INTRODUCTION
The prevalence and incidence of dementia is rising, and it is projected to affect up to 14 million persons by 2050 in the United States alone (Hebert, Weuve, Scherr, and Evans, Reference Hebert, Weuve, Scherr and Evans2013). Biomarkers of vascular health have been associated with the risk of dementia and cognitive impairment. For example, stroke and hypertension are among the most important predictors of vascular dementia (VD), but they have also been associated with incident Alzheimer disease (AD) (Gorelick et al., Reference Gorelick, Scuteri, Black, Decarli, Greenberg, Iadecola and Seshadri2011; Peters et al., Reference Peters, Beckett, Fagard, Thijs, Wang, Forette and Bulpitt2013). Other biomarkers of small artery disease such as white matter hyperintensities and silent brain infarcts have been associated with VD and AD (de Leeuw et al., Reference de Leeuw, Richard, de Groot, van Duijn, Hofman, Van Gijn and Breteler2004; Vermeer et al., Reference Vermeer, Prins, den Heijer, Hofman, Koudstaal and Breteler2003).
In our prior work, brain arterial diameters related non-linearly to vascular events. Individuals with the smallest brain arterial diameters had a higher risk of death and any vascular events, while individuals with the largest brain arterial diameters had a higher risk of vascular death, myocardial infarction and any vascular events (Gutierrez, Cheung, et al., Reference Gutierrez, Cheung, Bagci, Rundek, Alperin, Sacco and Elkind2015). Similarly, brain arterial pathologies that relate to either very small or large brain arterial luminal diameters relate to cognition. For example, intracranial atherosclerosis (which decreases luminal diameter) increases the risk of stroke, and stroke is one of the main risks for vascular dementia (Sacco et al., Reference Sacco, Boden-Albala, Gan, Chen, Kargman, Shea and Hauser1998; Sacco, Kargman, Gu, and Zamanillo, Reference Sacco, Kargman, Gu and Zamanillo1995).Intracranial atherosclerosis has also been associated with AD pathology and risk of dementia (Gutierrez, Honig, et al., Reference Gutierrez, Honig, Elkind, Mohr, Goldman, Dwork and Marshall2016; Honig, Kukull, and Mayeux, Reference Honig, Kukull and Mayeux2005; Roher et al., Reference Roher, Esh, Kokjohn, Kalback, Luehrs, Seward and Beach2003). Data from our group suggest that brain arterial aging, consisting of arterial dilatation and elastin loss, is associated with AD pathology independent of chronological age, brain infarcts, and large artery atherosclerosis (Gutierrez, Honig, et al., Reference Gutierrez, Honig, Elkind, Mohr, Goldman, Dwork and Marshall2016), and brain arterial diameters relate non-linearly with Mini-Mental State Examination scores (Gutierrez, Cheung, et al., Reference Gutierrez, Honig, Elkind, Mohr, Goldman, Dwork and Marshall2016).
It is not known whether there exists an association between brain arterial diameters supplying a vascular territory and a cognitive domain thought to be predominantly located in such vascular territory. It is plausible that left sided anterior arteries [i.e., middle (MCA) and anterior (ACA) cerebral arteries] relate to semantic memory. First, semantic memory (i.e., language) is predominantly localized to the left frontal lobe, most of which is perfused by the left MCA and ACA. There is evidence suggestive of activation, using functional MRI, of the middle temporal gyrus, inferior frontal and parahippocampal gyri of the dominant hemisphere with completion of parts of the Boston Naming test (Tomaszewki Farias, Harrington, Broomand, and Seyal, Reference Tomaszewki Farias, Harrington, Broomand and Seyal2005) and strong left lateralized (frontal) activity for phonemic fluency (Meinzer et al., Reference Meinzer, Wilser, Flaisch, Eulitz, Rockstroh, Conway and Crosson2009).
Also, there is an association between poorer Boston naming test performance in patients with left peri-Sylvian cortex damage due to stroke, as determined by voxel-based lesion symptom mapping (Baldo, Arévalo, Patterson, and Dronkers, Reference Baldo, Arévalo, Patterson and Dronkers2013). Furthermore, even in left handed-individuals, the majority of language-related tasks are localized to the left hemisphere (Knecht et al., Reference Knecht, Dräger, Deppe, Bobe, Lohmann, Flöel and Henningsen2000). Another plausible regional link between cognitive function and arterial diameter includes verbal episodic memory, attributed to the left hippocampus, which is supplied by the left posterior cerebral artery (PCA) and/or left posterior communicating artery (Pcomm). This hypothesis is supported by the fact that in the presence of a fetal PCA the anterior circulation dominates the hippocampal blood flow and function (Hendrikse, van Raamt, van der Graaf, Mali, & van der Grond, 2005) and in the reciprocal relationship between left PCA with left Pcomm (Gutierrez et al., Reference Gutierrez, Sultan, Bagci, Rundek, Alperin, Elkind and Wright2013).
The objective of this study is to tests the hypothesis that regional brain arterial diameters are associated with cognitive performance presumably localized or related to the vascular territory supplied by such arteries.
METHODS
The Northern Manhattan Study (NOMAS) is a population-based cohort of self-reported stroke-free individuals who have been followed prospectively since 1993. In 2003, NOMAS participants were invited to undergo a brain MRI if they remained stroke-free, had no contraindication to MRI, and provided informed consent. The study was approved by the local IRB. Age, sex, ethnicity, and years of education were self-reported. Hypertension, diabetes and hypercholesterolemia were defined by either self-reported diagnosis, treatment, or blood pressure or laboratory measurements as previously reported (Gutierrez, Cheung, et al., Reference Gutierrez, Cheung, Bagci, Rundek, Alperin, Sacco and Elkind2015). Smoking was labeled current if participants reported smoking at the time of the MRI. Apolipoprotein ε4 (ApoE4) carriers (i.e., ApoE4/2, ApoE4/4, or ApoE4/3) were identified in DNA samples from peripheral blood white cells using HhaI digestion and amplified by polymerase chain reaction.
Cognitive Assessment at the Time of the Brain MRI
Trained research staff administered a battery of cognitive tests (in Spanish or English) the day of the MRI. We did not exclude any NOMAS participant based on their cognitive status at MRI, but the participants did need to be able to give informed consent to be enrolled. Thus, participants recognized to have severe dementia were excluded from the NOMAS MRI substudy. We used factor analysis to cluster neuropsychological testing in four domains, with factor loading >0.3 as the criterion to include a test in a given domain. Executive function was represented by Color Trails Test Form 1 and Form 2 and the sum of the Odd-Man-Out subtests 2 and 4 scores (D’Elia and Satz, Reference D’Elia and Satz1996; Frearson and Eysenck, Reference Frearson and Eysenck1986). The Odd-Man-Out measures reaction time and consist in asking the participant to select which item in a set of three does not belong with the other items (D’Elia and Satz, Reference D’Elia and Satz1996).
Semantic memory was assessed with the Boston Naming test, Animal Naming, and C, F, L in English speakers and F, A, S in Spanish speakers (phonemic fluency) (Kaplan, Goodglass, and Weintraub, Reference Kaplan, Goodglass and Weintraub2001; Tombaugh, Kozak, and Rees, Reference Tombaugh, Kozak and Rees1999). Episodic memory was evaluated by using three subscores on a 12-word five-trial list-learning task: list learning total score, delayed recall score, and delayed recognition score (Stuss et al., Reference Stuss, Alexander, Palumbo, Buckle, Sayer and Pogue1994). Processing speed was assessed with the Grooved Pegboard task with the non-dominant hand, and the Color Trails Test Form 1 (Bryden and Roy, Reference Bryden and Roy2005; D’Elia and Satz, Reference D’Elia and Satz1996). We calculated the mean and standard deviation of each neuropsychological test and performed a within sample Z-score transformation. We averaged the Z-scores of all tests clustered by domain (as above) to obtain the domain Z-score. We adjusted all models for a measure of crystallized abilities based on tests of vocabulary and literacy.
Brain Arterial Diameters
Brain MRIs were performed in 1290 NOMAS participants, of whom 1034 had brain time-of-flight MRAs available. The MRAs were acquired in a 1.5 T MRI system (Phillips Medical Systems) with the following parameters: field of view 15 cm, 1 mm effective slice thickness, acquisition matrix interpolated to 256 × 228 matrix, flip angle of 25 degrees, repetition time/echo time 20 and 2.7 ms, respectively, and one average. We used in-house software that allows for the tridimensional reconstruction of the Circle of Willis for arterial measurements. We measured 13 arterial segments: cavernous portion of the intracranial internal carotid artery (ICA); the first segments of the MCA, ACA, and PCA; the fourth segment of the vertebral artery (VA V4); the proximal segment of the basilar artery (BA); and the Pcomm. The location of measurement was aimed at the largest portion of a given segment free of focal stenosis, with good to excellent reliability (Gutierrez et al., Reference Gutierrez, Bagci, Gardener, Rundek, Ekind, Alperin and Wright2014).
We created a separate variable for focal stenosis defined as any luminal irregularity or stenoses suggestive of atherosclerosis as determined by the neuroradiologists who interpreted the brain MRA as part of the Institutional Review Board-mandated safety read. For arteries visualized in the axial source MRA images but not thick enough to be reconstructed, we systematically assigned the smallest measured diameter for that artery in the sample minus 0.1 mm. For arteries not visualized in axial source MRA images, we assigned a diameter of zero. We obtained the average of the 13 arterial segments and transformed each distribution to normal using a rank-based Blom method to be able to weight equally the 13 brain arteries with naturally occurring various diameters (Li, Yuan, Han, Gao, and Li, Reference Li, Yuan, Han, Gao and Li2014). We summed and averaged the 13 normally distributed brain arterial diameters to obtain the “global Brain Arterial Remodeling (BAR) score.” We also used regional arterial scores to relate to cognitive function localized downstream from that artery (or arteries). We used right-sided (normally distributed) arterial diameters as control for left-sided arterial diameters relating to presumed left-dominant cognitive domains.
We also obtained other MRI covariates reportedly associated with cognitive impairment/ dementia. White matter hyperintensity volumes were quantified by pixel thresholding if pixel intensity was 3.5 SDs above the mean of the fitted distribution of brain parenchyma using FLAIR MRI sequences (DeCarli et al., Reference DeCarli, Maisog, Murphy, Teichberg, Rapoport and Horwitz1992). Brain infarcts and dilated perivascular spaces were rated using a method that incorporated MRI characteristics validated in radio-pathological correlation studies from other groups to differentiate pathologically informed silent brain infarcts (PI-SBI) from small (SPVS) and large perivascular spaces (LPVS) (Gutierrez, Elkind, et al., Reference Gutierrez, Elkind, Cheung, Rundek, Sacco and Wright2015). We used WMHV, any PI-SBI, SPVS, and LPVS scores as covariates.
Longitudinal Measurements
Incident vascular events
Participants were followed prospectively after their brain MRI and baseline cognitive assessment for evaluation of vascular outcomes. Participants were contacted annually by telephone to screen for possible new events for more detailed evaluation. Case ascertainment for incident stroke was carried out independently by two vascular neurologists blinded to the baseline MRI, upon reviewing the stroke inpatient evaluation. Myocardial infarction was ascertained by a study cardiologist using the criteria from the Cardiac Arrhythmia Suppression Trial and the Lipid Research Clinics Coronary Primary Prevention Trial (Echt et al., Reference Echt, Liebson, Mitchell, Peters, Obias-Manno and Barker1991; Schaefer et al., Reference Schaefer, Lamon-Fava, Jenner, McNamara, Ordovas, Davis and Levy1994).
Cognitive tests
The same battery of cognitive tests was repeated longitudinally (after their brain MRI) in surviving participants who agreed to return for cognitive testing.
Statistical Analyses
The differences in continuous and categorical variables between participants included in these analyses compared to the overall MRI subsample were determined using student t test or chi square, respectively. We used generalized additive models to determine the linear and non-linear associations of the studied variables. The first analysis focused on the relationship between the global BAR score and the covariates used in this study using a simple model adjusting for age, sex, ethnicity and head size. We reported the linear beta estimate and its p-value under a linear assumption, and report the p-value of the chi square of the spline regression with three degrees of freedom for non-linear associations.
In the second analysis, we used each cognitive score at the time of the brain MRI as the dependent variable and the global and regional arterial diameters as the main independent variables, adjusting progressively for the reported covariates. For regional brain arterial diameters, we averaged the normalized diameter of the ACA plus MCA and the PCA plus the Pcomm on each side to represent all the flow to the supratentorial brain. We used eigenfactor and variance inflation to evaluate for collinearity among regional brain arterial diameters.
In the third analysis, we used longitudinal cognitive change after MRI as the dependent variable based on the regression-based method described here briefly. We used the mean and standard deviation from visit 1 to Z-transform the raw scores of the same neuropsychological tests from visit 2, and calculated the domain Z-score by averaging the standardized test scores of the domain. For each domain, we regressed domain Z-score at visit 2 on the domain Z-score at visit 1, age at visit 1, education (in years) and time between visit 1 and visit 2, and defined the cognitive change by the standardized residuals in the regression models. This approach was previously described by several authors (Duff and Ramezani, Reference Duff and Ramezani2015; Duff, Schoenberg, Mold, Scott, and Adams, Reference Duff, Schoenberg, Mold, Scott and Adams2011; McSweeny, Naugle, Chelune, & Lüders, Reference McSweeny, Naugle, Chelune and Lüders1993).
A global Z-score for cognitive decline was then obtained by averaging the four domain Z-scores. Because some NOMAS participants developed vascular events between visit 1 and visit 2, we also adjusted for incident stroke and myocardial infarction. Cox proportional hazards models were used to assess competing risks by determining whether participants who did not return for cognitive testing had a higher hazard ratio (HR) for vascular events.
A p value ≤.05 was used for statistical significance. The analyses were carried out with SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
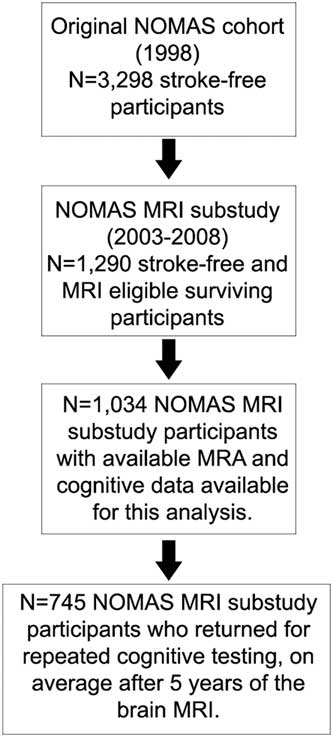
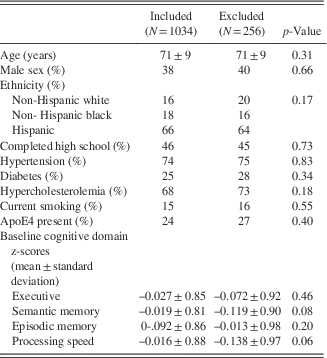
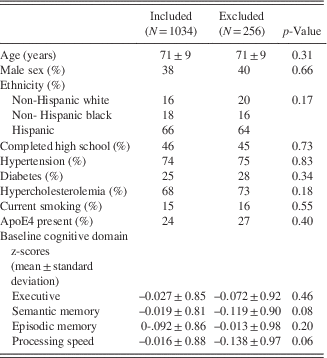
Among the complete NOMAS MRI subsample (N=1290), there were 1034 participants with MRA and cognitive data available for analyses (Figure 1). There were no significant differences in any of the variables studied (Table 1).
Fig. 1 Flow chart describes the selection of the NOMAS sample studied in this analysis.
Table 1 Demographics of the subsample of the NOMAS MRI substudy included in this analysis compared to the excluded sample
Global Brain Arterial Remodeling Score in Relation to Covariates
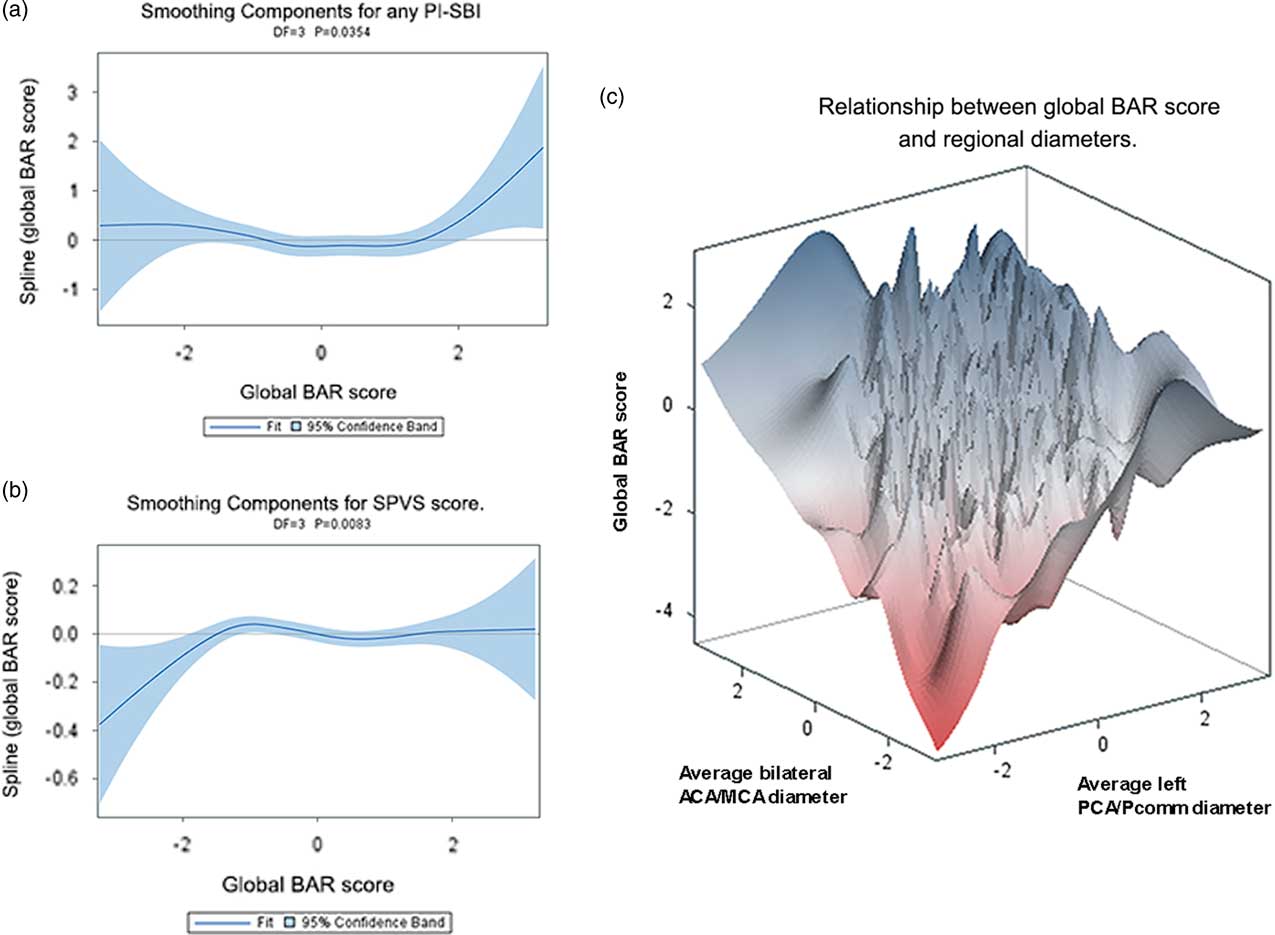
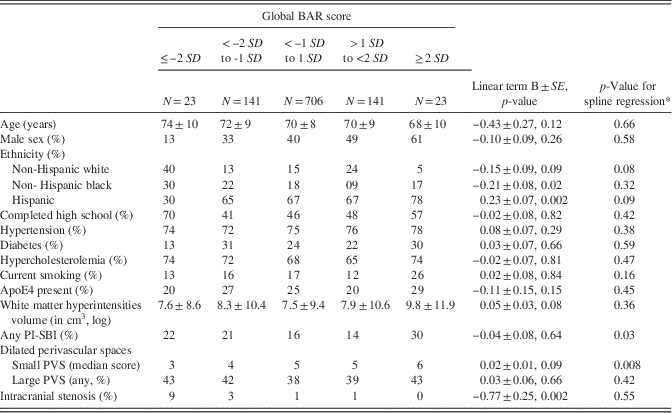
In a model adjusted for age, Hispanics had a higher global BAR score compared with non-Hispanics (white or black), and Non-Hispanic blacks had lower global BAR score compared with Hispanics and non-Hispanic whites (Table 2). Participants with the smallest and the largest global BAR score had higher prevalence of any PI-SBI, suggestive of a parabolic relationship (Figure 2a). The global BAR score was related exponentially to SPVS score, so that those with lower global BAR score had less SPVS compared with participants with average and large global BAR scores (Figure 2b).
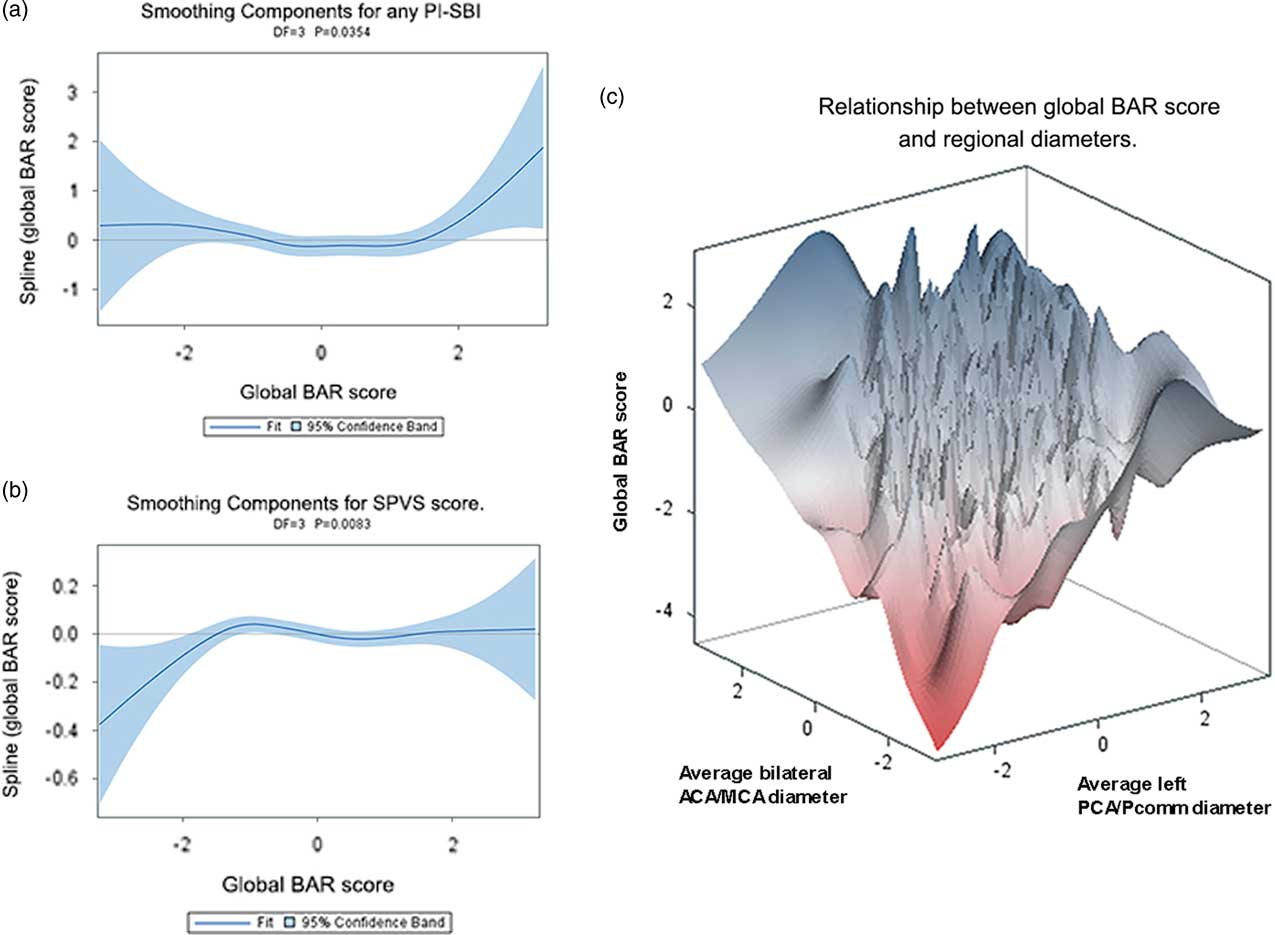
Fig. 2 A: Plot between the presence of any PI-SBI and the global BAR score, showing a U-shaped relationship with highest prevalence of PI-SBI among those with the largest scores. B: Non-linear relationship between SPVS score with the global BAR score. In C, we related the global BAR score to the average bilateral ACA/MCA and the average left PCA/Pcomm diameters as graphical evidence of heterogeneous relationships between anterior and posterior circulation.
Table 2 Relationship between global BAR score and covariates used in this study (N=1034)
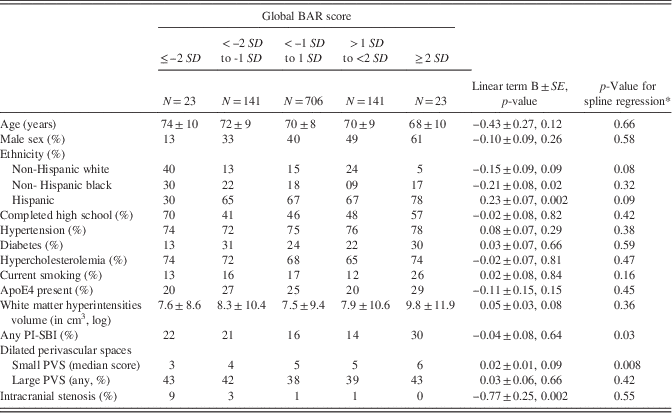
Note. All estimates were adjusted for sex, age, ethnicity, and head size.
*p-Value is calculated as a chi squared test with df=3.
Plotting the global BAR score against the bilateral ACA/MCA mean diameter and the left PCA/Pcomm mean diameter demonstrated that some participants scored high marks in all three scores, while others had either a small left PCA/Pcomm mean diameter and a large bilateral ACA/MCA mean diameter, or vice versa (Figure 2c). Consequently, we used all four regional brain arterial diameters together in subsequent models. The association between radiographically defined arterial stenosis with a small BAR score suggests that atherosclerosis may be the underlying pathology in arteries with small luminal diameters.
Cross-sectional Relationship Between BAR Scores and Cognition at the Time of the Brain MRI
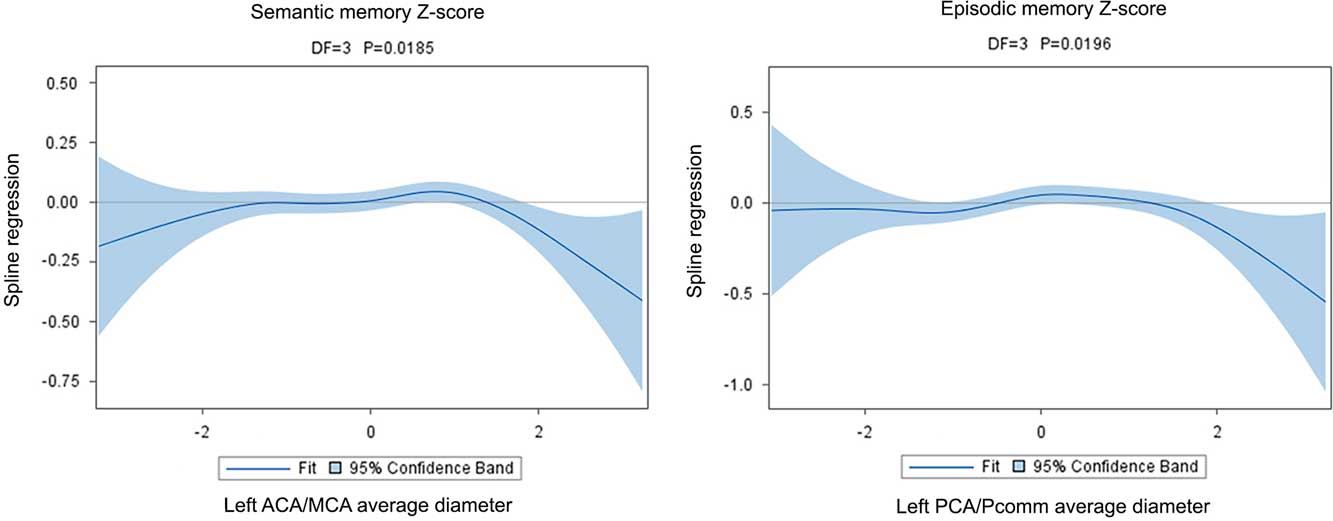
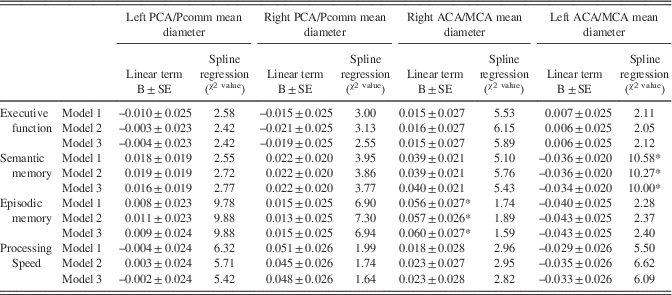
There were no associations between the global BAR score and cognitive performance at the time of the brain MRI (Table 3). Regional brain arterial diameters were associated with cognitive performance, however (Table 4; Figure 3).
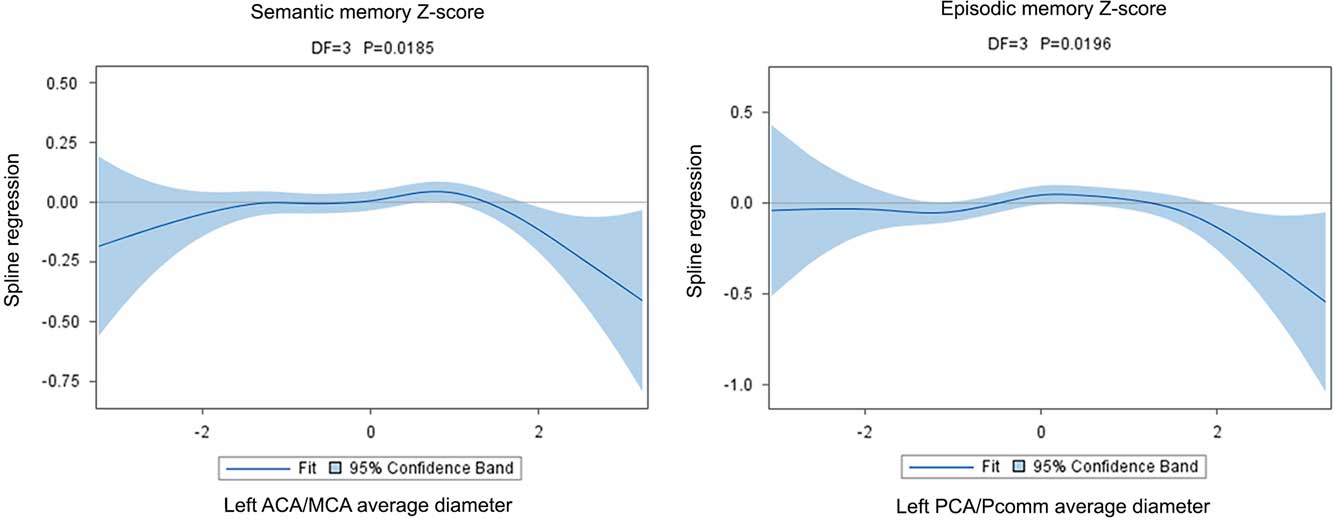
Fig. 3 The graphical display of the regression of the spline confirms the non-linear association between cognition and brain arterial diameters. Although in both instances there appears to be a U-shaped relationship between Z-scores and brain arterial diameters, the point estimate for the regression is more clearly different than zero for participants with the largest brain arterial diameters.
Table 3 Relationship between cognitive performance and global brain average arterial diameter
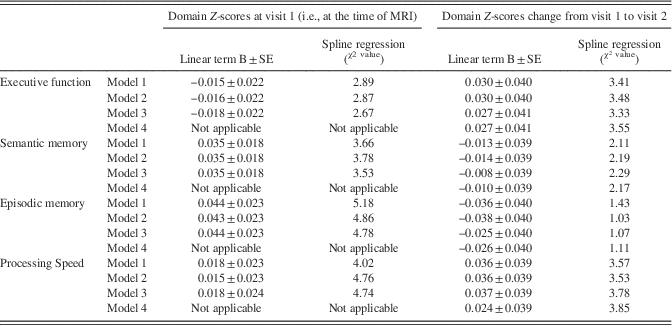
Note. BAR scores and left middle cerebral diameter distributions were normalized.
Models: Model 1: Adjusted for age, sex, ethnicity, head size, ApoE4, years of education, follow-up time, and IQ (crystallized intelligence). Model 2: Model 1 plus hypertension, diabetes, dyslipidemia, active smoking. Model 3: Model 2 plus white matter hyperintensities volume, any pathologically informed silent brain infarct, and small and large perivascular spaces and intracranial stenosis. Model 4: Model 3 plus Incident vascular event (myocardial infarction, any stroke [ischemic or hemorrhagic]).
The association between the left ACA/MCA mean diameter with semantic memory Z-scores was U-shaped (χ2=10.00; DF=3; p=.019). Although graphically both participants with the largest or smallest diameters had lower Z-scores than those with more average diameters, it was those with the largest diameters that had more consistently a lower score (Figure 3). The association was independent of the covariates used in this analysis and reported in Table 4. There were no other significant associations between the other three regional brain arterial diameters with semantic memory.
Table 4 Cross-sectional relationship between cognitive performance at visit 1 (by domain) with regional normalized brain arterial diameters
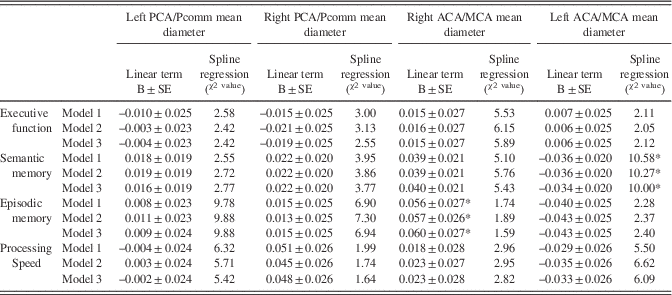
Note. BAR scores and left middle cerebral diameter distributions were normalized. Models: Model 1: Adjusted for age, sex, ethnicity, head size, ApoE4, years of education, and IQ (crystallized intelligence). Model 2: Model 1 plus hypertension, diabetes, dyslipidemia, active smoking. Model 3: Model 2 plus white matter hyperintensities volume, any pathologically informed silent brain infarct, and small and large perivascular spaces and intracranial stenosis.
*p-Value ≤0.05.
Similarly, the relationship between the left PCA/Pcomm mean diameter with episodic memory Z-scores was non-linear (χ2=9.88; DF=3; p=.020), with participants with the largest left PCA/Pcomm mean diameter performing poorer than the rest of participants (Figure 3). The association was independent of the covariates used in this study. In this same model, the right ACA/MCA mean diameter associated linearly with episodic memory (B=0.60±0.27; p=0.03).
There were no associations between executive function and processing speed with any of the four regional brain arterial diameters at the time of the brain MRI.
Brain Arterial Diameters at Baseline Were Associated With Cognitive Change During Follow-up
There were 745 participants who had repeated cognitive testing, on average 5.0±0.4 years (range, 4.4–8.3 years) after their MRI, with a total of 3837 person-years follow-up. Participants who did not return for repeated cognitive testing more often had a global BAR score in either tail of the normal distribution (χ=7.94; DF=3; p=.047), and had a higher risk of death (HR 3.95; 95% CI [3.03, 5.14]), vascular death (HR 3.62; 95% CI [2.36, 5.54]), myocardial infarction (HR 3.09; 95% CI [1.65, 5.78]), and any stroke (HR 3.86; 95% CI [2.01, 7.39]) compared to those who did come back for cognitive testing.
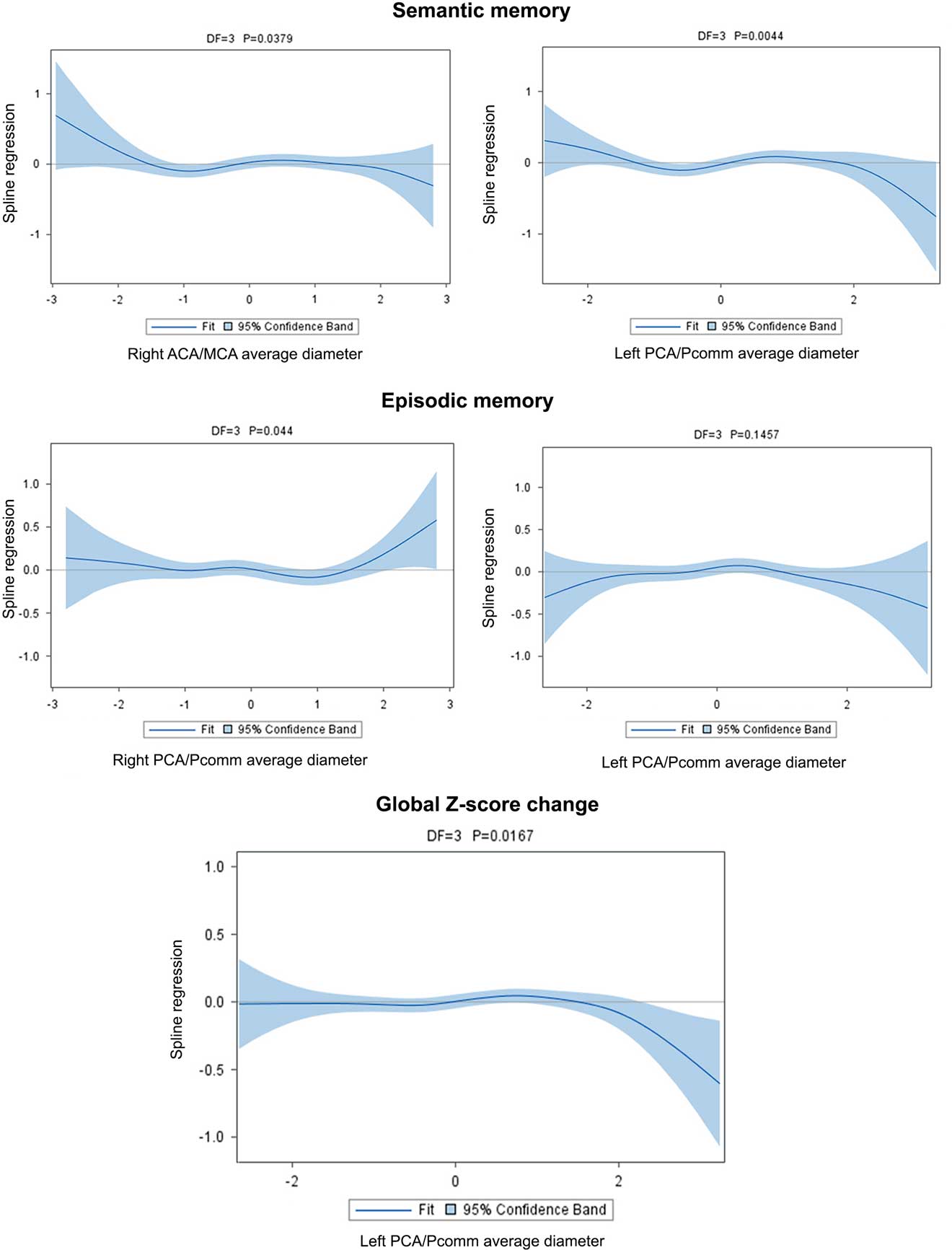
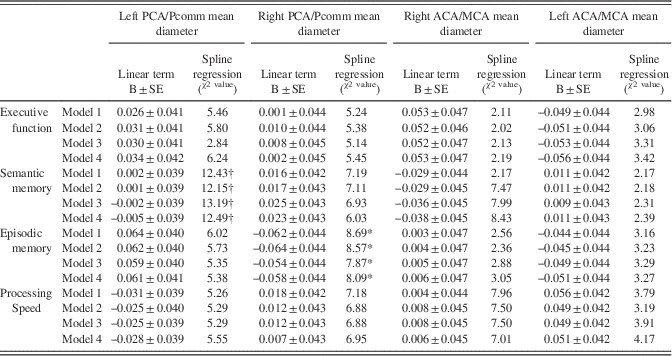
The association between semantic memory with the left PCA/Pcomm mean diameter (χ2=13.09; DF=3; p=0.004) and with the right MCA/ACA mean diameter (χ2=8.43; DF=3; p=0.03) was non-linear, with those with the largest left PCA/Pcomm mean diameter showing greater decline than the rest of participants (Figure 4; Table 5).
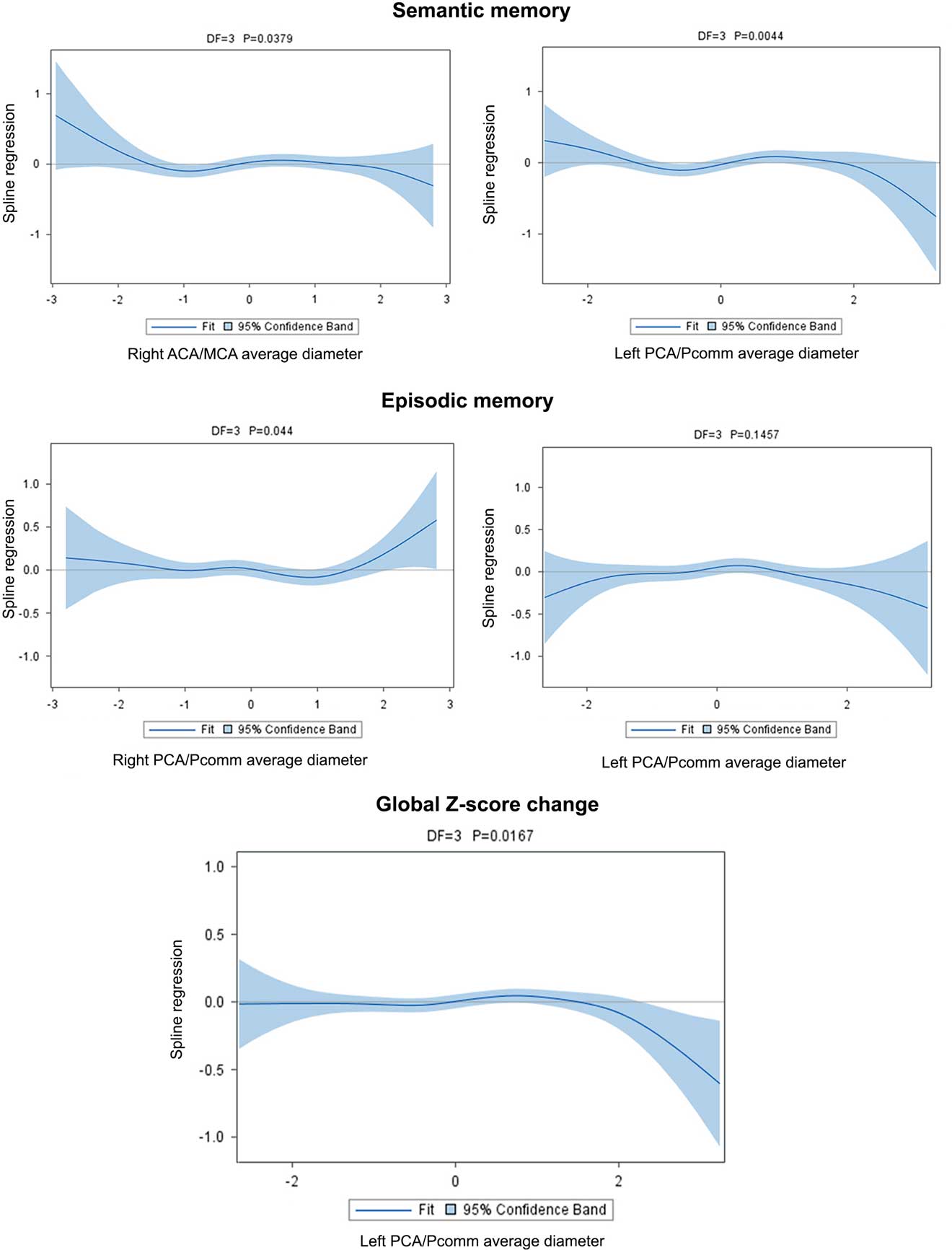
Fig. 4 Brain arterial diameters at visit 1 (simultaneously to the brain MRI) were associated with cognitive change over time. For semantic memory, participants with the largest diameters in the right ACA/MCA mean diameter or the left PCA/Pcomm mean diameter had the greatest decline, while those with smaller arteries in these same segments had less decline. For episodic memory, those with larger right PCA/Pcomm mean diameter had less decline, while those with larger left PCA/Pcomm mean diameter had a non-significant trend toward more decline. Overall, those with larger left PCA/Pcomm mean diameter had the greater cognitive decline over time.
Table 5 Cognitive performance change from visit 1 to visit 2 in relationship to regional brain arterial diameters
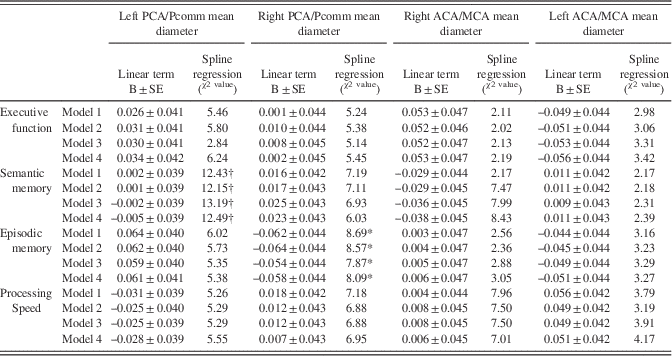
Note. BAR scores and left middle cerebral diameter distributions were normalized. Models: Model 1: Adjusted for sex, ethnicity, head size, ApoE4, and IQ (crystallized intelligence). Model 2: Model 1 plus hypertension, diabetes, dyslipidemia, active smoking. Model 3: Model 2 plus white matter hyperintensities volume, any pathologically-informed silent brain infarct, and small and large perivascular spaces and intracranial stenosis. Model 4: Model 3 plus incident vascular event (myocardial infarction, ischemic or hemorrhagic stroke).
* p-Value ≤0.05–0.01.
† p-Value <0.01.
The association between right PCA/Pcomm mean diameter and episodic memory decline was non-linear (χ2=8.09; DF=3; p=.04), with those with the largest right PCA/Pcomm mean diameter showing less decline than the rest of participants (Figure 4). The association between left PCA/Pcomm mean diameter and episodic memory decline had a non-linear trend (χ2=5.38; DF=3; p=.14), with those with the largest left PCA/Pcomm mean diameter showing greater decline than the rest of participants (Figure 4).
Using the all-domain Z-score for cognitive decline as the outcome disclosed a non-linear association with left PCA/Pcomm mean diameter (χ2=10.23; DF=3; p=.01), with participants with the largest left PCA/Pcomm mean diameters showing the greatest all-domain cognitive decline (Figure 4).
In model 4 (Table 5), incident stroke was associated with lower processing speed Z-scores (B=−0.34±0.14; p=.01) but not with executive function (B=0.10±0.15; p=.52), semantic memory (B=−0.17±0.14; p=.22), or episodic memory (B=−0.08±0.15; p=.58), independent of brain arterial diameters.
DISCUSSION
In this study, we report cross-sectional and longitudinal associations between brain arterial diameters and cognitive performance. We report a cross-sectional non-linear association between the left MCA/ACA mean diameter and semantic memory (language-related tasks) and between the left PCA/Pcomm mean diameter and episodic memory. In these cross-sectional models, participants with the largest diameters had lower scores compared with the rest of the participants. Longitudinally, surviving participants with the largest left PCA/Pcomm mean diameter had greater decline in all-domain Z-scores, as well as in semantic memory and possibly episodic memory compared with participants with more average diameters. These associations were independent of vascular events during follow up. These findings suggest that both extremes of arterial diameters may be detrimental to parenchymal homeostasis and affect cognitive performance, especially larger left-sided brain arterial diameters over predominantly left-sided cognitive tasks. Together these results offer partial validation to the overarching hypothesis that brain arterial diameters may be important biomarkers of parenchymal cognitive function.
In the absence of repeated measures of brain arterial diameters, we considered possible explanations for the associations between brain arterial diameter and cognition. First, it may be postulated that modification of the brain arterial diameter precedes the parenchymal disruption. This hypothesis is supported by data showing loss of cerebral autoregulation in the setting of proximal arterial stenoses (Chmayssani, Lazar, Hirsch, and Marshall, Reference Chmayssani, Lazar, Hirsch and Marshall2009). Dilatation of arterioles is the presumed pathological correlate of loss of cerebral autoregulation, and relates to blood brain barrier disruption (Forteza et al., Reference Forteza, Echeverria, Haussen, Gutierrez, Wiley and De Gusmao2010; Ogasawara et al., Reference Ogasawara, Yukawa, Kobayashi, Mikami, Konno, Terasaki and Ogawa2003; Marmarou, Takagi, and Shulman, Reference Marmarou, Takagi and Shulman1980). Hypoperfusion to left frontal cortex due to left MCA stenosis is associated with aphasia after non-thalamic left-sided subcortical strokes (Hillis et al., Reference Hillis, Barker, Wityk, Aldrich, Restrepo, Breese and Work2004), and similarly left posterior cerebral artery small diameters relate to episodic memory (Szabo et al., Reference Szabo, Forster, Jager, Kern, Griebe, Hennerici and Gass2009).
Alternatively, small brain arterial diameters could be the result of adaptive remodeling due to loss of demand, secondary to stroke or neuronal loss or dysfunction due to, for example, a primary neurodegenerative disorder (Brown and Thore, Reference Brown and Thore2011; Kubis, Checoury, Tedgui, and Levy, Reference Kubis, Checoury, Tedgui and Levy2001). Prospective data acknowledging arterial diameters and regional atrophy and metabolic rates would help clarify the directionality of these associations.
Fewer data exist to relate large arterial diameters to parenchymal health or cognition. Under physiological circumstances, the diameter of the arteries in the Circle of Willis is directly proportional to the territory supplied and the capillary density in that territory (van der Zwan, Hillen, Tulleken, and Dujovny, Reference van der Zwan, Hillen, Tulleken and Dujovny1993). Inherited or acquired conditions that lead to either increased flow or weakness of the arterial wall may also lead to outward remodeling (Gutierrez, Reference Gutierrez2014). With aging, there is evidence that brain arteries dilate, their walls thicken concentrically, and elastin is lost, leading to stiffer arteries even in the absence of atherosclerosis (Bouissou, Emery, and Sorbara, Reference Bouissou, Emery and Sorbara1975; Gutierrez, Honig, et al., Reference Gutierrez, Honig, Elkind, Mohr, Goldman, Dwork and Marshall2016; Sorbara, Reference Sorbara1972) This form of non-atherosclerotic aging is associated with AD, brain infarcts, and large artery amyloidosis (Gutierrez, Honig, et al., Reference Gutierrez, Honig, Elkind, Mohr, Goldman, Dwork and Marshall2016).
In the presence of systemic arterial stiffness, it is possible that stiffer dilated brain arteries will transmit greater pulsatility to the brain parenchyma and lead to arteriolar remodeling and capillary flow disruption (Mitchell et al., Reference Mitchell, van Buchem, Sigurdsson, Gotal, Jonsdottir, Kjartansson and Launer2011; Ochi et al., Reference Ochi, Kohara, Tabara, Nagai, Kido, Uetani and Miki2010; Ott, Raff, Harazny, Michelson, and Schmieder, Reference Ott, Raff, Harazny, Michelson and Schmieder2013). This hypothesis is supported theoretically by the Moens-Korteweg equation of pulse wave velocity in elastic conduits, in which a drop in the elastic modulus would lead to higher pulse-wave velocities even in the presence of a dilated artery (Gosling and Budge, Reference Gosling and Budge2003). Clinical data also support a functional link between systemic arterial stiffness and parenchymal disruption, as suggested by the association between higher pulse wave velocities with higher brain amyloid burden, and with memory deficits typical of AD (Hughes et al., Reference Hughes, Kuller, Barinas-Mitchell, Mackey, McDade, Klunk and Lopez2013; Mitchell et al., Reference Mitchell, van Buchem, Sigurdsson, Gotal, Jonsdottir, Kjartansson and Launer2011; Tsao et al., Reference Tsao, Seshadri, Beiser, Westwood, Decarli, Au and Mitchell2013). Alternatively, chronic hypotrophic arteriolar remodeling leading to dilatation may be the initial trigger, as in pregnancy, leading to lower flow resistance and increased flow (Zeeman, Hatab, and Twickler, Reference Zeeman, Hatab and Twickler2003).
Our study has several strengths. The data derives from unselected participants representing a multiethnic urban community in New York. The measurements of brain arterial diameter, white matter hyperintensities, PI-SBI, and perivascular spaces was carried out systematically, blinded to clinical outcomes, and with reliable methods (Gutierrez et al., Reference Gutierrez, Bagci, Gardener, Rundek, Ekind, Alperin and Wright2014; Gutierrez, Elkind, et al., Reference Gutierrez, Elkind, Cheung, Rundek, Sacco and Wright2015). Despite a survival bias for those who returned for repeat cognitive assessments, we were able to demonstrate associations between brain arterial diameters and cognitive performance, and we hope that as brain arterial diameters are incorporated in ongoing epidemiological studies, collaborative effort can overcome the limitation of underpowered comparisons.
We recognize that performance on cognitive tests that represent particular domains do not localize only to discrete regions of the brain, as we suggested in our lateralized analyses, and are part of wider networks that also involve other regions (e.g., language tasks variably involve the right hemisphere) (Baldo et al., Reference Baldo, Arévalo, Patterson and Dronkers2013; Meinzer et al., Reference Meinzer, Wilser, Flaisch, Eulitz, Rockstroh, Conway and Crosson2009; Tomaszewki Farias et al., Reference Tomaszewki Farias, Harrington, Broomand and Seyal2005). The fact that there were positive associations between arterial diameters supplying a brain region and cognitive performance despite non-differential error supports the dominant role of the left hemisphere in semantic and episodic memory reported by others (Knecht et al., Reference Knecht, Dräger, Deppe, Bobe, Lohmann, Flöel and Henningsen2000; Meinzer et al., Reference Meinzer, Wilser, Flaisch, Eulitz, Rockstroh, Conway and Crosson2009; Szabo et al., Reference Szabo, Forster, Jager, Kern, Griebe, Hennerici and Gass2009). We did not adjust for multiple comparisons because linking specific arteries with cognitive domains was in itself a single hypothesis, in which we used the other cognitive domains and diameters as controls.
Finally, we do not have details about vascular risk factor control over time, which is a limitation. In is worth pointing that adjustment for incident vascular events did not totally eclipse the association between brain arterial diameters and cognitive change and that the progressive adjustment of the prevalence of vascular risk factor modified little the strength of the associations. This observations suggest to us that the associations found in this study may be independent of vascular risk factor control. In other words, either very small or very large brain arterial diameters may represent end-organ (arterial) damage, either due to vascular risk factor and/or genetic influences, and their present is more directly related to the brain parenchyma and its function that the factor that lead to these phenotypes.
In summary, brain arterial diameters are radiological biomarkers of cerebrovascular health, and they are intimately related to the functionality of the brain parenchyma they perfuse. Extreme forms of arterial remodeling manifested with smaller or larger arterial diameters (compared with the population average) may have downstream effects on parenchymal integrity and function, which manifests as poorer cognitive performance in the domain localized in that territory. Identifying the mechanisms and the directionality of such interactions may increase the understanding of the vascular contribution to cognitive impairment and dementia.
ACKNOWLEDGMENTS
We thank Dr. Kevin Duff from providing clarification regarding the regression-based methods used here to describe cognitive change.
Authors contributions: Jose Gutierrez: Study design, data analyses and interpretation, manuscript draft. Erin Kulick, MPH: Data analyses, revising manuscript for intellectual content. Yeseon Park Moon: Data analyses, revising manuscript for intellectual content. Chuanhui Dong: Data analyses, data interpretation, revising manuscript for intellectual content. Ken Cheung: Data analyses, data interpretation, revising manuscript for intellectual content. Bagci Ahmet: data acquisition, revising manuscript for intellectual content. Yaakov Stern: Data interpretation, revising manuscript for intellectual content. Noam Alperin: data acquisition, revising manuscript for intellectual content. Tatjana Rundek: Data interpretation, revising manuscript for intellectual content. Ralph L Sacco: Study design, data interpretation, revising manuscript for intellectual content, obtaining funding. Clinton B Wright: Study design, data interpretation, revising manuscript for intellectual content. Mitchell SV Elkind: Study design, data interpretation, revising manuscript for intellectual content, obtaining funding. Sources of funding: This work was supported by the National Institutes of Health/ NINDS (5R01NS029993). Disclosures: Jose Gutierrez, MD, MPH : Dr Gutierrez receives compensation for providing consultative services for Pfizer, Prophase and medical litigation. Erin Kulick, MPH: None. Yeseon Park Moon: None. Chuanhi Dong, PhD: None. Ken Cheung, PhD: None. Bagci Ahmet, PhD: None. Yaakov Stern, PhD: None. Noam Alperin, PhD: Shareholder in Alperin Noninvasive Diagnostics, Inc. Tatjana Rundek, MD, PhD: None. Ralph L Sacco, MD, MS: None. Clinton B Wright, MD, MS: Dr. Wright receives royalties from UpToDate.com for two chapters on vascular dementia. Mitchell S.V. Elkind, MD, MS: Dr. Elkind receives compensation for providing consultative services for Biotelemetry/Cardionet, BMS-Pfizer Partnership, Boehringer-Ingelheim, and Sanofi-Regeneron Partnership; receives compensation for serving as an expert witness in litigation for BMS-Sanofi (Plavix), Merck/Organon (Nuvaring), and Hi-Tech Pharmaceuticals (dimethylamylamine); serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association; and receives royalties from UpToDate for chapters related to stroke.