Considerable research demonstrates that adverse childhood experiences (ACE) negatively impact physiological, behavioral, and emotional health well into adulthood (Danese et al., Reference Danese, Moffitt, Harrington, Milne, Polanczyk, Pariante and Caspi2009; Dube, Felitti, Dong, Giles, & Anda, Reference Dube, Felitti, Dong, Giles and Anda2003; Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998). Mounting evidence suggests ACE may also have long-lasting negative effects on cognition (Geoffroy, Pereira, Li, & Power, Reference Geoffroy, Pereira, Li and Power2016; Gould et al., Reference Gould, Clarke, Heim, Harvey, Majer and Nemeroff2012; Hart & Rubia, Reference Hart and Rubia2012; Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009). Developmental research provides support that pathophysiological changes, particularly hypothalamic-pituitary-adrenal (HPA) axis dysregulation and markers of allostatic load, can adversely impact the development and structural integrity of the brain (Colich, Rosen, Williams, & McLaughlin, Reference Colich, Rosen, Williams and McLaughlin2020; Hart & Rubia, Reference Hart and Rubia2012; Luecken, Reference Luecken2006; Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009; Teicher, Samson, Anderson, & Ohashi, Reference Teicher, Samson, Anderson and Ohashi2016). Studies also implicate mental health factors (Danese et al., Reference Danese, Moffitt, Harrington, Milne, Polanczyk, Pariante and Caspi2009) and hindrances to educational attainment (Almquist & Brännström, Reference Almquist and Brännström2018; Lansford et al., Reference Lansford, Dodge, Pettit, Bates, Crozier and Kaplow2002; Merrick et al., Reference Merrick, Ford, Ports, Guinn, Chen, Klevens and Mercy2019) as routes by which childhood adversity can weaken cognitive and intellectual function. However, despite a large body of research in children and adults, there is limited research regarding the long-term effects of early-life adversity in older adults.
Several direct and indirect routes link childhood adversity with cognitive dysfunction. Directly, adversity can lead to changes in brain structure and function (Kim & Diamond, Reference Kim and Diamond2002). Namely, HPA axis dysregulation stimulates the adrenal glands to overproduce glucocorticoids which cross the blood-brain barrier and bind to neuronal receptors. Prolonged exposure to glucocorticoids is thought to cause a neuronal loss in specific brain regions (Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009), which may lead to volumetric reductions in the hippocampus, anterior cingulate cortex, ventromedial prefrontal cortex (PFC), dorsomedial PFC, and parietal subregions, as well as volumetric increases in the amygdala across age groups (Ancelin et al., Reference Ancelin, Carrière, Artero, Maller, Meslin, Dupuy and Chaudieu2021; Hart & Rubia, Reference Hart and Rubia2012; Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009; Teicher et al., Reference Teicher, Samson, Anderson and Ohashi2016). In addition, cortical thinning in areas such as the ventromedial PFC, frontoparietal, default, and visual networks, can also exist in children and adolescents with a history of adversity (Colich et al., Reference Colich, Rosen, Williams and McLaughlin2020). Taken together, maltreatment may lead to disruptions in the development of brain areas, particularly the prefrontal cortex and hippocampus (Petchtel & Pizzagalli, Reference Pechtel and Pizzagalli2011). These regions are also sensitive to cognitive aging processes (Salthouse, Reference Salthouse2010). Thus, it is plausible that older adults with ACE may experience a “double hit” for the risk of cognitive decline.
Indirectly, links between ACE and cognitive dysfunction may occur through socioeconomic disadvantages, decreased opportunities for educational attainment, and increased risk for psychopathology (Almquist and Brännström Reference Almquist and Brännström2018; McLaughlin, DeCross, Jovanovic, & Tottenham, Reference McLaughlin, DeCross, Jovanovic and Tottenham2019; Merrick et al., Reference Merrick, Ford, Ports, Guinn, Chen, Klevens and Mercy2019; Turecki, Ota, Belangero, Jackowski, & Kaufman, Reference Turecki, Ota, Belangero, Jackowski and Kaufman2014). Large cohort studies suggest a dose-dependent relationship between the number of adverse events and socioeconomic status (SES) in adulthood (Font & Maguire-Jack, Reference Font and Maguire-Jack2016; Merrick et al., Reference Merrick, Ford, Ports, Guinn, Chen, Klevens and Mercy2019). This is a problematic link given the importance of higher educational attainment in bolstering greater levels of cognitive reserve, thereby reducing the risk for dementia (Stern, Reference Stern2006). In addition, consistent associations exist between psychopathology and early adversity. Children raised in aversive households are more likely to experience emotion regulation difficulties which may later persist into dispositional negative affect (Taylor, Way, & Seeman, Reference Taylor, Way and Seeman2011). These vulnerabilities may help explain the increased prevalence of depression across the life span in those with high ACE (Danese et al., Reference Danese, Moffitt, Harrington, Milne, Polanczyk, Pariante and Caspi2009; Rao et al., Reference Rao, Chen, Bidesi, Shad, Thomas and Hammen2010). Moreover, depression may mediate the relationship between maltreatment and later cognitive dysfunction in adults (Toyoshima et al., Reference Toyoshima, Inoue, Masuya, Fujimura, Higashi, Tanabe and Kusumi2020). Within older adult populations, there is a high correlation between depression and subjective cognitive concerns (SCC; Jessen et al., Reference Jessen, Amariglio, Van Boxtel, Breteler, Ceccaldi, Chételat and Glodzik2014). Together, these factors may heighten the risk for cognitive impairment and decline (Mendonça, Alves, & Bugalho, Reference Mendonça, Alves and Bugalho2016; Rabin, Smart, & Amariglio, Reference Rabin, Smart and Amariglio2017). Furthermore, resiliency factors such as mastery, self-esteem, and problem-solving abilities tend to be less prevalent in individuals with ACE (Taylor et al., Reference Taylor, Way and Seeman2011), which may negatively impact the building of cognitive reserve (Boyle et al., Reference Boyle, Buchman, Wilson, Yu, Schneider and Bennett2012).
Throughout childhood and adulthood, cross-sectional evidence suggests worse performance on measures of attention, working memory, executive function, and processing speed among individuals with a history of early adversity (Geoffroy et al., Reference Geoffroy, Pereira, Li and Power2016; Gould et al., Reference Gould, Clarke, Heim, Harvey, Majer and Nemeroff2012; Majer, Nater, Lin, Capuron, & Reeves, Reference Majer, Nater, Lin, Capuron and Reeves2010; Nikulina & Widom, Reference Nikulina and Widom2013; Nolin & Ethier Reference Nolin and Ethier2007; Sheridan, Peverill, Finn, & McLaughlin, Reference Sheridan, Peverill, Finn and McLaughlin2017; Vasilevski & Tucker, Reference Vasilevski and Tucker2016). Steeper rates of cognitive decline have been observed in older adults with both adverse childhoods and current depressive symptomology (Korten, Penninx, Pot, Deeg, & Comijs, Reference Korten, Penninx, Pot, Deeg and Comijs2014). On a global cognitive screener measure, there are suggestions of specific effects of ACE on memory but not on other cognitive functions in older adults (Kobayashi et al., Reference Kobayashi, Farrell, Payne, Mall, Montana, Wagner and Berkman2020). However, others have found that early traumatic events are associated with worse performance on measures of processing speed, attention, and executive functioning in anxious and depressed older adults (Petkus et al., Reference Petkus, Lenze, Butters, Twamley and Wetherell2018). Ritchie and colleagues (Reference Ritchie, Jaussent, Stewart, Dupuy, Courtet, Malafosse and Ancelin2011) comprehensively investigated cognitive function with specific types of abuse and found that poor environmental conditions are associated with worse verbal retrieval and visuospatial memory; however, there was evidence of better performance on measures of verbal fluency. Similarly, others have found an association between better word retrieval performance and childhood sexual abuse (Feeney, Kamiya, Robertson, & Kenny, Reference Feeney, Kamiya, Robertson and Kenny2013). There is also evidence that adults and older adults with childhood adversity demonstrate relatively similar cognitive abilities and rates of decline (Barnes et al., Reference Barnes, Wilson, Everson-Rose, Hayward, Evans and De Leon2012; Dunn et al., Reference Dunn, Busso, Raffeld, Smoller, Nelson, Doyle and Luk2016).
Finally, ACE may also impact intellectual development in children (Hart & Rubia, Reference Hart and Rubia2012), which could influence cognitive reserve. However, this relationship is inconsistent in adolescent and older adult populations (Ritchie et al., Reference Ritchie, Jaussent, Stewart, Dupuy, Courtet, Malafosse and Ancelin2011; Vasilevski & Tucker, Reference Vasilevski and Tucker2016).
ACE are associated with an increased risk for dementia, but this relationship and modifying factors are poorly understood. To address gaps in the literature, this study is the first to our knowledge to comprehensively examine the effect of ACE on specific cognitive functions and measures associated with greater risk and resiliency to cognitive decline in independent community-dwelling older adults. We aimed to determine the effect of ACE on cognitive function in community-dwelling older adults while adjusting for relevant demographic variables of age and education. We hypothesized ACE would associate with worse performance on measures of intellectual function, memory, and executive attention. We also examined interrelationships among ACE, depression, SES, SCC, and self-efficacy, as these factors have been implicated in both early life adversity and cognitive deficits. Follow-up analyses tested whether education mediated the relationship between specific cognitive functions and ACE.
METHOD
Participants
Participants were recruited as part of the Maine-Aging Behavior Learning Enrichment (M-ABLE) Study at the University of Maine. This study used community-based participatory research (CBPR) methods to enhance the recruitment of a socioeconomically diverse community-dwelling older adult sample. To reduce participation barriers, study visits occurred at easily accessible locations within the community and were offered on the weekends. The present study was interested in obtaining a representative sample of independent community-dwelling older adults with a range of cognitive functions (normal to MCI). Study inclusion criteria were intentionally broad to improve the generalizability of findings to more diverse older adults. Inclusion criteria included: ages 55–90 years old, willing to undergo neuropsychological assessment, and willingness to provide income information. Exclusion criteria included moderate to severe cognitive impairment (Montreal Cognitive Assessment scores < 18; Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead, Collin and Chertkow2005), severe depression (Geriatric Depression Scale scores > 10; Sheikh & Yesavage, Reference Sheikh and Yesavage1986), moderate to severe neurological impairments (e.g., moderate-to-severe traumatic brain injury), recent stroke (defined as in the past year), neurodegenerative disorder (e.g., Parkinson’s disease or Alzheimerʼs disease), or physical limitations (e.g., loss of visual field or unable to hold a pencil) that prohibited cognitive testing. Intellectual disability, dementia disorder, or any untreated or severe psychiatric conditions (e.g., psychotic disorders) were also excluded. Participants were compensated up to 70 U.S. dollars for the completion of the study. To attenuate time of day effects on cognitive performance, most appointments were scheduled in the morning, starting between 8:00 a.m. and 9:00 a.m.
Ethics of human subjects
Participants were screened for eligibility and underwent informed consent procedures approved by the University of Maine Institutional Review Board. All procedures performed in this study were in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments. Trained research assistants administered neuropsychological assessments.
Measures
Clinical characteristics
Participants provided demographic information (age, sex, years of education, income level) and clinical/medical history via interview. Clinical/medical history was collected via the National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) Subject Health History form (Form A5; Morris et al., Reference Morris, Weintraub, Chui, Cummings, DeCarli, Ferris and Beekly2006). This form measures various medical conditions that participants endorse as either “absent,” “actively present,” “remotely present,” or “unknown.” Current depressive status was determined by the “active” depression variable (endorsing a major depressive episode within the past two years). Other psychiatric disorders (e.g., generalized anxiety disorder, post-traumatic stress disorder) were represented by the endorsement of an “active” response on the psychiatric disorder variable. A cardiovascular risk composite score was created by summing the variables of cardiac arrest, atrial fibrillation, congestive heart failure, angina, and other evidence of coronary disease. The cerebrovascular risk composite score represented the sum of the stroke, transient ischemic attack, or evidence of other cerebrovascular disease variables.
Anthropometrics including waist circumference (centimeters), height (inches), and weight (pounds) were also collected. Body mass index (BMI) was derived with a formula to convert English to metric measurements. Sex-specific waist circumference cutoff measurements were used in conjunction with BMI to form relative disease risk (RDR) categories (National Heart, Lung, Blood Institute, National Institute of Diabetes, Digestive, & Kidney Diseases, 2013). Participants with a BMI in the obese or overweight range were classified as high RDR, while those within normal-to-underweight ranges were classified as normal RDR based on these guidelines.
Measures of psychological risk and resilience
The ACE questionnaire retrospectively collects information on experiences of abuse, neglect, and household dysfunction occurring in one’s life before the age of 18 (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998). The ACE is a dichotomous (yes or no) ten-question inventory, composed of 17 sub-items with demonstrated good to excellent reliability (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998). Total scores range from 0 to 10, with higher scores indicating more adverse events. Domains include physical abuse, sexual abuse, neglect, and household dysfunction. Questions of physical and sexual abuse assess the nature of the abuse, neglect questions ascertain the degree of emotional and physical neglect, while household dysfunction questions determine the presence of substance abuse, marital discord, and family mental illness. Risk is commonly approximated by the total ACE score, considering most individuals exposed to one type of adversity are also exposed to a second type of adversity (Felitti et al., Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards and Marks1998). Therefore, the current study used the continuous variable of the total ACE score as a measure of abuse.
The National Institute of Health-Toolbox for the Assessment of Neurological and Behavioral Function Quality of Life in Neurological Disorders (NIH-TB Neuro-QOL, Version 2.0) Cognitive Function, Depression, and Self-Efficacy self-report questionnaires were administered to assess modifiable risk factors associated with risk and resiliency for cognitive decline (Gershon et al., Reference Gershon, Wagster, Hendrie, Fox, Cook and Nowinski2013). The 18-item Cognitive Function questionnaire evaluated SCC within the past seven days (National Institute of Neurological Disorders and Stroke [NINDS], 2015). Participants rated perceived levels of difficulty in memory, attention, decision-making, and everyday functions (e.g., reading, planning tasks, or remembering names) according to a 5-point scale ranging from 1 (cannot do) to 5 (none/no difficulty). Total scores represent the sum of all items and range from 18 to 90, with lower scores indicating greater SCC. Scale reliability in the current sample was excellent (α = .905). The Depression questionnaire is a 24-item scale measuring how often participants experience depressive symptoms (National Institute of Neurological Disorders and Stroke (NINDS), 2015). Each of the 24 items is rated on a 5-point scale, ranging from 1 (never) to 5 (always). The ratings are summed, yielding a total score ranging from 24 to 120, in which higher scores reflect greater depressive symptomatology. Participants selected responses that best described their mood, thoughts, and behaviors during the past seven days. Scale reliability in the current sample was excellent (α = .946). The General Self-Efficacy scale (National Institute of Neurological Disorders and Stroke (NINDS), 2015) contains four items that evaluate confidence in the ability to problem-solve and handle unexpected situations, ranging from 1 (I am not confident at all) to 5 (I am very confident). Summed ratings yield a total score ranging from 4 to 20, where higher scores indicate higher confidence levels regarding perceived self-efficacy. Scale reliability for the current sample was excellent (α = .904).
Neuropsychological assessment
Intellectual function
The 70-item noncontextual word reading test, a subtest of the Wide Range Achievement Test-Fourth Edition (WRAT-4), served as a measure of verbal intellectual functioning (Wilkinson & Robertson, Reference Wilkinson and Robertson2006). The Peabody Picture Vocabulary Test—Fourth Edition (PPVT-4), a measure of receptive vocabulary, and the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) Block Design subtest, a measure of visuospatial construction, were used to assess nonverbal intelligence (Dunn & Dunn, Reference Dunn and Dunn2007; Wechsler, Reference Wechsler2008).
Verbal memory
The Rey Auditory Verbal Learning Task (RAVLT) served as a measure of episodic memory and verbal learning (Schmidt, Reference Schmidt1996). The RAVLT is composed of two 15-item word lists with five successive recall trials of List A (Trials 1–5), an interference trial (List B), and 20-minute delayed recall and recognition trials. The sum of scores from Trial 1 through Trial 5 reflected immediate recall, while the total number of words freely recalled 30 min after initial registration of the word list represented delayed recall.
Visual memory
The Brief Visuospatial Memory Test—Revised (BVMT-R) measured visuospatial learning and memory (Benedict, Reference Benedict1997). The task contains three learning trials and a 25-minute delayed recall trial. The immediate memory score reflected the sum of the learning trials, while the delayed memory score represented the amount of information remembered after a 25-minute delay.
Executive attention
The Trail Making Test B (TMT-B; Reitan, Reference Reitan1958) total time in seconds assessed set-shifting ability. The WAIS-IV Digit Span task total score examined auditory working memory. Auditory digit sequences were repeated in forward, reverse, and numeric order, and the sum of these three tasks represented the total score (Wechsler, Reference Wechsler2008). The WAIS-IV Coding subtest and the total seconds on the Trail Making Test A (TMT-A) served as estimates of visual scanning and rote attention ability (Reitan, Reference Reitan1958; Wechsler, Reference Wechsler2008). TMT-A and B were appropriately scaled such that larger numbers reflected slower (worse) performance, and smaller numbers reflected faster (better) performance.
Analyses
Descriptive statistics were generated for demographic variables, neuropsychological test scores, and ACE scores to determine whether assumptions of normality were violated and to obtain characteristics of the sample. Data were visually inspected and examined for outliers, skew, and kurtosis. Significant outliers (z-scores exceeding ± 3.29 standard deviations from the mean) were winsorized. Neuropsychological variable raw scores were converted into normally distributed scaled scores via Rankit’s method (Soloman & Sawilowsky, Reference Soloman and Sawilowsky2009). Ranked scores were transformed into normally distributed z-scores. Scores were then converted to scaled scores with a mean of ten and a standard deviation of three.
Tests chosen for the composite tests were selected based on prior theoretical framework regarding gold standard measures for assessing cognitive domains, and appropriate composite item reliability (Heaton et al. Reference Heaton, Akshoomoff, Tulsky, Mungas, Weintraub, Dikmen and Gershon2014, Scott, Sorrell, & Benitez, Reference Scott, Sorrell and Benitez2019; Weintraub et al., Reference Weintraub, Dikmen, Heaton, Tulsky, Zelazo, Slotkin and Havlik2014). An Intellectual Function composite was created by forming an average of the standardized and scaled scores of the WRAT-4, PPVT-4, and WAIS-IV Block Design tests and was again transformed and rescaled to have a mean of ten and a standard deviation of three. This procedure was repeated to form Verbal Memory (average of RAVLT immediate recall and delayed recall scores), Visual Memory (average of BVMT-R immediate memory and delayed memory scores), and Executive Attention (average of TMT-A, TMT-B, WAIS-IV Coding, and WAIS-IV Digit Span Total scores) composites. Cronbach’s alpha assessed the internal consistencies of each composite. Scale reliability of the Intellectual Function (α = .772), Verbal Memory (α = .863), Visual Memory (α = .945) and Executive Attention (α = .751) composites ranged from acceptable to excellent and were deemed adequate for study aims.
The composites served as dependent variables in a series of hierarchical regressions to assess the unique contribution of ACE scores on domains of intellectual function, verbal memory, visual memory, and executive attention while adjusting for relevant demographic variables of age and education. Adjusted R 2 and standardized beta values are reported for the final regression models. Spearman’s rank correlations explored relationships among ACE scores and clinical, demographic, and psychological measures. Mediation analyses were performed using Hayes’ PROCESS macro for SPSS (Hayes, Reference Hayes2017), posited to be a more advanced approach than traditional causal steps analyses (e.g., Baron & Kenny, Reference Baron and Kenny1986). The PROCESS approach is a regression-based method that allows for the simultaneous evaluation of the direct and indirect effects, as well as bootstrapped 95% Confidence Intervals for these estimates that serve as measures of effect size (Hayes, Reference Hayes2017). Bonferroni corrections (.05/4 = .0125) were applied to regression and mediation analyses to account for multiple comparisons.
Statistical power was computed a priori using G * Power 3.1 and was sufficient to test a linear multiple regression using three predictors (Faul, Erdfelder, Buchner, & Lang, Reference Faul, Erdfelder, Buchner and Lang2009). Pearson r values served as effect sizes for correlations; r values of .10, .30, and .50 corresponded to small, medium, and large effect sizes, respectively (Cohen, Reference Cohen1988). For hierarchical regressions, f 2 values of .02, .15, and .35, represented small, medium, and large effect sizes, respectively (Cohen, Reference Cohen1988). All tests of significance were two-tailed. Statistical analyses were performed using SPSS Version 27.
RESULTS
Descriptive statistics on demographics and clinical characteristics
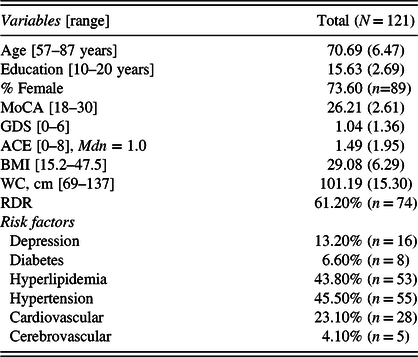
Table 1 presents descriptive statistics for the demographic and clinical characteristics of the sample. There was a larger proportion of women, as well as a broad range of years of education and annual income level (overall range: <$10,000–>$100,00, Mdn: $50,000–$59,000). Global cognition scores fell within the mild cognitively impaired to normal cognition ranges. Overall, depression scores were in the normal range. Hypertension and hyperlipidemia were the most endorsed vascular risk factors. Average BMI was in the overweight range, and over half of the sample fell in the High RDR category when considering the relative contributions of waist circumference and BMI.
Table 1. Clinical and demographic characteristics of sample
Adverse childhood experiences
Table 2 presents descriptive information on the prevalence of ACE events. Over 56% of participants experienced at least one adverse childhood event. Men and women did not statistically differ in ACE scores, F (1, 119) = .18, p = .670. Similarly, there were no sex differences in type of abuse, as men and women experienced relatively equal levels of physical/verbal abuse [χ 2 (1, N = 121) = .33, p = .568], sexual abuse [χ 2 (1, N = 121) = 1.51, p = .219], neglect [χ 2 (1, N = 121) = 2.08, p = .149], and household dysfunction, χ 2 (1, N = 121) = 2.78, p = .095. Household dysfunction represented the most endorsed domain, followed by physical abuse/verbal abuse. Parental substance abuse was the most endorsed type of adverse event.
Table 2. Frequency of ACE responses by categories and items

Neuropsychological performance
A series of hierarchical regression analyses were used to determine whether the number of ACE events associated with verbal/nonverbal intelligence, verbal memory, visual memory, and executive attention while adjusting for relevant age and education differences in neuropsychological test scores in the models. A rudimentary model was performed to test whether ACE was associated with intellectual function, visual/verbal memory, and executive function while adjusting only for age. These results revealed that ACE score was a significant predictor of intellectual function (β = −.24, p = .010) executive attention (β = −.21, p = .012), and a trend towards visual memory (β = −.17, p = .058). ACE scores were not significantly associated with verbal memory (β = −.07, p = .449). Intellectual function and executive attention results remained significant after Bonferroni correction; however, the visual memory trend dissipated. Analyses next adjusted for both age and education in the model. Results indicated ACE scores were no longer a significant predictor of intellectual function or executive attention (ps > .05) once the significant effect of education was adjusted for in the model (Table 3).
Table 3. Summaries of hierarchical regression analyses

To explore the possibility of a mediating role of education between ACE and cognitive abilities, a mediation path analysis was performed using the PROCESS macro-Version 3 (Hayes, Reference Hayes2017). Figures 1 and 2 present the conceptual relationships. As previously indicated, higher ACE scores predicted lower levels of education (path a: β = −.56, p < .001) within both models. These results indicated approximately 16.30% of the variance in education was explained by ACE scores (β = −.56, 95% CI [−.79, −.32], p < .001). There was a direct effect of higher ACE scores on lower levels of intellectual function (path c: β = −.10, p = .010). However, when the significant effect of education on intellectual function (path b: β = .18, p < .001) was included in the model, the relationship between ACE score and intellectual function (path c’: β = −.003, p = .927) was no longer significant. These results provide support that education mediated the relationship between ACE scores and intellectual function (β = −.10, 95% CI [−.14, −.06]; this indirect effect accounted for approximately 23.22% of the variance in intellectual function. A direct effect of ACE scores was also found on executive attention (path c: β = −.06, p = .046) when education was not adjusted for in the model. However, when the significant effect of education on executive attention (path b: β = .08, p < .001) was included in the model, the relationship between ACE score and executive attention (path c’: β = −.02, p = .585) was no longer significant. These results provide support that education mediated the relationship between ACE scores and executive attention (β = −.05, 95% CI [−.07, −.02]; this indirect effect accounted for approximately 12.50% of the variance in executive attention. While the direct effect of ACE on intellectual function survived Bonferroni correction, the direct effect of ACE on executive attention did not.

Fig. 1. Education mediates the relationship adverse childhood experiences and premorbid intellectual function. Higher ACE scores were significantly associated with less years of education (path a) and lower premorbid intellectual function (path c). However, this relationship was no longer significant (path c’) once adjusting for the significant effect of years of education on premorbid intellectual function (path b).

Fig. 2. Education mediates the relationship adverse childhood experiences and executive attention. Higher ACE scores were significantly associated with less years of education (path a) and lower executive attention (path c). However, this relationship was no longer significant (path c’) once adjusting for the significant effect of years of education on executive attention (path b).
Relationships among ACE with risk/resilience factors
Table 4 presents the interrelationships among ACE and risk/resilience factors. Higher ACE scores were significantly associated with fewer years of education, lower annual income, and higher levels of SCC and depressive symptoms (Table 4). Higher levels of depression symptoms were associated with fewer years of education, lower annual income level, less sense of self-efficacy, and higher levels of SCC. Higher perceptions of self-efficacy were associated with fewer SCC.
Table 4. Associations among total ACE scores, risk and resilience factors

Note. 1NeuroQOL (Version 2.0) Depression Questionnaire; 2Higher scores reflect less subjective cognitive concern (SCC); 3Patient Reported Outcomes Measurement Information System (PROMIS) Self-Efficacy Scale; *p<.05; **p<.001.
DISCUSSION
The present study is the first to our knowledge to comprehensively examine the effect of ACE on specific cognitive functions and measures associated with greater risk and resiliency to cognitive decline in independent community-dwelling older adults. ACE scores were significantly associated with lower intellectual function and worse executive attention, yielding large effect sizes. Subsequent analyses revealed that these relationships were fully mediated by years of education. However, after adjusting for multiple comparisons, the direct effect of ACE on executive attention was no longer significant. Results are thus interpreted within the context of large effect sizes and limitations of the conservative nature of Bonferroni corrections (i.e., risk of increased type II errors; Perneger, Reference Perneger1998). Significant associations, with small to medium effect sizes, also emerged among ACE with lower SES, greater depression symptoms, and greater SCC. Taken together, childhood adversity may serve as a distal basis by which risk factors build and interact to influence the risk of cognitive decline.
SES and ACE
A potential route by which ACE may increase the risk of dementia is through lower levels of early education, thereby hindering development for greater cognitive reserve. This is in line with a 15-year longitudinal study suggesting educational disparities have the most prominent influence on functional limitations and negative health outcomes during early to middle old age (House, Lantz, & Herd, Reference House, Lantz and Herd2005). Relatedly, longitudinal evidence demonstrates that educational attainment is a powerful mediator between early adversity and various poor social, economic, and health-related outcomes (Almquist & Brännström, Reference Almquist and Brännström2018). Even when adjusting for relevant family and child characteristics, evidence indicates that school attendance rate and perceptions regarding the likelihood of attending college are negatively impacted by childhood maltreatment (Lansford et al., Reference Lansford, Dodge, Pettit, Bates, Crozier and Kaplow2002).
Theoretical models suggest that children raised in environments of deprivation, characterized by lack of cognitive stimulation or opportunities for learning, may exhibit worse attention and executive function abilities (McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016; Sheridan et al., Reference Sheridan, Peverill, Finn and McLaughlin2017). Further, research suggests that higher educational attainment is an important socioeconomic contributor towards preserving memory function in older adults, while low annual income is associated with risk for cognitive decline (Marden, Tchetgen Tchetgen, Kawachi, & Glymour, Reference Marden, Tchetgen Tchetgen, Kawachi and Glymour2017). Thus, heightened risk for late-life cognitive dysfunction may exist through lack of opportunities for learning and enrichment in those with early adversity (Marden et al., Reference Marden, Tchetgen Tchetgen, Kawachi and Glymour2017; McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016; Sheridan et al., Reference Sheridan, Peverill, Finn and McLaughlin2017). While inherent genetic vulnerability for lower intellectual abilities may exist (Deary, Spinath, & Bates, Reference Deary, Spinath and Bates2006), educational attainment appears to play a consistent mediating role between childhood adversity and poor health, social, economic, and cognitive outcomes. Future research should target ways to increase educational opportunities in efforts to attenuate enduring negative effects of early life disadvantages.
Clinical implications
Individuals with more than one ACE reported higher levels of SCC. While these concerns can occur irrespective of objective cognitive deficits, they are often predictive of future cognitive decline (Mendonça et al., Reference Mendonça, Alves and Bugalho2016; Rabin et al., Reference Rabin, Smart and Amariglio2017). However, SCC may also reflect a negative attention bias and is highly correlated with subclinical levels of depression (Jessen et al., Reference Jessen, Amariglio, Van Boxtel, Breteler, Ceccaldi, Chételat and Glodzik2014; Rabin et al., Reference Rabin, Smart and Amariglio2017). These findings warrant further investigation in older adults with ACE to improve understanding of the interrelationships among heightened levels of depression, perceived cognitive difficulties, and objective impairment.
Within this study, current subclinical depression symptoms, but not a clinical history of depression, were significantly associated with greater ACE and current perceptions of lower self-efficacy. Notably, subclinical depression has emerged as a strong predictor of cognitive function in older adults (MacAulay et al., Reference MacAulay, Halpin, Cohen, Calamia, Boeve, Zhang and Keller2020). Research suggests early life adversity strongly predicts risk for developing mental illness such as depression, anxiety, and substance abuse disorders (Turecki et al., Reference Turecki, Ota, Belangero, Jackowski and Kaufman2014). It is plausible that predispositions to psychopathology are directed by genes and shaped by early experiences creating an additive vulnerability (Schwartz, Wright, & Valgardson, Reference Schwartz, Wright and Valgardson2019; Turecki et al., Reference Turecki, Ota, Belangero, Jackowski and Kaufman2014). Even in circumstances in which adversity has dissipated, early life stress can have enduring negative effects (Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011) that are beyond that produced by more proximal negative life events (Korten et al., Reference Korten, Penninx, Pot, Deeg and Comijs2014). These enduring effects may speak to the complexity of gene-environment interactions and epigenetic influences such as prenatal and intergenerational exposure to adversity that increase the risk for vulnerability factors shared by both early adverse experiences and mental illness (Schwartz et al., Reference Schwartz, Wright and Valgardson2019). These effects may also impart a greater likelihood of negative response bias in those individuals reporting high numbers of ACE. As such, investigating the potential cascading effects of early adversity on future psychopathology is warranted.
Strengths and Limitations
A strength of this study included the use of CBPR approaches, leading to increased enrollment of socioeconomically diverse participants. Recruitment efforts targeted older adults, and the time of testing was controlled to provide optimal times for cognitive performance in this population. The use of a comprehensive neuropsychological battery to test specific cognitive domains also highlights a strength of this study. Single global measures of cognitive function are commonly used to represent cognitive status. However, these measures can lack the sensitivity and specificity needed to detect subtle cognitive differences appropriately. Global measures may also preclude detection of singular domain impairments when other domains are within normal limits.
Although the present study extends the literature by investigating the effects of childhood adversity on cognition in socioeconomically diverse older adults, limitations include a racially and ethnically homogenous sample, reflecting the 94.7% non-Hispanic white population estimate for the state of Maine (U.S. Census Bureau, 2018). Studying these factors within a racially and ethnically diverse sample is needed to determine if racial and ethnic disparities potentiate accumulative effects. Future research should also examine the role of education quality as it has been shown to predict cognitive abilities beyond years of education alone (Crowe et al., Reference Crowe, Clay, Martin, Howard, Wadley, Sawyer and Allman2013).
Conclusion
In summary, the current study found that the relationship between objective cognitive performance and childhood adversity was mediated by years of education. These results indicate that childhood adversity may indirectly impact cognitive functioning in older adults by reducing factors related to building and maintaining cognitive reserve, such as greater educational attainment. Interestingly, greater levels of SCC were reported in those with adverse childhoods. In line with prior research, greater ACE were also associated with greater levels of subclinical depression and lower socioeconomic status (e.g., less education and less annual income). Taken together, individuals with adverse backgrounds appear to be more vulnerable to experiencing numerous known risk factors for cognitive decline. An important goal will be to understand the additive effects of these risks toward late-life cognition in older adults. In addition, identification of resilience factors is also warranted, considering that specific coping styles, personality traits, or cognitive processes may elucidate approaches that can be used to foster self-esteem and reduce behavioral risk factors in this population. Future research should emphasize identifying mechanisms that may serve a mediating or moderating role in the relationship between ACE and late-life cognition.
FINANCIAL SUPPORT
This work was supported by startup funds from the University of Maine and Maine Economic Improvement Fund, and a National Academy of Neuropsychology Clinical Trial grant provided to the PI (Rebecca K. MacAulay). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.








