across different views was used to ensure atrophy, healthy brain space (e.g., fourth ventricle or gyri), and large sulci were not included in lesion masks. All lesion masks were verified and corrected by an experienced neuroradiologist (L.W.), who was blind to participant risk status, radiation history, and neuropsychological test results. Next, a script was used to summarize the lesion size to each lobe of the cerebellum and calculate total lesion size. Methods were modeled based on prior studies (e.g., Kirschen et al., Reference Kirschen, Davis-Ratner, Milner, Chen, Schraedley-Desmond, Fisher and Desmond2008; Küper et al., Reference Küper, Döring, Spangenberg, Konczak, Gizewski, Schoch and Timmann2013; Ravizza et al., Reference Ravizza, McCormick, Schlerf, Justus, Ivry and Fiez2006) in which the lesion was overlaid on top of an atlas template to determine the percentage of lesion size.
Atrophy Measure
Volume of the intracranial vault was calculated using SPM8 with Ashburner and Friston’s (Reference Ashburner and Friston2005) unified segmentation program. Then a script (Ridgway, Reference Ridgway2007; http://www0.cs.ucl.ac.uk/staff/G.Ridgway/vbm/get_totals.m) was used to obtain the voxel counts for participants’ white matter, gray matter, and cerebrospinal fluid. The sum of the total gray matter, white matter, and cerebrospinal fluid volume was used to calculate the volume of the intracranial vault (Sanfilipo, Benedict, Zivadinov, & Bakshi, Reference Sanfilipo, Benedict, Zivadinov and Bakshi2007). This measure of intracranial vault has been previously used in populations who experience both lesions and atrophy such as multiple sclerosis (e.g., Chard et al., Reference Chard, Griffin, Parker, Kapoor, Thompson and Miller2002). To quantify atrophy, the following formula was developed and corresponds with the prior literature. Volume of the intracranial vault was included in this measure to correct for any possible premorbid individual brain-size differences.
Equations [1–3] were adapted from Sanfilipo and colleagues (Reference Sanfilipo, Benedict, Zivadinov and Bakshi2007).
Equation [2] was adapted to calculate whole brain specific absolute parenchymal fraction using their original equation: (total gray + total white matter volume). For the cerebellar volume, the same script was used with the corrected cerebellar mask for the white and gray matter images.
Equation [3] was based on the original equation that was used to calculate whole brain volume to obtain brain parenchymal fraction ([gray matter + white matter]/ICV). We adapted this equation to obtain a cerebellar specific brain parenchymal fraction for controls.
Equation [4] was adapted from Chard and colleagues’ (Reference Chard, Griffin, Parker, Kapoor, Thompson and Miller2002) measure of atrophy with multiple sclerosis (MS) patients. The original equation ([total white matter + lesion volume (all lesions were in WM)]/ ICV) was adapted to fit the needs of our sample. Atrophy in this equation is an X factor that will be solved for in Equation [5].
Equation [5] was adapted from Chard and colleagues’ (Reference Chard, Griffin, Parker, Kapoor, Thompson and Miller2002) cross-sectional comparison between matched controls and MS patients. Their original equation (Group mean control total white matter]/ICV – [MS white matter + lesion volume]/ICV) was adapted for our sample and lesion size was added back into our survivors’ volume to make them comparable to controls.
Equation [6] was adapted from Chard and colleagues (Reference Chard, Griffin, Parker, Kapoor, Thompson and Miller2002) who reported the percent decline relative to healthy comparison group using their cross-sectional comparison between matched controls and MS patients. The denominator refers to the group average of healthy controls’ parenchymal fraction.
Analyses
Descriptive analyses were used to characterize the distribution of SES and brain damage in the sample. To characterize the distribution of brain damage, we investigated the percentage of damage in the whole cerebellum, the dentate, the vermis, and the frequency of lesion laterality. Then, we used t-tests to investigate whether survivors differed from controls with regard to brain volumetric measures (e.g., whole brain gray matter volume). Next, we looked at t-tests among survivors treated with radiation therapy, survivors treated without radiation therapy, and controls to determine if radiation therapy was the primary factor explaining differences between survivors and controls. Cohen’s D is reported because it is less sensitive to bias due to multiple comparisons.
To investigate the first hypothesis, we first computed Pearson correlations between age at diagnosis and cerebellar atrophy in the radiation group and no radiation group, respectively. Next we computed Pearson and point-biserial correlations to determine if there were any disease and treatment confounds or covariates. The first hypothesis was tested using a simultaneous entry multiple regression that includes age at diagnosis, radiation therapy, an interaction between age at diagnosis and radiation therapy, and any covariates as predictor variables of cerebellar atrophy. The second hypothesis was tested using two additional simultaneous entry multiple regressions that includes cerebellar lesion size, cerebellar atrophy, an interaction between cerebellar lesion size and cerebellar atrophy and any covariates as predictor variables of oral and written processing speed, respectively.
Results
In general, the survivor group reported a higher SES when compared to the controls. Chi square analyses indicate that 75% of controls reported low SES, whereas only 26% of survivors reported a low SES, χ2(1)=11.25, p<.01. With regard to brain damage, 80% of survivors of pediatric brain tumors had some degree of diffuse cerebellar damage; for instance, participants experienced on average 15% cerebellar atrophy (SD=16; range=0–42). The highest percentage of cerebellar lesion occurred in the dentate and the vermis, and participants evidenced 14% lesion damage to the cerebellum (range: 2–28%). On average, 10% of the right dentate (range: 0–100%), 4% of the left dentate (range: 0–30%), 7% of the total dentate (range: 0–50%), and 29% of the total vermis (range: 0–72%) was lesioned. Given the high degree of diffuse damage and lesion overlap, it was not possible to examine specificity of location of cerebellar damage.
For regional subdivisions of lesions and atrophy, see Appendix 1. In general, the highest percentage of lesion occurred in the vermis, and the cerebellar hemispheres had relatively smaller lesions. With regard to lesion laterality, 16 participants had midline lesions, 5 had left lateralized lesions, and 4 had right lateralized lesions.
The group comparison indicated that survivors differed from controls with regard to amount of CSF, cerebellar white matter, cerebellar gray matter, total cerebellar volume, whole brain gray matter, and the proportion of the cerebellum relative to the intracranial vault (ICV) as shown in Table 1. The size of tumor resection (i.e., lesion size) was matched between low and high grade tumor groups (see Table 2 for additional subgroup comparisons based on tumor grade).
Table 2 Subgroup descriptive statistics and effect sizes

Note. Volumetric measures (e.g., whole brain gray matter) are reported in voxel units. Cohen’s d: small=0.2–0.3, medium=0.5a, large≥0.8b.
* Indicates significant difference.
CSF=cerebrospinal fluid; ICV=intracranial vault; CB=cerebellar; inc=including.
In the current sample, it is impossible to differentiate treatment related to the degree of tumor malignancy because no low grade tumors were treated with radiation, and only one high grade tumor was not treated with radiation; therefore, the effect of radiation therapy may be confounded with tumor grade. However, for participants who received radiation treatment, age at diagnosis was correlated with a greater percentage of cerebellar atrophy (n=14; r=−.64; p=.01). In contrast, age at diagnosis was not related to the percentage of atrophy for individuals who were not treated with radiation (n=11; r=−.06; p=.86).
As reported in Table 3, Pearson and point-biserial correlations revealed that some disease and treatment factors, such as radiation, hormone disorder, age at diagnosis, correlated with the percentage of atrophy in the cerebellum. Due to small sample size, we looked at both alpha values and large r values (≥.3) to determine whether variables were related. Atrophy and lesion size were not significantly correlated and displayed a small r value (r=−.26; p=.21). Several treatment variables were correlated with processing speed (see Table 4); therefore, a cumulative measure of total treatments and sequelae that includes radiation, hydrocephalus, seizure disorder, and hormone deficiency (i.e., NPS) was used as a covariate in the analyses for investigating processing speed.
Table 3 Correlation coefficients for potential confound analyses
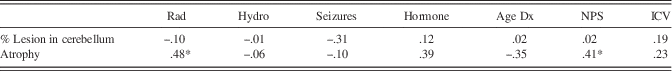
Note. N=25.
* Indicates p<.05.
Rad=radiation therapy; Hydro=hydrocephalus; Dx=diagnosis; NPS=Neurological Predictor Scale; CB=cerebellum; ICV=intracranial vault; Hormone=presence of hormone deficiency; Seizure=prescribed seizure medication.
Table 4 Correlation coefficients for potential covariate analyses
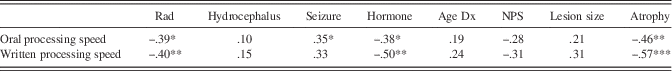
Note. N=25.
* Indicates trend <.10.
** Indicates p<.05.
*** Indicates p<.01.
Rad=radiation; Dx=diagnosis; NPS=Neurological Predictor Scale; Hormone=presence of hormone deficiency; Seizure=prescribed seizure medication.
To test the hypothesis that age at diagnosis would change the relationship between radiation therapy and the percentage of cerebellar atrophy, such that survivors who are diagnosed and treated with radiation therapy at a younger age will have a higher percentage of atrophy, an interaction between age at diagnosis and radiation therapy predicting the percentage of atrophy was investigated. A three predictor regression model, which included the simultaneous entry of age at diagnosis, presence of radiation, and an interaction term (age at diagnosis*radiation) as predictors (Figure 1), accounted for 44% of the variance in the percentage of cerebellar atrophy, Adj R 2 =.44, F(3,21)=7.30, p <.01. A main effect of age at diagnosis was not significant. Presence of radiation was associated with a higher percentage of cerebellar atrophy, B=11.99, SE=3.52, p<.01. Radiation uniquely explained 27% of the variance in the percentage of cerebellar atrophy (Table 5). An interaction effect between age at diagnosis and radiation was present in the sample, B=−1.51, SE=0.72, p=.049, and accounted for 10% of the variance in the percentage of atrophy. The interaction term indicated that, for individuals treated with radiation, younger age at diagnosis was associated with a higher percentage of cerebellar atrophy. When NPS was added to the model, it was not a significant predictor of the percentage of cerebellar atrophy (B=−.92, SE=1.67, p=.59); therefore, it was removed for parsimony.

Fig. 1 Radiation versus No Radiation treatment groups: interaction between age at diagnosis and cerebellar atrophy. Note. Visual depiction of the interaction between age at diagnosis and radiation as it relates to cerebellar atrophy. For the radiation group, younger age at diagnosis was associated with higher degrees of atrophy; however, in the no radiation group age at diagnosis was not associated with atrophy.
Table 5 Regression coefficients for age at diagnosis and radiation predicting cerebellar atrophy
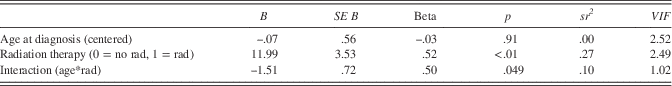
Note. This model accounted for 44% of the variance in percentage of cerebellar atrophy, Adj R 2 =.44, F(3,21)=7.30, p<.01.
To test the second hypothesis that an interaction between the percentage of cerebellar lesion size and cerebellar atrophy will predict written and oral processing speed, we used a four predictor regression model (i.e., NPS, atrophy, lesion, the interaction term of lesion and atrophy), which accounted for 33% of the variance in oral processing speed, Adj R 2 =.33, F(4,20)=3.95, p=.02 (Table 6). An interaction effect between the atrophy and the lesion size indicated that the effect of the percentage of atrophy was different based on the percentage of cerebellar lesion size, B=.01, SE=0.00, p=.01. This interaction accounted for 21% of the variance, and the interaction term indicated the slope of the regression line between oral processing speed and atrophy changed based on the lesion size (see Figure 2).

Fig. 2 Interaction between total cerebellar lesion size and atrophy predicts oral processing speed performance. Note. Continuous interaction is probed at three levels (small, medium, and large lesion).
Table 6 Regression coefficients for total lesion size and atrophy predicting oral processing speed performance

Note. This model accounted for 33% of the variance in oral processing speed, Adj R 2 =.33, F(4,20)=3.95, p=.02.
Specifically, when estimating oral processing speed, individuals with a smaller lesions were more affected by a greater percentage of cerebellar atrophy, whereas individuals with larger lesions were less affected by the percentage of atrophy. The results were not supportive of the hypothesis that large lesions would be more affected by a larger percentage of atrophy; rather, our analyses suggested that individuals with smaller lesions had oral processing speed that was more negatively affected by a larger percentage of cerebellar atrophy.
A model with the same predictors was used to test written processing speed, and these predictors accounted for 48% of the variance in written processing speed, Adj R 2 =.48, F(4,20)=6.49, p<.01 (Table 7). Percentage of cerebellar lesion size was positively associated with written processing speed at an average level of the percentage of cerebellar atrophy after including NPS in the regression equation, B=.08, SE=0.03, p=.03, and uniquely explained 12% of the variance. An interaction effect between the percentage of atrophy and the lesion size was present in the sample, B=.01, SE=0.00, p=.01, and accounted for 20% of the variance in written processing speed. Similar to the previous model, the interaction term indicated that the slope of the regression line between the written processing speed and the percentage of atrophy changed based on lesion size, where individuals with a smaller lesion sizes were more affected by a greater percentage of cerebellar atrophy (see Figure 3).

Fig. 3 Interaction between cerebellar lesion size and atrophy predicts written processing speed performance. Note. Continuous interaction is probed at three levels (small, medium, and large lesion).
Table 7 Regression coefficients for total lesion size and atrophy predicting written processing speed performance
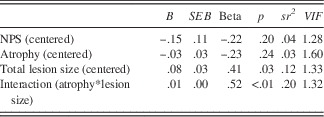
Note. This model accounted for 48% of the variance in oral processing speed, Adj R 2 =.48, F(4,20)=6.49, p<.01.
Post hoc analyses investigated group differences in overall cerebellar gray and white matter in relation to processing speeds to make comparisons with previous studies. We found that in controls cerebellar white matter and gray matter were not correlated to oral or written processing speed (white matter: r=.23; p=.26 and r=.23; p=.26; gray matter: r=−.01; p=.98 and r=−.06; p=.78). In contrast, cerebellar white matter was correlated to both oral and written processing speed in survivors (r=.40; p=.05; and r=.53; p<.01), and cerebellar gray matter was correlated to both oral and written processing speed in survivors (r=.53; p<.01 and r=.63; p<.01).
Discussion
The results of the current study provide evidence that radiation therapy and age at diagnosis are contributing factors of cerebellar atrophy, such that adult survivors of pediatric cerebellar tumors who were diagnosed at a young age and treated with radiation therapy displayed the highest amount of cerebellar atrophy (see Figure 4), consistent with previous research (Spanos et al., Reference Spanos, Wilde, Bigler, Cleavinger, Fearing, Levin and Hunter2007; Szathmari et al., Reference Szathmari, Thiesse, Galand-desmé, Mottolese, Bret, Jouanneau and Frappaz2010). Survivors who were not treated with radiation therapy did not display a correlation between age at diagnosis and cerebellar atrophy. The analyses suggested that 44% of the statistical variance in cerebellar atrophy was related to age at diagnosis and radiation therapy.

Fig. 4 Proposed model based on the relationships among lesion size, age at diagnosis, radiation treatment, atrophy, and processing speed. Note. Gray arrows indicate significant relationships among independent, moderating, and dependent variable.
Furthermore, greater cerebellar atrophy was associated with poorer oral and written processing speed for individuals with smaller lesion sizes. Together, the regression equation that included NPS, lesion size, atrophy, and the interaction term (age at diagnosis*radiation) explained a large portion of variance in processing speed (33% of oral processing speed and 48% of written processing speed). Importantly, radiation therapy could not be differentiated from tumor grade in the sample. Therefore, it is possible that factors related to higher tumor grade also contributed to these results.
The results of the current study are consistent with literature on brain tumor survivors who underwent radiation, which suggests that young age at cranial radiation results in lower white matter volume, increased atrophy (Dietrich et al., Reference Dietrich, Wanke, Mueller, Wieland, Moellers, Forsting and Stolke2001), reduced white matter integrity (King, Wang, & Mao, Reference King, Wang and Mao2015), and slower processing speed (Palmer et al., Reference Palmer, Armstrong, Onar-Thomas, Wu, Wallace, Bonner and Gajjar2013). These results suggest that radiation therapy has significant negative ramifications for the developing brain and processing speed and should, therefore, continue to be delayed for young children when medically appropriate and when it does not increase the risk of death or impairment. While results were interpreted as related to radiation therapy, it is important to highlight that radiation therapy was confounded with higher tumor grade and additional treatments in this sample. Therefore, future research should work to differentiate the effect of treatment and tumor grade in relation to cerebellar atrophy in brain tumor populations.
Inconsistent with the hypotheses based on Rohkamm’s (Reference Rohkamm1977) animal study, there was an unexpected finding that survivors with larger lesion sizes had processing speed that was less affected by cerebellar atrophy. This is likely related to the diffuse nature of cerebellar injury in the current population. It appears that smaller lesion sizes have more remaining cerebellar volume to be affected by atrophy, and atrophy may be more closely related to processing speed when the lesion size is small. It is possible that lesion location, rather than lesion size was an important factor, as has been reported in prior research (Konczak et al., 2005; Küper et al., Reference Küper, Döring, Spangenberg, Konczak, Gizewski, Schoch and Timmann2013). Therefore, future studies with more focal lesion populations also should investigate if the specific location (e.g., the dentate nucleus; Perreault et al., Reference Perreault, Lober, Cheshier, Partap, Edwards and Yeom2014), within the cerebellum explains greater variance in processing speed when compared to lesion size.
Research on the human brain suggests that subcortical structures are uniquely vulnerable to radiation treatment and that compromise of these structures corresponds with poorer behavioral outcomes. In a prior study on this sample, significant volumetric differences were found in radiation versus no radiation groups for the subcortical structures such as the hippocampus and putamen, and lower hippocampal volume was specifically related to poorer attention performance in adult survivors of childhood brain tumors (Jayakar, King, Morris, & Na, Reference Jayakar, King, Morris and Na2015). Palmer (Reference Palmer2008) proposed that processing speed may be upstream in a developmental cascade in which processing speed affects attention. Thus, it is likely that the processing speed findings reported in this study are related to, perhaps even cause, the attention difficulties that have been observed in prior studies on this sample.
Although our study highlighted important findings and implications, the results are based on brain tumor survivors who were diagnosed and treated 15 years before participating in this study. Therefore, caution must be used when generalizing neuroimaging and neurocognitive findings to more recent brain tumor cohorts, as treatment approaches have changed over time.
While the current study had a large number of participants for this patient population, it was limited by a relatively small sample size that restricted the amount of variables that could be statistically modeled. An inevitable limitation of using neuroimaging data, particularly with this population, was exclusion based upon poor quality imaging data (n=4) or inability to obtain an MRI scan due to medical implant (n=4). Small sample size resulted in underpowered models (Power=.51–.69), therefore, results that did not reach significance should be interpreted with caution. Although it would be desirable to replicate these findings with a larger sample, it is challenging to follow brain tumor survivors this long post diagnosis due to difficulty tracking patients over time and across transition to adulthood.
Despite these limitations, the findings regarding atrophy are noteworthy and heretofore understudied. Animal research provides some evidence that the process of atrophy may be different from that of focal brain lesions (Rohkamm, Reference Rohkamm1977), which would be consistent with the current findings. Atrophy secondary to cerebellar brain tumors is unique relative to other populations with brain atrophy because it is more difficult to differentiate due to presence of tumor resection. CSF does not differentiate between brain lesion and brain atrophy; therefore, methodological constraints have limited investigations of lesion and atrophy together (Dietrich et al., Reference Dietrich, Wanke, Mueller, Wieland, Moellers, Forsting and Stolke2001; Schmahmann, Reference Schmahmann2004; Szathmari et al., Reference Szathmari, Thiesse, Galand-desmé, Mottolese, Bret, Jouanneau and Frappaz2010; Timmann et al., Reference Timmann, Brandauer, Hermsdörfer, Ilg, Konczak, Gerwig and Schoch2008). As a result, this study is among the first to provide empirical information regarding the relationships among demographic and treatment correlates of cerebellar atrophy and how atrophy and lesion relate to behavioral performance.
Existing literature on traumatic brain injury and animal studies suggest that cerebellar atrophy occurs even when there are no direct injuries to the cerebellum (Finnie et al., Reference Finnie, Van den Heuvel, Gebski, Manavis, Summersides and Blumbergs2001; Spanos et al., Reference Spanos, Wilde, Bigler, Cleavinger, Fearing, Levin and Hunter2007). To date, we are not aware of any studies that have investigated cerebellar atrophy as a result of brain tumors in regions other than the cerebellum. Given that cerebellar brain tumor survivors showed a unique vulnerability to cerebellar atrophy in the current study, it will be important for future studies to investigate whether tumors in other locations similarly result in cerebellar atrophy. This research will be important to differentiate how cerebellar atrophy correlates with general neurological insult (e.g., surgical resection), brain tumor treatment, and tumor location.
As many as 43% of individuals with childhood cerebellar medulloblastoma experience atrophy five years post diagnosis (Szathmari et al., Reference Szathmari, Thiesse, Galand-desmé, Mottolese, Bret, Jouanneau and Frappaz2010). Although medulloblastoma survivors tend to experience the most significant reductions in brain volume and corresponding neurobehavioral deficits (Mulhern et al., Reference Mulhern, Reddick, Palmer, Glass, Elkin, Kun and Gajjar1999), results from this study suggest that survivors with a diverse group of tumor pathologies experience cerebellar atrophy. Therefore, an important future direction will be to explore possible etiologies of cerebellar atrophy in an effort to understand and prevent atrophy secondary to brain tumor diagnosis and treatment when possible. Researchers suggest that cerebellar structure is sensitive and may be at high risk for atrophy (e.g., Finnie et al., Reference Finnie, Van den Heuvel, Gebski, Manavis, Summersides and Blumbergs2001; Rohkamm, Reference Rohkamm1977; Spanos et al., Reference Spanos, Wilde, Bigler, Cleavinger, Fearing, Levin and Hunter2007). Others propose that damage to the dentate and vermis may result in disconnection and may be related to atrophy in other brain regions (Spanos et al., Reference Spanos, Wilde, Bigler, Cleavinger, Fearing, Levin and Hunter2007). These and other theories should be explored to best identify and prevent cerebellar atrophy and its deleterious consequences.
Acknowledgments
We thank the individuals and families who participated in this study and generously contributed their time and effort. Also, we thank the Developmental Neuropsychology Research Team for helping with data acquisition and management, the staff at the Georgia State University / Georgia Institute of Technology Joint Center for Advanced Brain Imaging, and the Brain Tumor Foundation for helping share information about this clinical research opportunity with long-term brain tumor survivors. This research was supported by a Research Scholar Grant from the American Cancer Society to T.Z.K. (#RSGPB-CPPB-114044). A. Ailion is supported by the Georgia State University Language Literacy Initiative and M. Fox is supported by the Georgia State University Brains and Behavior Initiative. No conflicts of interest exist.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1355617716000138













