INTRODUCTION
According to the World Health Organization (WHO), cannabis is among the most widely cultivated, trafficked, and consumed psychoactive substance, with approximately 2.5% of the world’s population reporting past-year cannabis use (World Health Organization, 2016). Even higher prevalence rates of cannabis use are reported in North America, as the rates of cannabis use in Canada and the United States were approximately 14% and 16%, respectively, as reported in 2017 (United Nations Office of Drug Control, 2017). Given the legalization of recreational cannabis use in Canada, and across several states in the United States, there continues to be an increasing interest in the risks associated with cannabis use, including the impact of cannabis on cognitive functioning.
A substantial body of literature has attempted to clarify how various cannabis use parameters, such as Δ9-tetrahydrocannabinol (THC), frequency of use, severity of cannabis involvement, and age of initiation of cannabis use impact cognitive performance. For instance, systematic reviews point toward an association between higher frequency of use and poorer performance on a measure of working memory (Broyd, Van Hell, Beale, Yücel, & Solowij, Reference Broyd, Van Hell, Beale, Yücel and Solowij2016). When Thames etal. (Reference Thames, Arbid and Sayegh2014) examined frequency of use by categorizing participants as recent users (n = 68), past users (i.e., more than 28 days; n = 41), and non-users (n = 49), recent users performed worse compared to the non-users and past users on measures of global neurocognitive performance, attention, learning/memory, information processed speed, and executive functioning. Further, the number of times cannabis was used in the last four weeks among recent users was negatively correlated with performance level on the aforementioned neurocognitive domains. Similarly, adolescents who were regular cannabis users (used more than once per week) had poorer performance than less frequent users across measures of attention, learning, and spatial working memory (Harvey, Sellman, Porter, & Frampton, Reference Harvey, Sellman, Porter and Frampton2009), suggesting that effects on cognition extend beyond acute use. Although most studies have been cross-sectional, a growing number of longitudinal studies are informing the question of whether cannabis impacts cognition. Two quasi-experimental longitudinal co-twin studies of young adults recently concluded there was minimal impact of cannabis on cognition (Meier etal., Reference Meier, Caspi, Danese, Fisher, Houts, Arseneault and Moffitt2018; Ross etal., Reference Ross, Ellingson, Rhee, Hewitt, Corley, Lessem and Friedman2020), but a recent meta-analysis found that frequent use or cannabis use disorder was indeed associated with global intelligence declines (Power etal., Reference Power, Sabherwal, Healy, O’ Neill, Cotter and Cannon2021).
The association between cannabis use and symptoms of attention deficit hyperactivity disorder (ADHD) has also been of recent interest. For example, ADHD and lifetime cannabis use have been shown to have shared genetic contributions, and a causal pathways between ADHD and later onset of cannabis use has been identified (Artigas etal., Reference Artigas, Sanchez-Mora, Rovira, Richarte, Garcia-Martinez, Pagerols and Ribases2020). Recent findings from a large sample of community adults suggested associations between ADHD symptoms and cannabis use (Petker etal., Reference Petker, DeJesus, Lee, Gillard, Owens, Balodis and Mackillop2020). This finding is consistent with other studies pointing toward a relation between cannabis use severity and ADHD symptomatology (Loflin, Earleywine, De Leo, & Hobkirk, Reference Loflin, Earleywine, De Leo and Hobkirk2014; Notzon etal., Reference Notzon, Pavlicova, Glass, Mariani, Mahony, Brooks and Levin2016; Van de Glind etal., Reference Van de Glind, Van Emmerik-van Oortmerssen, Carpentier, Levin, Koeter, Barta and van den Brink2013). Poorer inhibitory functioning is a core feature of ADHD and, during early adolescence, has been found to predict greater substance use (including cannabis) at age 18 (Squeglia & Gray, Reference Squeglia and Gray2016). In addition to assessing the relationship between cannabis use and clinical syndromes associated with inhibitory deficits, such as ADHD, research has also explored how cannabis use relates to impulsivity on other behavioral tasks, such as delay discounting. For instance, consistent with a recent meta-analysis (Amlung, Vedelago, Acker, Balodis, & MacKillop, Reference Amlung, Vedelago, Acker, Balodis and MacKillop2017), Petker etal. (Reference Petker, DeJesus, Lee, Gillard, Owens, Balodis and Mackillop2020) found that the severity of cannabis-related problems was related to higher levels of impulsivity on a measure of delay discounting, in addition to symptom endorsement on a self-report measure of ADHD. Across these neurocognitive findings, however, considerable inconsistency is present (Broyd etal., Reference Broyd, Van Hell, Beale, Yücel and Solowij2016), which may be a function of relatively small sample sizes. This is unsurprising as neurocognitive testing is time and resource intensive, but small samples reduce statistical power and the ability to incorporate potential confounders, such as alcohol and tobacco use.
A further factor that may influence the relation between cannabis and cognition is age of initiation. Studies examining age of cannabis initiation as a parameter of interest have shown that earlier age of initiation is related to increased frequency and quantity of cannabis use (Gruber, Sagar, Dahlgren, Racine, & Lukas, Reference Gruber, Sagar, Dahlgren, Racine and Lukas2012) and has been linked to more severe cognitive impairments (Gruber etal., Reference Gruber, Sagar, Dahlgren, Racine and Lukas2012; Pope etal., Reference Pope, Gruber, Hudson, Cohane, Huestis and Yurgelun-Todd2003). However, other studies suggest that there is little or no impact of age of initiation on neurocognitive functioning (Ganzer, Bröning, Kraft, Sack, & Thomasius, Reference Ganzer, Bröning, Kraft, Sack and Thomasius2016; Petker etal., Reference Petker, DeJesus, Lee, Gillard, Owens, Balodis and Mackillop2020; Scott etal., Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018). Thus, whether age of first use acts as a moderator between current cannabis use and neurocognitive performance remains unclear.
Finally, a factor that is increasingly recognized to have a substantial influence is the residual effect of recent cannabis use. In a meta-analysis of studies on cannabis and cognition in adolescents and young adults, a significant negative association was present overall, but studies using an abstinence period of greater than 72 hr had a significantly smaller effect size that was not significantly different from zero (Scott etal., Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018). This suggests that cannabis-related cognitive effects dissipate after stopping cannabis use. Similarly, recent studies using urinary THC as a marker of recent use have revealed significant negative associations with cognition. In a sample of more than 1100 participants, Petker etal. (Reference Petker, Owens, Amlung, Oshri, Sweet and MacKillop2019) found that THC+ status, but not overall cannabis use, cannabis use disorder, or age of initiation, was selectively associated with worse performance in episodic memory and processing speed. Similarly, in a functional magnetic resonance imaging study, Owens etal. (Reference Owens, McNally, Petker, Amlung, Balodis, Sweet and Mackillop2019) found that THC+ status was associated with poorer working memory performance that was mediated by deficits in task-positive activity and excess task-negative activity. Thus, recent use (such that THC is still detectable in urine) appears to be highly relevant to whether cannabis involvement is associated with poorer cognitive functioning.
The current study seeks to examine the associations between cannabis use and neurocognitive performance in a relatively large sample of emerging adults (ages 21–25), who were initially recruited for a study on high-risk drinking. Concurrent cannabis use and high-risk drinking is common in this age group (Pape, Rossow, & Storvoll, Reference Pape, Rossow and Storvoll2009; Terry-McElrath, O’Malley, & Johnston, Reference Terry-McElrath, O’Malley and Johnston2014; Terry-McElrath etal., Reference Terry-McElrath, O’Malley, Johnston, Bray, Patrick and Schulenberg2017) and confers greater risk than either individually (Brière, Fallu, Descheneaux, & Janosz, Reference Brière, Fallu, Descheneaux and Janosz2011; Subbaraman & Kerr, Reference Subbaraman and Kerr2015). Those with comorbid use may be an especially vulnerable group given that alcohol and cannabis use have both been shown to have effects on neuropsychological test performance, brain function, and brain structure in adolescents (Squeglia & Gray, Reference Squeglia and Gray2016). Using a cross-sectional design, the study first examined complementary aspects of cannabis use by examining a categorical current cannabis use variable: frequency of use (i.e., daily use, occasional use, and none), followed by examining a more continuous current use variable (severity of cannabis-related problems). The study then examined whether age of cannabis initiation positively moderated the relation between current use variables (frequency of use and severity of use) and neurocognitive performance, using a broad neurocognitive test battery with a particular emphasis on domains previously implicated in the cannabis use literature (e.g., working memory, attention, probability and delay discounting). The current study aims to contribute to the literature via its comparatively large sample size, broad battery of objective and subjective measures of neurocognitive functioning, and incorporation of numerous potential confounders that have been often overlooked in previous studies.
METHODS
Participants and Procedure
Participants were emerging adults recruited using public advertisements in Memphis, Tennessee, for a study investigating high-risk drinking (n = 603). Due to missing cannabis use data, five participants were excluded from the study, with a total sample size of n = 598. Eligibility criteria included fluency in written English; no current or past diagnosis of a psychotic disorder; age range of 21 to <25; and heavy episodic drinking (>4/3 standard drinks for males/females) at least 2–3 times/month over the past six months. With regard to cannabis use, of the 598, 276 (46.2%) reported no use of cannabis during the past month. Among the participants who reported any cannabis use in the past month (53.8%), those who endorsed using it monthly or weekly were categorized as occasional users, and those who reported using it daily or multiple times a day were classified as daily users. These aggregations were applied to maximize cell sizes, and in turn statistical power, according to conceptually compatible designations. Thus, the current study used three groups: 1) non-users (n = 276; 46.2%), 2) occasional users (i.e., monthly or weekly users; n = 201; 33.6%), and 3) daily users (i.e., daily or multiple times daily users; n = 121; 20.2%). Among those who reported cannabis use, the mean age of initiation was 16.8, with a standard deviation of 2.6 and a range of 8–23 years old. Other substance-use frequency was assessed for cocaine, methamphetamine/amphetamine, heroin, prescription sedatives/anti-anxiety medications (taken not as prescribed), prescription stimulants (taken not as prescribed), prescription painkillers (taken not as prescribed), prescription sleep aids (taken not as prescribed), inhalants, and Lysergic acid diethylamide (LSD)/magic mushrooms, but was infrequent, with the medians and modes being none in the last month for all three groups. Participants provided written informed consent followed by in-person assessments and a comprehensive demographic questionnaire, with data provided in Table 1. With regard to race (using National Institutes of Health categories), participants were 47.01% White, 41.53% African American, 4.98% more-than-one-race, 3.82% Asian, 1% American Indian/Alaskan Native/Pacific Islander, and 1.66% other. Non-mutually exclusive with race, 5.60% reported Hispanic/Latino ethnicity. All study procedures were reviewed and approved by the University of Memphis Institutional Review Board (Protocol #4320).
Table 1. Demographic characteristics across the three categorical groups

Note: CUD = Cannabis Use Disorder; WHO ADHD = World Health Organization Adult ADHD Self-Report Scale.
Test statistics are provided for variables that differed significantly between groups.
*p < .05; **p < .01.
a 12 or greater on the Cannabis Use Disorders Identification Test – Revised.
b “Non-Users” group includes those with positive use history but no use in past month (n = 146); never users excluded from age of initiation calculation in the “Non-Users” group.
Assessments
Frequency of cannabis and other drug use
The WHO’s Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), modified by the National Institute on Drug Abuse (NIDA) and the American Psychiatric Association to only include the frequency of substance use (NIDA) was used to quantify the frequency of cannabis and other illicit drug use over the past month. Standard alcoholic drinks per week was assessed using the Drinking Days Questionnaire (Collins etal., Reference Collins, Parks and Marlatt1985).
Cannabis use disorder identification test-revised
Severity of cannabis-related problems was assessed using The Cannabis Use Disorder Identification Test-Revised (CUDIT-R; Adamson etal., Reference Adamson, Kay-Lambkin, Baker, Lewin, Thornton, Kelly and Sellman2010). This is an eight-item self-report questionnaire assessing cannabis use over the past six months. Higher scores are indicative of greater severity of cannabis use and related problems. For the purpose of the current study, the CUDIT-R scores were examined as a continuous variable.
Age of cannabis initiation
Participants who reported any lifetime use of cannabis were asked to report their age of initiation. Participants were then classified into one of two groups: i) used at age 15 or younger (n = 138, 29.4%); and ii) used at age 16 or older (n = 330; 70.5%), a commonly used demarcation (Batalla etal., Reference Batalla, Lorenzetti, Chye, Yücel, Soriano-Mas, Bhattacharyya and Martín-Santos2018; Gruber, Dahlgren, Sagar, Gönenç, & Lukas, Reference Gruber, Dahlgren, Sagar, Gönenç and Lukas2014; Gruber etal., Reference Gruber, Sagar, Dahlgren, Racine and Lukas2012; Sagar etal., Reference Sagar, Dahlgren, Gönenç, Racine, Dreman and Gruber2015). Never users were excluded from analyses that included age of cannabis initiation to avoid imputing an arbitrary age of initiation among those who have not yet used cannabis (e.g., setting the age cut-off for the current study, age 25, as the age of initiation for never users).
Shipley verbal IQ
Verbal intellectual quotient (IQ) was measured using the Shipley Institute of Living Scale – Second Edition (Shipley, Reference Shipley2009), which is a brief computerized task assessing crystallized intelligence. During this task, participants are required to select the correct synonym associated with English words over 40 progressively difficult trials. The number of correct responses was summed to calculate raw scores. Standardized verbal IQ scores were generated using age norms and participants with scores < 70 were excluded from analyses (n = 3).
Digit span forward and backward
Basic auditory attention and working memory were assessed using a computerized digit span task, composed of two subtests: digits forward (DS-F) and digits backward (DS-B), measuring basic auditory attention and working memory, respectively. During both tasks, participants were presented with a string of numbers read out loud by a recorded voice via headphones. For DS-F, they were required to immediately type the numbers in the same order in which they were presented. For DS-B, they were required to enter the numbers in the reverse order of what had been presented. Notably, scores on DS-F and DS-B were significantly correlated when the entire sample was combined (r = .351, p < .001). The raw maximum number of correct DS-F and DS-B were used in the present analysis. Although the current study used a modified digit span task, individuals with spans ≤2 for the forward condition, and <2 for the backward condition, or a total of ≤5 between the two conditions (i.e., reliable digit span = 6;) were excluded from analyses, based on research suggesting individuals falling below a certain threshold might suggest variable effort or engagement (Greiffenstein, Baker, & Gola, Reference Greiffenstein, Baker and Gola1994). Three participants had invalid DS-F performance, 14 had invalid DS-B performance, and 6 had invalid total DS scores when the forward and backward conditions were summed. A total of 15 participants were excluded overall from the digit span analyses based on the aforementioned criteria (e.g., three related to DS-F scores; 14 related to low scores on DS-B; and 6 when the scores were summed). Among those excluded due to low digit span scores, seven were non-users, three were occasional users, and five were daily users.
Go/No-Go task
A computerized Go/No-Go task (Liddle, Kiehl, & Smith, Reference Liddle, Kiehl and Smith2001) was used to assess behavioral inhibition. Participants were instructed to respond as quickly as possible to a target stimulus (X; “Go”), and to refrain from responding to a distractor stimulus (K; “No-Go”), with a ratio of targets-to-distractors of 85:15. Outcome variables of interest included number of commission errors (i.e., responding when not supposed to on a “No-Go” trial), number of omission errors (i.e., failure to respond to a “Go” trial), and mean reaction time for “Go” trials. Performance on this task was considered invalid for respondents who had >80% commission errors (n = 67 excluded) and >50% omission errors (n = 2 excluded), with a total of 69 participants excluded overall based on either commission or omission errors. Among those excluded due to invalid Go/No-Go data 32 were non-users, 26 were occasional users, and 11 were daily users.
Delay and probability discounting
The five-trial adjusting delay task (Koffarnus & Bickel, Reference Koffarnus and Bickel2014) was used to measure both probability and delay discounting using computerized administration. The measure uses the effective delay 50% value (ED50) to pose choices between larger delayed rewards and smaller immediate rewards of 50% value, with rewards held constant at varying delays to rapidly infer an individual’s temporal discounting function. ED50, which is a measure of discount rate, is converted to a k-value (i.e., rate of discounting delayed rewards) for delay discounting trials, and an h-value (i.e., rate of discounting uncertain rewards) for probability discounting trials. The procedure for the delay discounting task is as follows: the first of five trials was held constant between the amount of the full reward delayed available after three weeks, and half of the same reward available immediately. The dollar amounts presented in the proceeding trials were always either the full reward ($100 and $1000) available after a titrating amount delay, or half of the reward ($50 and $500) available immediately. Depending on the choice made by the participant, the delay adjusts up (i.e., more time between the choice and reward) or down (i.e., less time between choice and reward), with delays ranging from “now” to 25 years. For probability discounting, participants were instructed to choose between two outcomes; one with $50 delivered “for sure” (i.e., 100% probability of receipt), and $100 delivered with probabilities ranging from 1% to 99%. The first of five trials was held constant between the amount of the full reward ($100) with a 50% probability, and half of the full reward delivered “for sure.” In the remaining trials, the reward amount was titrated as a result of participants’ choices.
WHO Adult ADHD Self-Report Scale
ADHD symptomatology was assessed with the WHO Adult ADHD Self-Report Scale (Kessler etal., Reference Kessler, Adler, Ames, Demler, Faraone, Hiripi and Walters2005), a self-report screening tool used to assess severity of inattention and hyperactivity/impulsivity associated with ADHD. Subscale scores of the inattention and hyperactivity/impulsivity symptom subtypes were used in the present analysis. In addition, as per the scoring instructions, participants who endorsed four or more items on the screener portion of this self-report questionnaire were categorized as endorsing symptoms highly consistent with ADHD. Non-users, occasional users, and daily users did not differ with respect to proportion of participants in each group who endorsed symptoms highly consistent with ADHD.
Data Analysis
Data were first examined for missingness, with less than 1% missing for all variables of interest. Data were then screened for patterns of low-effort responses within the neurocognitive variables of interest, and only participants with valid data were included in the final analysis (n = 598 included). Outliers were identified (Z scores > 4.0) among dependent variables, and these data points were winsorized to one unit greater than the closest nonoutlying value (Tabachnick & Fidell, Reference Tabachnick and Fidell2001). A total of 23 cases had outlying values (Go/No-Go omission errors: 1.6%; delay discounting $100:1.2%; delay discounting $1000:1.0%). Distribution normality was then examined. Scores pertaining to omission errors on the Go/No-Go task were square-root transformed, and h-values and k-values were transformed using logarithmic transformation. Preliminary analyses were performed to assess if the frequency of participants with invalid performance who were excluded from study analyses differed as a function of frequency of cannabis use, as differing distributions according to frequency of use could suggest retaining these data in the analyses. The primary analyses examined both categorical frequency of cannabis use, as well as continuous severity of cannabis use and their effects on neurocognitive variables. First, a series of one-way analyses of covariance (ANCOVAs) were conducted to examine differences in the three classifications of past-month frequency of cannabis use (no use, occasional use, and daily use) on neurocognitive variables. The following covariates were incorporated as they significantly correlated with cannabis use variables of frequency of use and severity of cannabis-related problems in the full sample (see Supplementary Table 1), supporting their incorporation into analyses: age, race (i.e., dichotomized to reflect membership in the most common self-reported race for the sample, White), sex, income, years of education, number of cigarettes per month, and the number of standard alcoholic drinks per week. With regard to race, dichotomization was used because participants cannot have more or less race dimensionally, and dummy variables reflecting all permutations of categories were not used because of increasingly lopsided and small cell sizes. Second, hierarchical regression analyses were run, incorporating the same covariates in the first block, followed by CUDIT-R scores in the second block, to assess the amount of variance explained in neurocognitive scores by severity of cannabis use and related problems. Secondary analyses were performed in order to examine potential effects of age of initiation on neuropsychological task performance, self-reported attention abilities, and delay and probability discounting. Secondary analyses therefore included a series of 3 (frequency of cannabis use) × 2 (age of initiation) ANCOVAs using the categorical frequency of use data, as well as a series of hierarchical regressions using the severity of use and related problems (i.e., CUDIT-R scores) as well as age of initiation and their interaction term. Never users (n = 130) were excluded from these secondary analyses to avoid having to impute an arbitrary age of initiation for individuals who reported never using cannabis. Age of initiation was examined categorically for compatibility with the previous literature, but was also examined as a continuous variable in supplementary analyses. Specifically, hierarchical regressions examined neuropsychological task performance, self-reported attention abilities, and delay and probability discounting as dependent variables, with CUDIT-R scores (centered), age of initiation as a continuous variable (centered), and their interaction as independent variables in a second block (covariates entered in a first block).
RESULTS
Preliminary Analyses
To ensure data quality control, preliminary analyses were performed to assess if the frequency of participants with invalid performance who were excluded from study analyses differed as a function of frequency of cannabis use. Chi-square analyses were conducted to assess if participants with invalid neurocognitive data on the more challenging tasks (i.e., digit span and Go/No-Go) were equally distributed among the frequency of cannabis use groups. These analyses were included because low level of performance may be more related to frequency of cannabis use, rather than low or variable effort. With respect to those with questionable performance across digit span subtests (n = 15), the frequency of invalid performance did not differ between non-users (n = 7), occasional users (n = 3), and daily users (n = 5) (X 2(2) = 2.28, p = .32). In addition, when all participants’ (pre-winsorization) Go/No-Go scores were categorized as valid or invalid based on the aforementioned criteria, the frequency of participants with invalid performance on this task did not differ between non-users (n = 32), occasional users (n = 26), and daily users (n = 11) (X 2(2) = 1.08, p = .58). These exploratory analyses suggest that each group was as equally likely to have an “invalid” performance, and therefore, low scores on tasks observed in the current study sample is most likely a function of variable effort or engagement overall, rather than a function of frequency of cannabis use. Zero-order correlations among the neurocognitive indicators were generated for descriptive purposes (Supplementary Table 2) and revealed expectable associations (e.g., significant associations between both forms of ADHD symptoms in relation to impulsive delay discounting).
Cannabis Involvement and Neurocognitive Performance: Categorical Analyses
Separate univariate ANCOVAs analyzing the effect of frequency of cannabis use on the neurocognitive variables revealed a significant group effect on DS-B, F(2, 536) = 3.41, p = .034, η p 2 = .013 (Table 2). Follow-up comparisons demonstrated that DS-B was significantly higher among non-users compared to those who used cannabis daily (p = .029, Cohen’s d = .32), but not when compared to occasional users (Table 2; Figure 1).
Table 2. One-Way ANCOVAs contrasting individuals reporting no cannabis in the past month, occasional (monthly or weekly) use, or daily use. Covariates included age, race, sex, income, years of education, tobacco use, and alcohol use. Effect size is reported as ηp2

Notes: IQ = Intellectual Quotient; GNG = Go/No-Go; RT = reaction time; WHO ADHD = World Health Organization Adult ADHD Self-Report Scale.

Fig. 1. Differences in digit span performance among individuals reporting no past-month cannabis use, occasional use, and daily use. * p < .05 between daily users and non-users.
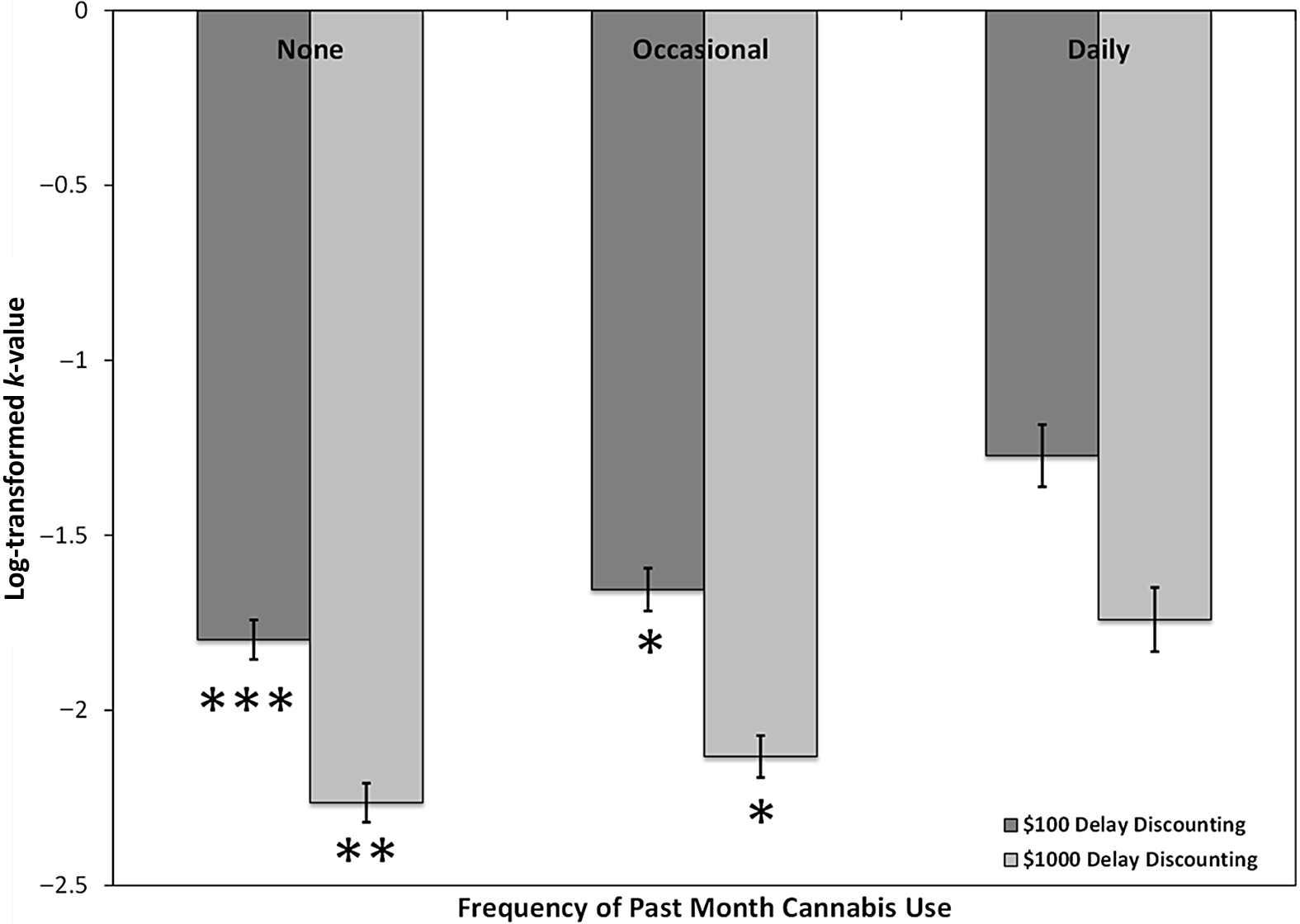
The ANCOVAs on the probability and delay discounting measures revealed a significant group effect on k-values for both the $100 delay discounting condition, F(2, 547) = 6.30, p = .002, η p 2 = .023, as well as the $1000 delay discounting condition, F(2, 549) = 5.27, p = .005, η p 2 = .019. Pairwise comparisons demonstrated that k-values for delay discounting at $100 differed between non-users and daily users (p = .001, Cohen’s d = .42), and also differed between occasional users and daily users (p = .032, Cohen’s d = .32), with daily users demonstrating the steepest k-values. Pairwise comparisons also demonstrated that delay discounting $1000 differed between non-users and daily users (p = .004, Cohen’s d = .45), and again between occasional users and daily users (p = .034, Cohen’s d = .39) with daily users once again demonstrating the highest (untransformed) k-values (see Figure 2). Groups did not differ with respect to the probability discounting rates.

Fig. 2. Differences in delay discounting performance among individuals reporting no past-month cannabis use, occasional use, and daily use. Significance reflects comparisons with daily users; * p < .05, **p < .01, ***p ≤ .001.
With respect to the measures of self-reported ADHD symptoms, separate ANCOVAs revealed a significant group effect on WHO ADHD hyperactive scores, F(2, 548) = 4.77, p = .009, η p 2 = .017, with non-users reporting less hyperactivity as compared to both the occasional users (p = .017, Cohen’s d = .22) and the daily users (p = .007, Cohen’s d = .32), shown in Figure 3. There was also a group effect approaching significance on WHO ADHD inattentive scores, F(2, 549) = 3.00, p = .051, η p 2 = .011, with the non-users reporting less inattention when compared with the occasional users (p = .018, Cohen’s d = .23), but not when compared with daily users.

Fig. 3. Differences in hyperactive/impulsive symptoms of attention deficit hyperactivity disorder (ADHD) among individuals reporting no past-month cannabis use, occasional use, and daily use. * p < .05 in contrast to non-users; ** p < .01 in contrast to non-users.
There were no significant main effects of group for Shipley Verbal IQ, DS-F, Go/No-Go commission or omission errors, or reaction time.
Cannabis Involvement and Neurocognitive Performance: Dimensional Analyses
After incorporating covariates, the severity of cannabis misuse (i.e., CUDIT-R scores) accounted for significant variance in performance on DS-B, the number of commission errors on the Go/No-Go Task, k-values for both $100 and $1000 delay discounting, as well as scores on the WHO ADHD Hyperactivity and Inattention Subscales (Table 3). Individual regression coefficients indicated that higher severity of cannabis use and related problems were associated with worse performance across these tasks and greater endorsement of ADHD-related experiences (Table 4).
Table 3. Hierarchical regressions comprising covariates followed by the cannabis use disorder identification test (CUDIT) in relation to neurocognitive performance. Covariates were age, race, sex, income, years of education, tobacco use, and alcohol use

Notes: IQ = Intellectual Quotient; GNG = Go/No-Go; WHO ADHD = World Health Organization Adult ADHD Self-Report Scale.
Table 4. Individual hierarchical regressions of covariates and severity of cannabis use and related problems in relation to neurocognitive performance for models significantly implicating CUDIT-R

Notes: CUDIT-R = Cannabis-Use Disorder Identification Test – Revised; GNG = Go/No-Go; WHO ADHD = World Health Organization Adult ADHD Self-Report Scale.
Age of Initiation as a Moderator
The 3 (cannabis frequency of use group) × 2 (cannabis age of initiation) ANCOVAs did not reveal statistically significant main effects or interaction effects on any of the neurocognitive variables (Table 5).
Table 5. Results of 3 (Frequency of cannabis use) × 2 (Age of initiation) analyses of covariance. Covariates include age, race, sex, income, years of education, tobacco use, and alcohol use. Effect size are reported as ηp2

Notes: DD = delay discounting; IQ = Intellectual Quotient; GNG = Go/No-Go; PD = probability discounting; WHO ADHD = World Health Organization Adult ADHD Self-Report Scale.
In the hierarchical regressions, after adjusting for covariates, the severity of cannabis use and related problems (i.e., CUDIT-R scores), age of initiation, and the interaction term of cannabis use severity by age of initiation accounted for significantly more variance in k-values for $100 delay discounting, as well as scores on the WHO ADHD Hyperactivity Subscale (Table 6). Age of initiation and the interaction term alone, however, did not account for a significant proportion of variance in either of these outcome variables when added to the model (Table 7).
Table 6. Hierarchical regression comprising covariates followed by cannabis use variables (severity of cannabis use, age of initiation, and their interaction). Covariates included age, race, sex, income, years of education, tobacco use, and alcohol use

Notes: IQ = Intellectual Quotient; GNG = Go/No-Go; ADHD = World Health Organization Adult ADHD Self-Report Scale. *p < .05
Table 7. Individual hierarchical regressions of covariates and severity of cannabis use and related problems in relation to neurocognitive performance for models that were significant at the p < .05 level

Notes: CUDIT-R = Cannabis-Use Disorder Identification Test – Revised; WHO ADHD = World Health Organization Adult ADHD Self-Report Scale.
Age of initiation was also examined as a continuous variable in hierarchical regressions (replacing the categorical age of initiation in the model). After adjusting for covariates, the severity of cannabis use and related problems (i.e., CUDIT-R scores, which were centered for the purposes of the supplementary analysis), age of initiation as a continuous variable (also centered) and the interaction term of cannabis use severity by age of initiation accounted for significantly more variance in k-values for $100 delay discounting. Similar to the aforementioned findings with age of initiation set as a categorical variable, neither age of initiation (when set categorially) nor the interaction term alone accounted for a significant proportion of variance in k-values. Unlike when age of initiation was set as a categorical variable, the model with age of initiation set as a continuous variable no longer accounted for significantly more variance on the WHO ADHD Hyperactivity Subscale. A unique significant finding emerged with age of initiation set as a continuous variable included in the model, wherein this model accounted for significant variance in Go-No Go omission errors. However, neither age of initiation alone, nor the interaction term accounted for significant variance in omission errors, though CUDIT-R scores did. Inspection of the coefficients indicated that higher CUDIT-R scores were associated with significantly more omission errors (Supplementary Tables 3 and 4).
DISCUSSION
In a relatively large sample of nearly 600 young adults and incorporating a broad neurocognitive battery and a large number of potential confounders, the current study found that daily cannabis users had poorer working memory performance compared to non-users, and both daily and more occasional users endorsed more hyperactive ADHD symptoms as compared to non-users. Further, daily users demonstrated greater impulsivity as measured by delay discounting compared to occasional and non-users. When considered dimensionally, similar findings emerged, as greater severity was significantly associated with poorer working memory (i.e., DS-B), lower inhibitory control (commission errors), greater endorsement of hyperactivity and inattentive ADHD symptoms, and greater impulsivity (delay discounting). Neither frequency of use, nor severity of cannabis involvement, was associated with verbal intelligence, inattention (omission errors), or risk orientation (probability discounting). Age of initiation was not associated with neurocognitive performance scores on any of the measures when captured as a categorical variable or when captured as a continuous variable.
Although the evidence of the effect of cannabis use on working memory is inconsistent (Broyd etal., Reference Broyd, Van Hell, Beale, Yücel and Solowij2016), the current findings support those reported by Thames etal. (Reference Thames, Arbid and Sayegh2014) and suggest that greater frequency of use in particular may put one at risk for experiencing subtle (given the small effect size) declines in working memory ability. Whether this finding is functionally significant remains unknown, and while it may be that difficulty with working memory manifests in certain features of ADHD in daily life, future studies may wish to examine this question by understanding functional variables such as education, employment, and instrumental activities of daily living. Performance on the remaining objective neurocognitive tasks did not differ as a function of frequency of cannabis use, and therefore daily cannabis use does not appear to have a global effect across the other cognitive domains evaluated.
Regarding subjective attentional difficulties, non-users reported less hyperactivity as compared to both the occasional users and compared to the daily users. There was also a group effect approaching significance on self-reported inattention, with the non-users reporting less inattention as compared to the occasional users. These findings are in line with previous evidence of acute effects of cannabis exposure on objective and subjective measures of attention (Broyd etal., Reference Broyd, Van Hell, Beale, Yücel and Solowij2016; Loflin etal., Reference Loflin, Earleywine, De Leo and Hobkirk2014). Conflicting findings have been interpreted to indicate that daily users may have developed a tolerance to the acute effects of cannabis (Ramaekers, Kauert, Theunissen, Toennes, & Moeller, Reference Ramaekers, Kauert, Theunissen, Toennes and Moeller2009; Ramesh, Haney, & Cooper, Reference Ramesh, Haney and Cooper2013; Schwope, Bosker, Ramaekers, Gorelick, & Huestis, Reference Schwope, Bosker, Ramaekers, Gorelick and Huestis2012). Based on the current findings, however, attentional abilities are most vulnerable among daily users, at least subjectively, contradicting this common interpretation of increased tolerance. Another possible interpretation is that daily users are engaging in fewer tasks/roles that require sustained attention and might thus be less likely to self-report difficulties with inattention.
Furthermore, daily users were more impulsive than occasional users in that they discounted future rewards more steeply. This finding was consistent with a study by Sofis etal. (Reference Sofis, Budney, Stanger, Knapp and Borodovsky2020) in which the authors also classified participants based on frequency of use, and found the same pattern wherein greater frequency of use was associated with greater delay discounting. In the broader literature on addictive behaviors, various substance-use variables have been shown to be associated with greater delay discounting, including severity, dependence, and quantity/frequency (MacKillop etal., Reference MacKillop, Amlung, Few, Ray, Sweet and Munafo2011; Amlung, Vedelago, Acker, Balodis, & MacKillop, Reference Amlung, Vedelago, Acker, Balodis and MacKillop2017). Moreover, a recent systematic review and meta-analysis on cannabis and impulsive delay discounting identified a similar, albeit smaller association between cannabis use and delay discounting (Strickland, Lee, Vandrey, & Johnson, Reference Strickland, Lee, Vandrey and Johnson2020). Taken together, the current findings and the broader literature on delay discounting provide robust evidence that cannabis use is associated with more impulsive decision-making, favoring smaller immediate rewards over larger delayed rewards.
Overall, the data suggest that daily cannabis use, but not occasional use, poses the greatest risk for adverse neurocognitive impacts. Of course, the cross-sectional design of the current study constrains our ability to address causality directly. It will be important for future longitudinal research to address the directional relationships between working memory, ADHD symptoms, and delay discounting in relation to cannabis use more definitively. These cognitive vulnerabilities could serve as risk factors for higher frequency and severity of cannabis use, but one longitudinal study nonetheless found that positive recovery effects on verbal memory can occur within one month of abstinence (Schuster etal., Reference Schuster, Gilman, Schoenfeld, Evenden, Hareli, Ulysse and Evins2018). This is also supported by meta-analytic findings that an abstinence period of longer than 72 hr is associated with improved cognition (Scott etal., Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018). Longitudinal designs will be needed to ascertain the extent to which adverse cognitive effects improves following abstinence.
Much like with frequency of use, more severe cannabis-related problems were associated with poorer performance on measures of working memory (DS-B) and response inhibition (number of commission errors on Go/No-Go), greater subjective hyperactivity and inattention (WHO ADHD), and greater delay discounting. These findings are congruent with those for frequency of use, but with the additional association of severity of cannabis involvement and commission errors on the Go/No-Go Task. The association between cannabis and attentional issues is well documented, with previous studies reporting an association between more severe cannabis use problems and presence of ADHD symptomatology (Loflin etal., Reference Loflin, Earleywine, De Leo and Hobkirk2014; Notzon etal., Reference Notzon, Pavlicova, Glass, Mariani, Mahony, Brooks and Levin2016; Petker etal., Reference Petker, DeJesus, Lee, Gillard, Owens, Balodis and Mackillop2020; Van de Glind etal., Reference Van de Glind, Van Emmerik-van Oortmerssen, Carpentier, Levin, Koeter, Barta and van den Brink2013). Collectively, greater frequency of use, and greater severity of cannabis-related problems give rise to greater neurocognitive vulnerabilities. Within the realm of ADHD, shared genetic contributions and causal pathways between ADHD and onset of cannabis use have been identified (Artigas etal., Reference Artigas, Sanchez-Mora, Rovira, Richarte, Garcia-Martinez, Pagerols and Ribases2020), suggesting that ADHD in itself may pose a greater risk for cannabis use. That is, ADHD symptoms may be contributing to increased cannabis use, rather than increased cannabis use accounting for greater ADHD symptoms, though the directionality of this relationship cannot be confirmed using the current cross-sectional design.
Based on the current findings, earlier age of initiation did not appear to be a risk factor for poorer cognitive performance across objective and subjective tasks, which is consistent with a meta-analysis which found that cognitive effects of cannabis use did not vary as a function age of first use (Scott etal., Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018). The lack of association between age of first use and neurocognitive functioning was also present when age of first use was examined as a continuous variable. However, the inconsistency in the literature suggests there may also be a need to consider patterns of use over time that influence the relation between age of first use and current cognitive performance, and for further studies that directly compare the effects of differing methods of age of first use classification.
These findings should be considered in the context of the study’s strengths and limitations. Notable strengths in the current study include its large sample size and use of a broad battery of objective and subjective measures of neurocognitive performance and functioning. There are several limitations in the current study that are important to acknowledge. As noted above, the cross-sectional study design limits us from disentangling cause from consequence in terms of the relationship between cannabis and cognition, and longitudinal studies are necessary to address causal versus consequential roles more definitively. Fundamentally, causality cannot be inferred in cross-sectional designs. In addition, urinary THC levels were not collected as part of this study, which would have provided an objective indicator of recent cannabis use. In addition, while age of initiation and current use were included in the current study, the degree of consistency of cannabis use frequency since age of initiation is unknown, as is the cumulative use. Other cannabis-related variables that could be informative but that were not included in the study are days since last use and number of recent use episodes. The current sample consists of high-risk drinkers, and while we analytically modeled the level of alcohol use, the results of this study might not generalize to cannabis users who are not high-risk drinkers. Similarly, while we adjusted for age, sex, income, education, and tobacco use, the daily users did differ somewhat demographically. Given the ADHD findings, the prevalence of ADHD psychostimulant medication status is unknown and could have affected the results. Lastly, we performed a sizable number of analyses, but did not apply an error correction strategy, increasing the risk of Type I error. However, given the inconsistencies in the literature, we elected to err on the side of minimizing Type II error to avoid potentially missing a finding that would be compatible with previous work. The potential for increased false positive findings is nonetheless a consideration.
Acknowledging these considerations, the findings from the current study nonetheless indicate several potential risks of cannabis use on neurocognitive functioning (working memory, subjective attention, and impulsivity), particularly for frequent or problematic cannabis use. On the other hand, these findings suggest that most areas of cognition exhibited no association with cannabis involvement and when a person started using cannabis had no bearing on cognitive performance. In both cases, the findings underscore the need for additional longitudinal studies to elucidate the complex relationships between cannabis involvement and neurocognitive functioning.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617721000618
Acknowledgements
The authors are very grateful for the contributions of the following staffs, Dr. Ashley Dennhardt, Ms. Allison Wallace, and Ms. Jessica Gillard, and for the time and effort of the study participants.
FINANCIAL SUPPORT
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA024930) and the Peter Boris Chair in Addictions Research.
CONFLICTS OF INTEREST
Dr. MacKillop is a principal and senior scientist in BEAM Diagnostics, Inc., but no BEAM products were used in the reported research. No other authors have declarations.