INTRODUCTION
Reading is a complex activity that requires our eyes to move in such a way as to allow for the extraction of spatially distributed visual information and the comprehension of written language (Findlay & Gilchrist, Reference Findlay and Gilchrist2003). The eye movements follow a typical scan path across the text, in the direction from left-to-right and from top-to-bottom for Western cultures (Schuett, Heywood, Kentridge, & Zihl, Reference Schuett, Heywood, Kentridge and Zihl2008). The eye movements during reading task can be described in terms of fixations and saccades. Fixations are movements made when the eye is relatively still and focused on a particular target. They typically last between 200 and 300 ms, but can range from 100 to over 500 ms (Eden, Stein, Wood, & Wood, Reference Eden, Stein, Wood and Wood1994). The first fixation on a line is typically 5–7 letter spaces from the beginning of a sentence, while the last fixation occurs approximately 5–7 letter spaces from the end of a sentence. The first fixation also tends to be longer in duration, while the last fixation of a line tends to be the shortest. Saccades can be defined as rapid movements that allow the eyes to move from one fixation point to another while scanning and processing the information between fixation points (Eden et al., Reference Eden, Stein, Wood and Wood1994). Saccades can range in length, but are typically 7–9 letter spaces in silent reading (Rayner, Reference Rayner1998). Western readers tend to make their first fixation between the beginning and the middle of the word (Li, Liu, & Rayner, Reference Li, Liu and Rayner2011). They are able to identify words in the area closest to the fixation point (this area is called the fovea); beyond that region (called parafovea), they are able to obtain more information such as the initial letters of words, letter features, and word length (Bellocchi, Muneaux, Bastien-Toniazzo, & Ducrot, Reference Bellocchi, Muneaux, Bastien-Toniazzo and Ducrot2013).
In the literature regarding eye movements, a large number of studies agree on the link between attention and oculomotor control during reading. In particular, attention seems to affect saccadic programming, that is, the position where the eyes land in a word. Moreover, both eye fixations and saccadic movements are involved in attention and parafoveal/peripheral vision. Kennedy and Pynte (Reference Kennedy and Pynte2005) and Rayner (Reference Rayner1998) showed that attention modulates the size of the region that is attended, that is, attention is not only restricted to the currently fixated word, but can also be extended to the word located in the parafovea (Rayner, Reference Rayner1998). Furthermore, attention and memory cannot operate without each other, in particular, working memory (WM) is involved in the reading process (Lonigan, Reference Lonigan, Lerner, Liben and Müeller2015). WM refers to the active, top-down manipulation of information held in short-term memory (Baddeley, Reference Baddeley2007), and includes interrelated functions, such as dual-processing, supervisory attentional control, updating, and reordering (Nee et al., Reference Nee, Brown, Askren, Berman, Demiralp, Krawitz and Jonides2013; Wager & Smith, Reference Wager and Smith2003). It has been well established that attention and WM closely interact during encoding and manipulation phases (Fabio, Iannizzotto, Nucita, & Caprì, Reference Fabio, Iannizzotto, Nucita and Caprì2019; Fougnie, Reference Fougnie and Johansen2008; Rutman, Clapp, Chadick, & Gazzaley, Reference Rutman, Clapp, Chadick and Gazzaley2010). With reference to the retrieval phase of information, it was found that the accuracy of the retrieval is strongly related to WM capacity, and attention plays a role in the retrieval of an item due to activation of maintained information (Ecker, Lewandowsky, Oberauer, & Chee, Reference Ecker, Lewandowsky, Oberauer and Chee2010). In addition, it was demonstrated a mechanistic overlap between the processes of attention and WM. Several studies have revealed a major role of WM in the control of visual selective attention (Caprì, Gugliandolo, Iannizzotto, Nucita, & Fabio, Reference Caprì, Gugliandolo, Iannizzotto, Nucita and Fabio2019; de Fockert, Rees, Frith, & Lavie, Reference de Fockert, Rees, Frith and Lavie2001; Desimone, Reference Desimone1996), whereas others have shown that attention is a key component of WM (Awh & Jonides, Reference Awh and Jonides2001). Moreover, the literature showed cross-sectional and longitudinal associations between WM and reading performance in developmental samples (Cain, Oakhill, & Bryant, Reference Cain, Oakhill and Bryant2004; Chan et al., Reference Chan, Guo, Zou, Li, Hu and Yang2006; Fabio, Martinazzoli, & Antonietti, Reference Fabio, Martinazzoli and Antonietti2005; Mayes & Calhoun, Reference Mayes and Calhoun2006; Peng et al., Reference Peng, Barnes, Wang, Wang, Li, Swanson and Tao2018; Sarver et al., Reference Sarver, Rapport, Kofler, Scanlan, Raiker, Altro and Bolden2012; Sesma, Mahone, Levine, Eason, & Cutting, Reference Sesma, Mahone, Levine, Eason and Cutting2009; Thorell, Reference Thorell2007; Wåhlstedt, Thorell, & Bohlin, Reference Wåhlstedt, Thorell and Bohlin2009). However, the majority of this literature focused on correlational associations, but the link between WM and reading can be conveyed by third variable that was not investigated by this literature, for example, the attention.
Given that a number of studies agree on the link between attention and WM, and between attention and eye movements, an important question that could be addressed to better understand these relationships is: to what extent can an attention deficit affect eye movements and, consequently, remembering a word during the reading process? The purpose of the present study is to contribute to this question.
Subjects with Attention Deficit Hyperactivity Disorder (ADHD) can have some reading problems; they also show WM impairments and poor oculomotor control (Casal et al., Reference Casal, Esposito, Martínez, Capdevila, Puig, de la Osa and Cañete2019; Fabio & Caprì, Reference Fabio and Caprì2015, Reference Fabio and Caprì2019; Halleland, Haavik, & Lundervold, Reference Halleland, Haavik and Lundervold2012; Kofler et al., Reference Kofler, Spiegel, Soto, Irwin, Wells and Austin2019). It has been proposed that WM may explain the reading difficulties in ADHD (Friedman, Rapport, Raiker, Orban, & Eckrich, Reference Friedman, Rapport, Raiker, Orban and Eckrich2017; Kasper, Alderson, & Hudec, Reference Kasper, Alderson and Hudec2012; Martino, Caprì, Castriciano, & Fabio, Reference Martino, Caprì, Castriciano and Fabio2017), but it seems that WM training does not improve reading performance for both children with ADHD and typically developing subjects (TD) (Fabio, Caprì, Iannizzotto, Nucita, & Mohammadhasani, Reference Fabio, Caprì, Iannizzotto, Nucita and Mohammadhasani2019; Mohammadhasani, Fardanesh, Hatami, Mozayani, & Fabio, Reference Mohammadhasani, Fardanesh, Hatami, Mozayani and Fabio2018; Rapport, Orban, Kofler, & Friedman, Reference Rapport, Orban, Kofler and Friedman2013). Although there is a consensus that an impairment of WM exists independently of language or learning disorders and independently of general intelligence in children with ADHD (Alloway & Gathercole, Reference Alloway and Gathercole2006; Antonietti, Monnier, Gatti, & Fabio, Reference Antonietti, Monnier, Gatti and Fabio2010; Fabio & Antonietti, Reference Fabio and Antonietti2012; Fabio & Caprì, Reference Fabio and Caprì2017; Fabio & Urso, Reference Fabio and Urso2014; Fabio et al., Reference Fabio, Caprì, Mohammadhasani, Gangemi, Gagliano and Martino2018), results related to WM deficits are inconsistent (Fabio, Reference Fabio2017; Martinussen & Tannock, Reference Martinussen and Tannock2006; Pazvantoğlu et al., Reference Pazvantoğlu, Aker, Karabekiroğlu, Akbaş, Sarısoy, Baykal and Şahin2012). This is possibly due to the heterogeneity of ADHD, and to the tasks used in studies (Fabio, Caprì, Campana, & Buzzai, Reference Fabio, Caprì, Campana and Buzzai2018; Kuntsi, Wood, Van Ded Meere, & Asherson, Reference Kuntsi, Wood, Van Ded Meere and Asherson2009; Maehler & Schuchardt, Reference Maehler and Schuchardt2016).
At the level of saccadic eye movement behavior, some studies showed that both children and adult with ADHD displayed more atypical eye movement compared with control subjects, (Deans O’Laughlin, Brubaker, Gay, & Krug, Reference Deans, O’Laughlin, Brubaker, Gay and Krug2010; Lee, Lee, Chang, & Kwa, Reference Lee, Lee, Chang and Kwa2015). Sun, Wang, Han and Zhu (Reference Sun, Wang, Han and Zhu2003) demonstrated that children with ADHD had a lower number of eye fixations, longer mean eye scanning length and lower responsive search scores than the healthy control subjects. Munoz, Armstrong, Hampton and Moore (Reference Munoz, Armstrong, Hampton and Moore2003) found that children with ADHD had unique patterns of eye movement in visual tracking tasks. In particular, they displayed longer reaction times, more variability, and slower saccades in the prosaccade task compared with participants in the control group. Also, research on scanning and ADHD has shown that subjects with ADHD have deficits in response inhibition and as a result are less able to voluntarily inhibit unwanted saccades as well as a decreased ability to govern the amount and duration of fixations they make (Karatekin & Asarnow, Reference Karatekin and Asarnow1999; Nada-Raja, et al., Reference Nada-Raja, Langley, McGee, Williams, Begg and Reeder1997; Ross, Harris, Olincy, & Radant, Reference Ross, Harris, Olincy and Radant2000). Moreover, individuals who have ADHD made significantly more large saccades during a visual fixation task than the control group (Gould, Bastain, Israel, Hommer, & Castellanos, Reference Gould, Bastain, Israel, Hommer and Castellanos2001; Michaelis, McConnell, & Smither, Reference Michaelis, McConnell and Smither2012) and their rate of microsaccades is slower than control subjects (Engbert & Kliegl, Reference Engbert and Kliegl2003; Friedman, Rapport, Raiker, Orban, & Eckrich, Reference Friedman, Rapport, Raiker, Orban and Eckrich2017).
With references to ADHD subtypes characteristics in visual working memory (VWM) processes, neuropsychological data are quite consistent. The studies of Geurts, Verte, Oosterlaan, Roeyers and Sergeant (Reference Geurts, Verte, Oosterlaan, Roeyers and Sergeant2005) and Hinshaw, Carte, Sami, Treuting and Zupan (Reference Hinshaw, Carte, Sami, Treuting and Zupan2002) found no or limited differences between combined ADHD subtype (ADHD-C) and ADHD predominantly inattentive (ADHD-I) on a series of executive functioning measures, among which there were VWM tasks. In another study, Liebel and Nelson (Reference Liebel and Nelson2017) compared three groups: ADHD-I, ADHD-C, and normal controls matched by age and IQ on VWM, planning, cognitive flexibility, and verbal fluency. Executive function measurements, including VWM, did not show significant differences between the ADHD-C and ADHD-I subtypes. Also, Gallego-Martínez, García-Sevilla and Fenollar-Cortés (Reference Gallego-Martínez, García-Sevilla and Fenollar-Cortés2018), examining the VWM, found no differences.
However, all these previous studies, examining visual scanning or WM in ADHD, have used eye movement paradigms that require tracking a visual stimulus rather than tasks that require reading skills. Therefore, in the present study, we focused on the precise dynamic of the visual scan on word stimuli presented with an eye-tracker to obtain more complete results about the relationship between WM, attention, and eye movement control in ADHD.
More precisely, there were three specific aims of this study: (1) to compare visual patterns of word stimuli between children with ADHD and TD subjects, during a visual task on word stimuli; (2) to examine the WM accuracy of the word stimuli; and (3) to compare the dynamic of visual scan path in both groups. To achieve these goals, we used eye-tracking technology. This is a valid methodology for examining viewing patterns during word processing and their relation with WM. Using this approach, some research has demonstrated that eye movements were related to visuo-attentional processes during reading both in TD subjects and children with ADHD or reading disorders (Deans et al., Reference Deans, O’Laughlin, Brubaker, Gay and Krug2010; Rayner, Reference Rayner1998).
As previously stated, we expected differences in eye movements between the ADHD and the TD groups. Precisely, the TD group should show a typical scan path across the visual text, whereas the ADHD group could present an atypical scan path. To highlight the scan path, two measures were used: the ordered direction of reading (ODR) and the entropy index. The first is useful to analyze the ordered scan path. The second is useful to measure the disorder or the variability of the replies. We also expected differences in WM accuracy to be attributable not just to general attention deficit (Booth et al., Reference Booth, Burman, Meyer, Lei, Trommer, Davenport and Mesulam2005; Rubia, Smith, Brammer, Toone, & Taylor, Reference Rubia, Smith, Brammer, Toone and Taylor2005; Valera, Faraone, Biederman, Poldrack, & Seidman, Reference Valera, Faraone, Biederman, Poldrack and Seidman2005) but to atypical visual patterns in the ADHD group, compared with the TD group.
METHOD
Participants
The participants in this study were selected from a sample of 1120 children (308 females and 812 males) attending public primary schools in Sicily, a region of Southern Italy. Students ranged in age from 9 to11 years (M = 8.5, SD = 4.52) and were Italian. For all participants, their teachers completed both the Italian version of the ADHD Rating Scale for Teachers (SDAI; Capodieci, Reference Capodieci2017; Marzocchi & Cornoldi, Reference Marzocchi and Cornoldi2000; Marzocchi, Re, & Cornoldi, Reference Marzocchi, Re and Cornoldi2010) and the Disruptive Behaviour Disorder Rating Scale (SCOD; Marzocchi et al., Reference Marzocchi, Oosterlaan, De Meo, Di Pietro, Pezzica, Cavolina and Zuddas2001) aimed at assessing the possible presence of, respectively, ADHD and/or learning disabilities (LD).
ADHD group
The first screening for ADHD diagnosis was based on SDAI scores. The SDAI is widely used in Italy and has been validated and standardized for the Italian population (Bianchini et al., Reference Bianchini, Bianchini, Postorino, Grasso, Santoro, Migliore and Mazzone2013; Capodieci, Lachinam, & Cornoldi, Reference Capodieci, Lachinam and Cornoldi2018; Re, Mirandola, Esposito, & Capodieci, Reference Re, Mirandola, Esposito and Capodieci2014), showing an interrater reliability of .80 (Inattentive subscale) and .74 (Hyperactive/Impulsive), optimal discriminatory power, and concurrent validity, obtained by correlating the scale with others (r > .95; Marzocchi, Re, & Cornoldi, Reference Marzocchi, Re and Cornoldi2010). The test–retest reliability is .83 and .81 for Inattentive and Hyperactive/Impulsive, respectively (Marzocchi & Cornoldi, Reference Marzocchi and Cornoldi2000). Eighteen items compose SDAI, corresponding to the symptom domain of ADHD as described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; American Psychiatric Association, 2013). Teachers were asked to monitor a child’s behavior for about 2 weeks and then report the frequency of the types of symptomatic behavior described in each item, through a Likert scale from 0 (problematic behavior never present) to 3 scores (very often present). Two scores can be obtained: a measure of distractibility or inattention (I) and a measure of hyperactivity (H). In this experiment, inclusion in the ADHD condition was based on cutoff scores (a score of 14 or higher) in both subscales (I and H) and on a clinical assessment carried out by a specialized psychologist. The presence of other disorders was excluded by normal SCOD scores (scores as 0) and by the clinical interview. This interview involved a series of open-ended questions and structured, science-based items, including accessing services (school), general medical situation, daily functioning, and ADHD-related impairment (Croskerry, Reference Croskerry2009; Ely, Graber, & Croskerry, Reference Ely, Graber and Croskerry2011; Frick, Barry, & Kamphaus, Reference Frick, Barry and Kamphaus2010). In addition, the interview element of the assessment process allows for behavioral observations that may be useful in describing the child, as well as discerning the convergence with known diagnoses. The interview lasted approximately 1 hr. Participants were free to respond in their own words. Prompts were used where necessary to elicit more detailed responses. The SCOD is composed of 13 items. Eight items provide a disruptive behaviour disorder index and five items provide an LP (learning problems) index in both mathematical and linguistic areas. Items are rated on a 4-point scale, where never or rarely is scored as 0, sometimes as 1, often as 2, and very often as 3. The total score on the disruptive behaviour disorder subscale ranges from 0 to 24 and the cutoff criterion is 12. The total score on the LP subscale ranges from 0 to 15 and the cutoff score is 8. Marzocchi et al. (Reference Marzocchi, Oosterlaan, De Meo, Di Pietro, Pezzica, Cavolina and Zuddas2001) reported a 1-month test–retest reliability of .92 for the disruptive behaviour disorder subscale and of .89 for the LP subscales. Internal reliability for the two subscales was .88 and .86, respectively.
Sixty-two participants met cutoff criteria for ADHD based on the questionnaire, and 49 of those also had a clinical diagnosis as confirmed by a specialised psychologist and had SCOD scores as 0. No child had a history of brain damage, epilepsy, psychosis, or anxiety disorders. Consequently, the final ADHD group included 49 children (Table 1).
Table 1. Demographic characteristics of the three groups participating in the experiment

TD group
The sample of the initial 1120 children who obtained SDAI and SCOD scores in the normal range (scores from 0 to 9), who were not included in any clinical group, and of children not diagnosed by the school psychologists with behavioral, emotional, and/or relational problems constituted the basis to form the control group, and a set of children was therefore randomly selected. Gender and age were considered so as to find students who could constitute a group whose male/female ratio and whose mean age approximately matched the male/female ratios and the mean ages of the clinical groups. Among TD children who were selected based on gender and age criteria, only children who also obtained SDAI and SCOD scores as 0 and had no clinical disorders (as diagnosed by the school psychologist), were included in the final TD group.
The final sample included 49 children with ADHD – 20 subtype inattentive (ADHD-I group) and 29 with subtype combined (ADHD-C group) – and 32 TD children (TD group) (total 81 children). We excluded seven children who met the criteria for ADHD-H criteria because the size of this group was small (Table 1). Informed consent was obtained from parents of the participants included in the study.
Instruments
Eye-tracking
An eye-tracker Tobii Series-I was used to record the subject’s visual scanning. This device records ocular movements such as the location and duration of ocular fixations (pause of eye movement on an object of interest) and saccadic movements (rapid movements between fixations). The participant was positioned at a distance of about 30 cm from the screen and the direction of the gaze was determined according to the Pupil Centre/Corneal Reflection Method of low-intensity infrared light. Passive gaze tracing (LC Technologies, Sao Paulo, Brazil) software was used to generate gaze data during visuals scanning. In addition, this device allows to to define the areas of interest (AOI) within the words chosen for the statistical analysis of eye tracking. An AOI cluster refers to selected specific areas that will be used for recalling details of the images.
The Word Memory Test (Green, Allen, & Astner, Reference Green, Allen and Astner1996; Green, Lees-Haley, & Allen, Reference Green, Lees-Haley and Allen2002)
The Word Memory Test (WMT) was implemented in the eye-tacker Tobii Series-I. The WMT is an immediate free recall test (FR) and consists of measures of verbal WM. The task involves learning 11 lists of 16 words in an array of 4 × 4, presented through 11 slides of the eye-tracker. The subject is requested to repeat the lists of words after each presentation.
Measures
To analyze the ocular movements, fixation length (FL) parameter was computed. It is the amount of time (seconds) spent by the subject when looking at the target. Fixations were extracted using a threshold of 100 ms.
To analyze the WM, the performance to the WMT was codified considering accuracy index, that is, the number of recalled correct words (RCW).
To understand the dynamic of eye movements of ADHD groups and TD group, scan path representations were used. A scan path is an ordered set of fixations points (depicted by circles) connected by saccades (depicted by lines). The saccades were divided into (1) progressive saccades (i.e., saccades in the direction of reading); (2) line returns (i.e., saccades in the opposite direction, landing to the next line of text); (3) regressive saccades (i.e., saccades in the opposite direction, landing to any previous line of text); and (4) external saccades (i.e., saccades in the direction out of the screen). Although the probability for two individuals to stare exactly at the same point of a stimulus is very low, comparison of scan paths is well documented (Privitera, Azzariti, & Stark, Reference Privitera, Azzariti and Stark2000).
To analyze the visual scan path, two different measures were used: the ODR and the entropy index of Shannon (Reference Shannon1948). To measure the ODR, we applied the ratio Progressive Saccades/Total Saccades × 100, where Progressive Saccades are saccades in the direction of reading and the Total Saccades are the sum of progressive saccades, line returns, regressive saccades, and external saccades.
To measure the entropy index of Shannon, this formula was computed:
 $$(\tilde H = {H \over {{H_{\max }}}} = - {{\sum\nolimits_{j = 1}^k {{p_j}} \log {\mkern 1mu} {p_j}} \over {\log k}})$$
$$(\tilde H = {H \over {{H_{\max }}}} = - {{\sum\nolimits_{j = 1}^k {{p_j}} \log {\mkern 1mu} {p_j}} \over {\log k}})$$where k is the number of categories of saccades, equal to four (progressive, line returns, regressive, and external), and p is the frequency in each category.
Procedure
Participants sat in a dimly lit room of the University of Messina, in front of the eye-tracker monitor at a distance of 30 cm. To have similar conditions, lights were switched off. The eye tracker was positioned in such a way that ambient lighting did not affect the recordings. The eye tracking equipment was calibrated (9 points, monocular) for each participant at the beginning of the experiment. Gaze fixations of at least 1000 ms within a region of 2°–3° around each calibration point were considered accurate. After which the eye-tracker was calibrated to the participants and they confirmed that they understood the task, then they performed the WMT. The ADHD groups and the TD group were tested separately. All participants were tested in the morning from 9.00 to 12.00 a.m.
Design
A mixed factorial design with repeated measures was used: 3 (groups: ADHD subtype Inattentive vs. ADHD subtype Combined vs. TD children) × 11 (WMT: first list of word, second, third, fourth, fifth, sixth, seventh, eighth, ninth, tenth, eleventh).
Statistical Analysis
Prior to analysis, all data were assessed to ensure normal distribution, homogeneity of variance, and sphericity. A 3 × 11 analysis of variance (ANOVA) with repeated measure [group (ADHD-I, ADHD-C, TD) × lists of word] was used to analyze all data. When appropriate, follow-up analyses included one-way repeated measures ANOVAs and LSD post hoc comparisons. An alpha level of p < .05 was used to determine statistical significance. The descriptive statistics of the dependent variables were tabulated and examined. In case of significant effects, the effect size of the test was reported. More precisely, for ANOVA, partial eta-squared ( ${(_p}{\eta ^2})$) was used, ans for χ 2, phi (ϕ) was used. The Greenhouse–Geisser adjustment for nonsphericity was applied to probability values for repeated measures. Data were analyzed using SPSS 20 software (SPSS Inc., Chicago, IL).
${(_p}{\eta ^2})$) was used, ans for χ 2, phi (ϕ) was used. The Greenhouse–Geisser adjustment for nonsphericity was applied to probability values for repeated measures. Data were analyzed using SPSS 20 software (SPSS Inc., Chicago, IL).
RESULTS
To compare visual patterns of word stimuli between ADHD and TD groups during a visual task through eye-tracking instruments, we analyzed the FL parameter in both groups. The FL showed no statistically significant differences between the two groups, even if it was found to have longer values for ADHD groups (Table 2).
Table 2. Means and (standard deviation) of the parameters considered in the eleven lists of words

FL, fixation length; RCW, recalled correct words; ODR, ordered direction of reading; Ⓗ, entropy index of Shannon.
With reference to RCW, the factor “group” showed significant effect  $([F\left( {2,74} \right) = 3.27,p = .042{,_p}{\eta ^2} = .08])$. This result indicated that the TD group recalled a higher number of words than the other two groups. This was an expected date because in ADHD literature it is known that children with ADHD have a poor WM.
$([F\left( {2,74} \right) = 3.27,p = .042{,_p}{\eta ^2} = .08])$. This result indicated that the TD group recalled a higher number of words than the other two groups. This was an expected date because in ADHD literature it is known that children with ADHD have a poor WM.
We also examined the eye movement patterns in both groups. With reference to ODR parameter, the group showed significant effect [χ 2 (2) = 12.32, p = .022, ϕ = .51]. This date indicated that the subjects in the TD group showed a typical scan path across the text with progressive saccades in the direction from left-to-right higher than ADHD groups.
With reference to Ⓗ index, the factor “group” showed a significant effect  $([F(274) = 5.29,p = .008{,_p}{\eta ^2} = .12])$. This result again indicated that the visual scan path was very different between the two groups. In particular, as shown in Table 2, the subjects in the ADHD groups tend to show an index of entropy among the four categories of saccades higher than the subjects in the TD group. In other words, children with ADHD tended to have unstable fixations and made more saccades in the opposite direction, landing to the next line of text or landing to any previous line of text and external saccades (Figures 1 and 2).
$([F(274) = 5.29,p = .008{,_p}{\eta ^2} = .12])$. This result again indicated that the visual scan path was very different between the two groups. In particular, as shown in Table 2, the subjects in the ADHD groups tend to show an index of entropy among the four categories of saccades higher than the subjects in the TD group. In other words, children with ADHD tended to have unstable fixations and made more saccades in the opposite direction, landing to the next line of text or landing to any previous line of text and external saccades (Figures 1 and 2).
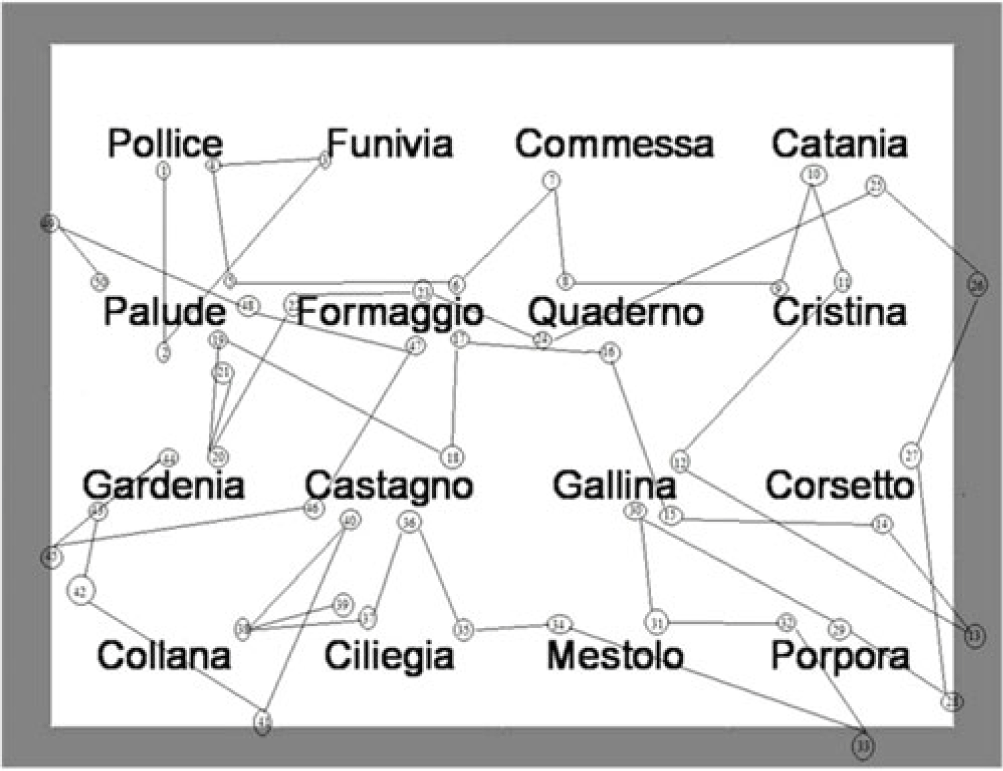
Fig. 1. Atypical visual scan of ADHD group.
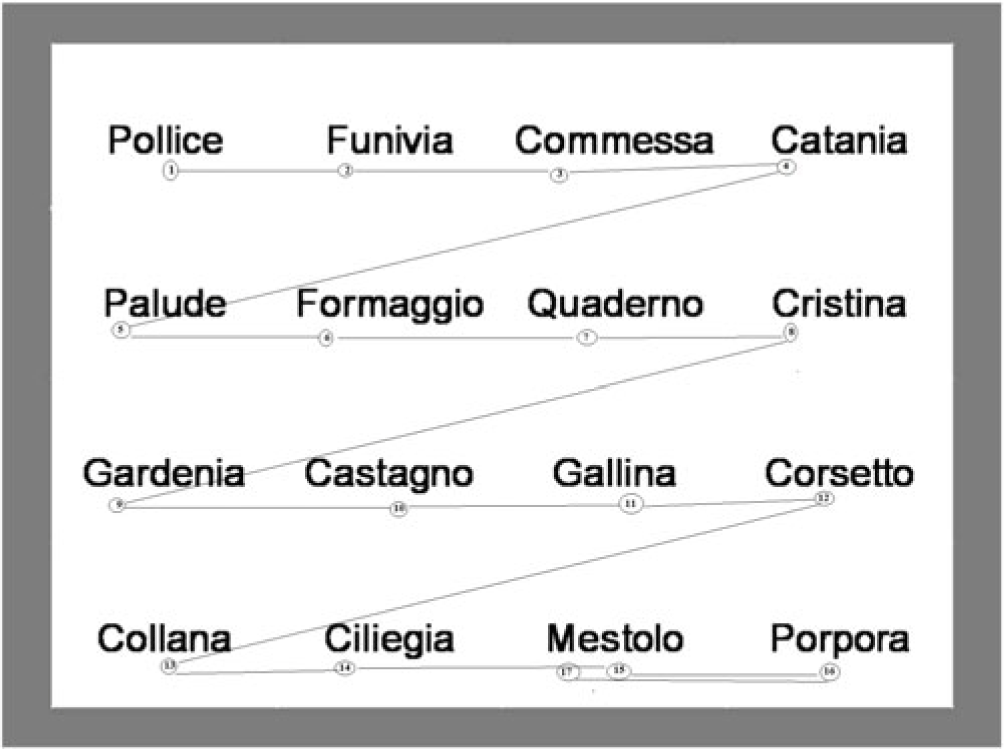
Fig. 2. Typical visual scan of TD group.
To see supplementary materials related to their videotracking, as example of ADHD visual tracking see https://www.youtube.com/watch?v=oOfNKGFz7Uw, https://www.youtube.com/watch?v=pRDpNnR39Ik&feature=youtu.be, and https://www.youtube.com/watch?v=e6mqdWgc9yo&feature=youtu.be, and as example of TD visual tracking see https://www.youtube.com/watch?v=aB_MEe8bzJ0&feature=youtu.be.
DISCUSSION
The current study aimed to compare visual patterns of word stimuli between children with ADHD and TD children, during a visual task implemented with an eye-tracker device; to examine the WM accuracy of the word stimuli; and to compare the dynamic of visual scan path in both groups.
The first hypothesis was not supported by the results which showed that both the ADHD and TD groups did not differ in the FL parameter. Despite the fact that the first hypothesis was not supported by the data, a difference was found for the subjects in the ADHD-I group that showed a FL parameter longer and larger than the other two groups, but it is not statistically significant. The lack of these significant differences in FL parameter may be attributed to the type of stimulus, that is, short words that did not require a complex encoding phase. Although this result differs from previous results reported in the literature, it is possible to argue that this difference is related to type of task. Previous studies used complex task, while eye movements were recorded during the experiment, for example, change detection task (Türkan, Amado, Ercan, & Perçinel, Reference Türkan, Amado, Ercan and Perçinel2016). Therefore, our finding suggests that children with ADHD do not show FL longer than TD children when the task or stimulus does not require a complex encoding phase.
The one significant difference between the ADHD and TD group was in CRW. As predicted, children in the ADHD group recalled a lower number of words than the TD group. Hence, the second hypothesis of this study was confirmed. This is an expected result because in ADHD literature, it is well known that these subjects had a WM impairment.
The most interesting results were the differences between ADHD and TD children in terms of visual scan path. With reference to ODR parameter, it was found that the ADHD groups did not follow a typical scan path across the words, but their visual scanning was discontinuous, uncoordinated, and chaotic. However, children in the TD group showed a typical, ordered visual scan. A prototype visual scan of both groups can be seen as supplementary material. These significant differences were confirmed by the results which indicated that the subjects in the ADHD groups tended to show an index of entropy among the four categories of saccades higher than the subjects in the TD group. Precisely, children with ADHD showed unstable fixations and made more saccades in the opposite direction, landing to the next line of text or landing to any previous line of text and external saccades. Hence, the third hypothesis was confirmed by the results in ODR parameter and index of entropy.
The present findings are consistent with previous studies in which children with ADHD displayed more atypical eye movement compared with TD children, using eye-tracking technology (Deans et al., Reference Deans, O’Laughlin, Brubaker, Gay and Krug2010; Mohammadhasani, Fabio, Fardanesh, & Hatami, Reference Mohammadhasani, Fabio, Fardanesh and Hatami2015; Munoz et al., Reference Munoz, Armstrong, Hampton and Moore2003; Sun, Wang, Han, & Zhu, Reference Sun, Wang, Han and Zhu2003). In particular, the current study yields support for significant differences between ADHD and TD children in terms of visual scan patterns, describing the specific features of the visual scan in subjects with ADHD. Taken together, the results of this study indicate that (1) children with ADHD have a WM deficit when the WM is measured using visual tasks with word stimuli, presented throughout the eye-tracker, and (2) the visual scanning of these children is characterized by specific type of saccades, such as line returns, regressive saccades, and external saccades.
A related question here is whether the impairment observed for visual scan path in ADHD may have been driven by an attention deficit, specified for subjects with ADHD. As stated in the introduction of this article, a number of studies agree on the link between attention and eye movements, and between attention and WM. The fact that the attention processes are involved in visual scan and WM may support the atypical visual patterns shown by children with ADHD. Therefore, it is reasonable to assume that an attention deficit can affect eye movements and, consequently, remembering a word. Thus, the poor WM performance of children with ADHD, in WMT presented throughout the eye-tracker, can be attributable not just to attention deficit (Fabio, Castriciano, & Rondanini, Reference Fabio, Castriciano and Rondanini2015; Liverta, Fabio, Tiezzi, & Cedro, Reference Liverta, Fabio, Tiezzi and Cedro2016; Martino, Caprì, Castriciano, & Fabio, Reference Martino, Caprì, Castriciano and Fabio2017) but also to atypical visual scan in ADHD.
This study has some treatment implications. Given that the meta-analysis evidences indicate that WM training fails to significantly improve reading and memory performance in children with ADHD and TD subjects (Melby-Lervåg, Redick, & Hulme, Reference Melby-Lervåg, Redick and Hulme2016; Rapport et al., Reference Rapport, Orban, Kofler and Friedman2013) and that these children show an atypical visual scan, we suggest training protocols focused on directing the visual scan to improve the WM performance. This means to design protocols following isolated training (eye movements are trained; attention is not trained) or combined training (both eye and attention are trained), in an effort to enhance a bidirectional relationship between eye movement control and attention and, consequently, to improve the WM.
The present study has a limitation related to the size of sample. In this work, the sample size is small and there may be constraints to the generalizability of the results. However, the effect size is adequate and, consequently, the results from groups can be considered reliable. Future research can use a larger ADHD sample to confirm or not the findings obtained by the current study. Future research might also include a larger ADHD sample and children who meet clinical criteria for both ADHD and reading disorder, to assess the specificity of our results in ADHD population.
In conclusion, this study has expanded previous knowledge about ADHD and scanning, and it has delineated the characteristics of visual scan path in children who have ADHD.
ACKNOWLEDGEMENTS
The authors thank all the children and their families who agreed to participate in this study. This article has no source of funding.
CONFLICT OF INTEREST
The authors have nothing to disclose.
ETHICAL STANDARDS
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.






