INTRODUCTION
Cannabis (CAN) impacts the brain by interacting with the endogenous endocannabinoid (eCB) system, which includes cannabinoid receptor 1 (CB1R, primarily in CNS) and two endogenous ligands [anandamide (AEA) and 2-arachidonoylglycerol (2-AG)] (Di Marzo, De Petrocellis, & Bisogno, Reference Di Marzo, De Petrocellis and Bisogno2005; Eggan & Lewis, Reference Eggan and Lewis2007; Herkenham et al., Reference Herkenham, Lynn, Little, Johnson, Melvin, de Costa and Rice1990). Regular exogenous CAN exposure downregulates the CB1Rs (Hirvonen et al., Reference Hirvonen, Goodwin, Li, Terry, Zoghbi, Morse and Innis2012) and is linked with neurocognitive deficits (see Lisdahl, Wright, Kirchner-Medina, Maple, & Shollenbarger, Reference Lisdahl, Wright, Kirchner-Medina, Maple and Shollenbarger2014). Despite these findings, daily CAN use amongst teens and young adults remains high (approximately 6% of 12th graders and 8% of young adults; Miech et al., Reference Miech, Johnston, O’Malley, Bachman, Schulenberg and Patrick2017).
Prior studies have often noted negative relationships between CAN use and neuropsychological functioning in emerging adults (for review, see Ganzer, Bröning, Kraft, Sack, & Thomasius, Reference Ganzer, Bröning, Kraft, Sack and Thomasius2016; Lisdahl et al., Reference Lisdahl, Wright, Kirchner-Medina, Maple and Shollenbarger2014). More specifically, after periods of brief (12+ hr) abstinence, findings suggest deficits in learning and memory, working memory, planning, and decision-making (Becker et al., Reference Becker, Collins, Schultz, Urosevic, Schmaling and Luciana2018) and in executive functioning (Dahlgren, Sagar, Racine, Dreman, & Gruber, Reference Dahlgren, Sagar, Racine, Dreman and Gruber2016). However, studies such as these do not account for more long-term changes in cognitive functioning even after cessation of use. Thames, Arbid, and Sayegh (Reference Thames, Arbid and Sayegh2014) found that, whether recent- (past 4 weeks) or past-use (greater than 4 weeks abstinence) and regardless of age, CAN users demonstrated decreased cognitive performance across attention, processing speed, and executive functioning, with mild recovery in the past-user group relative to the recent-user group.
Following a month of abstinence, emerging adult CAN users have also been found to have slower psychomotor speed, poorer complex attention, story memory, visual memory, visuospatial functioning, cognitive flexibility, working memory and sequencing (Jacobus, Squeglia, Sorg, Nguyen-Louie, & Tapert, Reference Jacobus, Squeglia, Sorg, Nguyen-Louie and Tapert2014; Jacobus et al., Reference Jacobus, Squeglia, Infante, Castro, Brumback, Meruelo and Tapert2015; Medina et al., Reference Medina, Hanson, Schweinsburg, Cohen-Zion, Nagel and Tapert2007; Winward, Hanson, Tapert, Brown, Reference Winward, Hanson, Tapert and Brown2014); similarly, we found poorer psychomotor speed, sustained attention, and cognitive inhibition in CAN users after a week of abstinence, with male users having greater psychomotor slowing (Lisdahl & Price, Reference Lisdahl and Price2012). A recent meta-analysis of CAN studies of emerging adults concluded that cognitive deficits do not persist beyond 72 hr of abstinence (Scott et al., Reference Scott, Slomiak, Jones, Rosen, Moore and Gur2018). However, some studies included did not adequately control for other substance use, used non-standardized neuropsychological measures, did not exclude for psychiatric comorbidities, or did not assess for dose-dependent relationships. As these differences in study design may have obscured the relationship between the chronic effects of cannabis and cognitive functioning, further assessment of the chronic impact of cannabis is warranted with careful consideration of other variables that may be important moderators (e.g., fitness level). Given prior neuropsychological findings after withdrawal and excluding for psychiatric comorbidities, there is increased interest in investigating more chronic impacts of CAN use and examining potential ameliorative tools that may reverse cannabis-related cognitive deficits.
Animal models demonstrate numerous positive effects of aerobic exercise (AE) on brain health, including improved cognition, neurogenesis, and protection of the nervous system from injury in regions including the prefrontal cortex (PFC; Cotman, Berchtold, & Christie, Reference Cotman, Berchtold and Christie2007; Ding et al., Reference Ding, Li, Luan, Ding, Lai, Rafols and Diaz2004; Stranahan et al., Reference Stranahan, Lee, Becker, Zhang, Maudsley, Martin and Mattson2010; van Praag, Christie, Sejnowski, & Gage, Reference van Praag, Christie, Sejnowski and Gage1999; Vaynman, Ying, & Gomez-Pinilla, Reference Vaynman, Ying and Gomez-Pinilla2004). Pre-clinical research has shown that AE of moderate intensity resulted in greater neurogenesis compared to high-intensity interval training (Leasure & Jones, Reference Leasure and Jones2008) or anaerobic resistance training (Nokia et al., Reference Nokia, Lensu, Ahtiainen, Johansson, Koch, Britton and Kainulainen2016). Notably, a series of studies have found that exercise blocks or reverses the negative effects of alcohol binge drinking on neurogenesis in the dentate gyrus and hippocampus in animals (Crews, Nixon, & Wilkie, Reference Crews, Nixon and Wilkie2004; Hamilton, Criss, & Klintsova, Reference Hamilton, Criss and Klintsova2015; Helfer, Goodlett, Greenough, & Klintsova, Reference Helfer, Goodlett, Greenough and Klintsova2009; Leasure & Nixon, Reference Leasure and Nixon2010), although to date no such research exists in CAN exposure for animals or humans. There are multiple possible mechanisms underlying the benefits of aerobic fitness, including decreased inflammatory response and oxidative stress (Radak, Kumagai, Taylor, Naito, & Goto, Reference Radak, Kumagai, Taylor, Naito and Goto2007; Sakurai et al., Reference Sakurai, Izawa, Kizaki, Ogasawara, Shirato, Imaizumi and Ohno2009), increased c-FOS expression (Dragunow, Reference Dragunow1996; He, Yamada, & Nabeshima, Reference He, Yamada and Nabeshima2002; Sim et al., Reference Sim, Kim, Shin, Chang, Shin, Ko and Kim2008; Vann, Brown, Erichsen, & Aggleton, Reference Vann, Brown, Erichsen and Aggleton2000), increased CB1R activity in the hippocampus (Ferreira-Vieira, Bastos, Pereira, Moreira, & Massensini, Reference Ferreira-Vieira, Bastos, Pereira, Moreira and Massensini2014), and improved catecholaminergic function in brain regions such as the PFC and limbic system (Chaouloff, Reference Chaouloff1989; Dunn, Reigle, Youngstedt, Armstrong, & Dishman, Reference Dunn, Reigle, Youngstedt, Armstrong and Dishman1996; Dunn & Dishman, Reference Dunn and Dishman1991; Elam, Svensson, & Thoren, 1987; Heyes, Garnett, & Coates, Reference Heyes, Garnett and Coates1985; Waters et al., Reference Waters, Emerson, Watt, Forster, Swallow and Summers2005). Converging lines of evidence also suggest changes in growth factors, such as brain-derived neurotrophic factor (BDNF), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF), may underlie AE-related neurocognitive changes (Castrén, Berninger, Leingärtner, & Lindholm, Reference Castrén, Berninger, Leingärtner and Lindholm1998; Cotman & Berchtold, Reference Cotman and Berchtold2002; Egan et al., Reference Egan, Kojima, Callicott, Goldberg, Kolachana, Bertolino and Weinberger2003; Kim, Lee, Kim, Yoo, & Kim, Reference Kim, Lee, Kim, Yoo and Kim2007; Lee et al., Reference Lee, Kim, Lee, Kim, Yang, Chang and Kim2006; Li, Jarvis, Alvarez-Borda, Lim, & Nottebohm, Reference Li, Jarvis, Alvarez-Borda, Lim and Nottebohm2000; Liu, Diorio, Day, Francis, & Meaney, Reference Liu, Diorio, Day, Francis and Meaney2000; Mueller et al., Reference Mueller, Moller, Horstmann, Busse, Lepsien, Bluher and Pleger2015; Whiteman, Young, Budson, Stern, & Schon, Reference Whiteman, Young, He, Chen, Wagenaar, Stern and Schon2014). More specific to CAN users, Koltyn and colleagues has reported that AE results in release of circulating eCB concentrations (Koltyn, Brellenthin, Cook, Sehgal, & Hillard, Reference Koltyn, Brellenthin, Cook, Sehgal and Hillard2014), resulting in improved mood (Brellenthin, Crombie, Hillard, & Koltyn, Reference Brellenthin, Crombie, Hillard and Koltyn2017). Therefore, AE may help restore circulating eCB concentrations in CAN users.
Prior research has suggested that AE may improve cognitive functioning in adolescents and young adults (for review, see Herting & Chu, Reference Herting and Chu2017). Aerobic fitness has been linked to better executive functioning, spatial memory, psychomotor speed, and attention in adolescents (Lee et al., Reference Lee, Wong, Lau, Lee, Yau and So2014; Wengaard, Kristoffersen, Harris, & Gundersen, Reference Wengaard, Kristoffersen, Harris and Gundersen2017), and attention and working memory in young adults (Hwang, Castelli, & Gonzalez-Lima, Reference Hwang, Castelli and Gonzalez-Lima2017). VO2 max (aka maximal oxygen consumption) provides a quantitative measure of one’s capability and capacity for AE (Bassett & Howley, Reference Bassett and Howley2000). By definition, it is the highest amount of oxygen that an individual can consume to make energy aerobically. It also provides a good indication of overall fitness, in that it requires unified and high-level functioning of multiple systems including the ventilaory, cardiovascular, hematologic, and muscular systems (Bassett & Howley, Reference Bassett and Howley2000).
VO2 max increases as a result of AE, such as running, walking, biking, etc. (Bassett & Howley, Reference Bassett and Howley2000). Therefore, the more aerobic activity a person engages in, the higher their VO2 max will be. Other research has linked VO2 max with neurocognitive function. For example, in healthy young adults, superior VO2 max performance was related to larger entorhinal cortex volume, which was in turn associated with better memory (Whiteman et al., Reference Whiteman, Young, Budson, Stern and Schon2016).
The relationship between aerobic fitness and CAN use has been understudied. A longitudinal study (Henchoz et al., Reference Henchoz, Dupuis, Deline, Studer, Baggio, N’Goran and Gmel2014) had young adult males self-report their frequency of exercise and complete a substance use screen. They found fewer cannabis use disorder (CUD) diagnoses and better mental health outcomes in those who maintained regular exercise habits, and lower prevalence of CUD in those who adopted new exercise routines. Another study of adult non-treatment seeking individuals with CUD found reduced cannabis use and craving following 2 weeks of AE (Buchowski et al., Reference Buchowski, Meade, Charboneau, Park, Dietrich, Cowan and Martin2011). Whether aerobic fitness level may moderate cognitive functioning in CAN users have not been previously studied. To date, no one has reported whether aerobic fitness moderates measures of cognitive function in CAN-using youth.
Here we aim to investigate the potential moderating effect of aerobic fitness level on neuropsychological outcomes in regular CAN users. We predict that following 3 weeks of monitored abstinence, greater past-year CAN use would be associated with greater cognitive deficits in a dose-dependent manner, especially on psychomotor speed, complex attention, and executive function tasks (Lisdahl & Price, Reference Lisdahl and Price2012; Medina et al., Reference Medina, Hanson, Schweinsburg, Cohen-Zion, Nagel and Tapert2007). We also predict that greater aerobic fitness would be associated with better neuropsychological performance. Finally, we hypothesize that CAN use would interact with aerobic fitness level, such that individuals with higher levels of past-year CAN use and lower VO2 max would have worse neuropsychological performance relative to individuals with higher VO2 max and greater levels of past-year cannabis use, and controls.
METHODS
Participants
Seventy-nine participants (37 CAN users; 42 controls) were recruited through local newspaper advertisements and fliers placed around Milwaukee, Wisconsin, for the larger parent study (Pricipal Investigaror, Lisdahl; R01 DA030354). Efforts were made to balance for gender and physical activity levels across substance use groups. Individuals were considered healthy controls if they had smoked less than 5 joints (or their equivalent) in the past year and no more than 20 joints in their lifetime. CAN users had smoked at least 52 times in the past year (approximately weekly users). Furthermore, each participant was classified as being high-fit if they were above the 50th percentile in VO2 max performance, and low-fit if they were below the 50th percentile by age and gender (Pescatello, Reference Pescatello2014).
Inclusion criteria included being a fluent English speaker between 16 and 26 years old. Exclusion criteria for all participants included: being left handed, MRI contraindications, past-year co-morbid independent Axis-I disorders, major medical or neurologic disorders, prenatal issues (e.g., gestation <35 weeks) or prenatal alcohol (>4 drinks/day or >7 drinks/week) or illicit drugs (>10 uses), or excessive illicit drug use in lifetime (>50 uses of any drug category except nicotine, alcohol, or CAN). Participants confirmed 3 weeks of abstinence from all alcohol and drug use (other than tobacco) through self-report and drug toxicology screen. The University of Wisconsin Milwaukee and Medical College of Wisconsin IRBs approved all aspects of this study.
Procedure
Screening
Eligibility criteria were established through a two-step screening process. First, participants called in to a study line after seeing fliers around the community advertising for active and sedentary participants. After receiving oral consent from the participant, or oral consent from the parent/guardian and oral assent from the participant (if under 18), they completed a 5- to 10-min phone screen. Each parent and participant were screened separately to answer initial eligibility question (including age, ethnicity, MRI contraindications, and yes/no questions regarding psychiatric and substance use history).
Next, written consent/assent (if under 18) was received via mail and a 45-min detailed phone or in-person screen was scheduled. Participants then completed lifetime history of substance use via the Customary Drinking and Drug Use Record (CDDR) (Brown et al., Reference Brown, Myers, Lippke, Tapert, Stewart and Vik1998; Stewart & Brown, Reference Stewart and Brown1995). Participants and their parents separately completed youth psychiatric history though the Mini International Psychiatric Interview (MINI) or MINI-Kid (if under 18) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998). All participants and their parents were compensated $20 for their time during the phone screen. If eligible, participants were scheduled for study sessions. If ineligible, participants were not informed of the specific reason for ineligibility to protect study integrity.
Study sessions
Five sessions were scheduled over the course of 3.5–4 weeks for all eligible participants. The initial three sessions each occurred 1 week apart. They consisted of a mini-neuropsychological battery to assess impact of CAN withdrawal symptoms on cognitive function (data not examined here), psychological questionnaires, and urinalysis and drug patch analysis to ensure abstinence. The fourth session (data presented here) occurred 1 week after the third session and consisted of a 3-hr fully neuropsychological battery and a VO2 max testing session. The final and fifth session occurred within 24–48 hr of the fourth session and consisted of MRI scanning. For the present study, only data from the fourth session, consisting of the neuropsychological battery and VO2 max, are included, to ensure no potential withdrawal symptoms impact findings (Budney, Hughes, Moore, & Vandrey, Reference Budney, Hughes, Moore and Vandrey2004).
Verifying abstinence
Abstinence was evaluated at each study session to ensure participants remained abstinent from all alcohol and drugs (other than nicotine) for the duration of the study. The ACCUTEST SplitCup 10 Panel drug test was used to measure amphetamines, barbiturates, benzodiazepines, cocaine, ecstasy, methadone, methamphetamines, opiates, phencyclidine (PCP), and delta-9-tetrahydrocannabinol (THC). Urine samples were also tested using NicAlert to test cotinine level, a metabolite of nicotine. Participants also wore PharmChek Drugs of Abuse Patches which continuously monitor sweat toxicology for the presence of cocaine, benzoylecgonine, heroin, 6-monoacetylmorphine, morphine, codeine, amphetamines, methamphetamine, THC, and PCP.
At the start of each session, participants also underwent breathalyzer screens. If positive for THC at Sessions 1–3, participants were considered eligible to remain in the study if their THC level, as measured by the PharmChek patch, went down over time. If positive for any drug or having a breath alcohol concentration greater than .000 at the start of Session 4 (neuropsychology battery and VO2 maximum testing) or Session 5 (MRI scan), participants were ineligible for study participation.
Measures
Psychiatric disorder screening
Participants and their parents separately completed youth psychiatric history though the Mini International Psychiatric Interview (MINI) (for 18 and older) or MINI-Kid (if under 18) (Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998).
Substance use
Lifetime Use. The CDDR (Brown et al., Reference Brown, Myers, Lippke, Tapert, Stewart and Vik1998; Stewart & Brown, Reference Stewart and Brown1995) was used to measure frequency/quantity of lifetime usage, age of first use, age of regular use, and symptoms of substance use disorder for alcohol, nicotine and cannabis. Past-year Use. A modified version of the Timeline Follow-Back (Lisdahl & Price, Reference Lisdahl and Price2012; Sobell & Sobell, Reference Sobell and Sobell1992) was used to measure past 365 days of CAN (joints), alcohol (standard drinks), and other drug use in standard units. For CAN, participants reported method and amount used (e.g., concentrates, hits, joints, etc.) and this was converted to a standard unit of joints. After being cued to memories, holidays, and personally significant events, participants recalled frequency and amount of drug use on each day or, if unable to recall day-by-day information, weekly averages. Last date of use was also captured to measure length of abstinence from substances other than tobacco.
Mood
Participants completed the 21-item Beck Depression Inventory - 2nd Edition (BDI) to measure current depressive symptoms from the past 2 weeks (Beck, Reference Beck1996).
Neuropsychological battery
Cognitive tasks and variables included the following:
1. Select subtests from the Delis-Kaplan Executive Functioning Scale (D-KEFS; Delis, Kaplan, & Kramer, Reference Delis, Kaplan and Kramer2001) were used. Specifically: Color-Word Interference, which consisted of subtests with measuring reading of words, then colors, then two tasks which required inhibiting aspects of reading/naming (e.g., naming color without reading the word). Variables assessed included total reading time in seconds for word reading, color naming, color-word interference, and color-word interference with switching; Verbal Fluency, wherein the participant had 60 s to list as many words as they could that started with a specific letter (variable assessed: FAS total correct); and Trail Making Tests (TMT), which consisted of various connect-the-dot tasks designed to measure simple attention, psychomotor speed, information processing speed, and cognitive flexibility (variables included time to complete in seconds for each of the five conditions).
2. California Verbal Learning Test-II (CVLT-II; Delis, Kramer, Kaplan, & Ober, Reference Delis, Kramer, Kaplan and Ober2000) is a 16-item verbal learning and recall measure with five learning trials, a distractor list, and both a brief and long delay. Variables assessed in this study included initial learning (trial 1), total learning (total trials 1–5), free recall (long delay free recall), and recognition (hits).
3. Rey Complex Figure Task (RCFT; Meyers & Meyers, Reference Meyers and Meyers1995) is a figure which a participant first copies then later has to recall from memory. Variables assessed include visuospatial skills (direct copy score) and visual recall (immediate recall, long delay recall).
4. Penn Emotional Faces Memory Task of the Penn’s Computerized Neurocognitive Battery (PennCNP; Gur et al., Reference Gur, Richard, Hughett, Calkins, Macy, Bilker and Gur2010) measures immediate recognition of faces following a learning trial and was assessed by facial memory total correct.
5. The Weschler Adult Intelligence Scale, Third Edition (WAIS-III; Wechsler, Reference Wechsler1997) Letter-Number Sequencing (LNS) subtest was used to measure working memory through total raw sequencing score.
6. Conner’s Continuous Performance Task, 2nd Edition (CPT-II; Conners, Reference Conners2000) is a task designed to assess sustained attention. Number of omission errors and number of commission errors were used in the present analyses.
7. Iowa Gambling Task (IGT) is a measure of decision making through trying to maximize money in a gambling card task earned and examined here by total net money (won-borrowed; Bechara, Damasio, Damasio, & Anderson, Reference Bechara, Damasio, Damasio and Anderson1994).
8. To estimate intelligence and quality of education (Manly, Jacobs, & Touradji, Reference Manly, Jacobs and Touradji2002), the Wide Range Achievement Test - 4th edition (WRAT-4) Word Reading subtest was used, with normed scores calculated. This test was only used for group comparisons in selection of covariates to ensure groups did not differ in premorbid IQ.
Neurocognitive variables that were not normally distributed (RCFT direct copy; CVLT-II long delay free recall; D-KEFS Trails Condition 4; CPT-II Omission errors) were log-transformed and used in all analyses. All raw scores were used, unless otherwise noted (i.e., WRAT-4 Word Reading). Measurement of Effort: An embedded effort measure from the CVLT-II, the Forced Choice trial, was assessed to ensure proper engagement by participants. No participants were below cutoff scores.
Body size and composition
Body height and mass were measured using standard procedures (Pescatello, Reference Pescatello2014). In addition, a Tanita SC-331S Body Composition Monitor (Tanita, Arlington Heights, IL) was used to measure additional fitness characteristics, such as weight, body mass index (BMI), and fat percentage.
Aerobic fitness (VO2 maximum)
Participants were asked to refrain from food and caffeine for 4 hr before the exercise tests. Before each exercise test, the metabolic measurement system, ParvoMedics TrueOne 2400 (ParvoMedics, Salt Lake City, UT) was calibrated according to the manufacturer’s instructions using a 3-liter syringe for the pneumotachometer, and a two-point calibration for the gas analyzers (room air and a certified gas 4.08% CO2, 115.98% O2, balance N2). Participants were fitted with the rubber mouthpiece connected to a Hans Rudolf 2700 series two-way nonrebreathing valve (Kansas City, MO), noseclip, and heart rate strap (Polar Wearlink 31, Finland) for the collection of expired gases and measurement of heart rate. Participants completed a maximal incremental exercise test on a treadmill (Full Vision Inc., TMX425C Trackmaster, Newton, KS) following the Bruce Protocol until volitional fatigue.
Expired gases were measured continuously using a ParvoMedics TrueOne 2400 metabolic measurement system (ParvoMedics, Salt Lake City, UT); this has been shown to be a valid measure of expired gases at rest and during increasing intensities of activity (Bassett et al., Reference Bassett, Howley, Thompson, King, Strath, McLaughlin and Parr2001). Trained exercise physiology research assistants, supervised by AS, used Howley, Bassett, and Welch (Reference Howley, Bassett and Welch1995) criteria to determine attainment of VO2 max. Metabolic data were averaged over 1 min and exported into a spreadsheet for analysis.
Data Analysis
To determine selection of covariates, two groups were formed: a CAN user group with at least an average of one joint per week over the past year, and a control group with less than 5 joints (or equivalent) in the past year and no more than 20 in their lifetime. Continuous variables reflecting total past-year CAN use (not groups) were used in the primary analyses. In addition, participants were again divided into a high (at or above 50th percentile) or low (below 50th percentile) fitness group, based on normed performance on VO2 max (Pescatello, Reference Pescatello2014), although again continuous variables, not group, were used in analyses. Between-group differences on demographic variables by both CAN group and fitness group were measured with analyses of variance and χ2 tests. Controlling for potential confounds (i.e., past-year alcohol use, cotinine level, BDI-II score, gender, and BMI), multiple regressions were run to examine whether past-year cannabis use or VO2 max performance independently related to cognitive functioning after the required abstinence period.
The potential interactive effect of past-year CAN use and VO2 max performance was also assessed as a second block in the regression; for ease of presentation, the interaction term used is CAN*VO2, though total past-year joints and actual VO2 max performance, not groups, were used to calculate the interaction. Twenty-two separate multiple regressions were run, with covariates and independent variables assessed in the first block of the regression, and the interaction variable added in the second block. DFBetas were examined to rule out outliers. Notably, results remain consistent regardless of whether or not length of abstinence is included as a covariate. No correction for multiple comparisons was used due to power limitations.
RESULTS
Demographic, Drug Use, and Fitness Differences According to Drug Group
Demographics
Substance use groups did not differ significantly on age [F(1,77) = .96; p = .33], education [F(1,77) = .87; p = .35], reading level (from the WRAT-4) [F(1,77) = .94; p = .34], race (χ2 = 5.81; p = .44), or ethnicity (χ2 = 3.00; p = .22). They differed by gender (χ2 = 3.98; p = .05) and depression symptoms as measured by the BDI-II [F(1,77) = 8.89; p = .004] (these are included as covariates in all analyses). See Table 1. Fitness Characteristics: Substance use groups did not differ significantly in weight [F(1,76) = .04; p = .85], body fat percentage [F(1,76) = 2.63; p = .11], BMI [F(1,76) = .00; p = .99], height [F(1,77) = .08; p = .78], waist circumference [F(1,76) = .99; p = .32], or VO2 max [F(77) = 1.55; p = .22]. Drug Use Patterns: CAN use groups differed significantly in drug use patterns, including past-year CAN use [F(1,77) = 41.11; p<.001], lifetime cannabis use [F(1,77) = 31.48; p<.001], cotinine level [F(1,77) = 11.65; p = .001], and past-year alcohol use [F(1,77) = 17.26; p<.001]. The latter two are covariates in all analyses.
Table 1 Demographics, substance use, and fitness characteristics by substance group
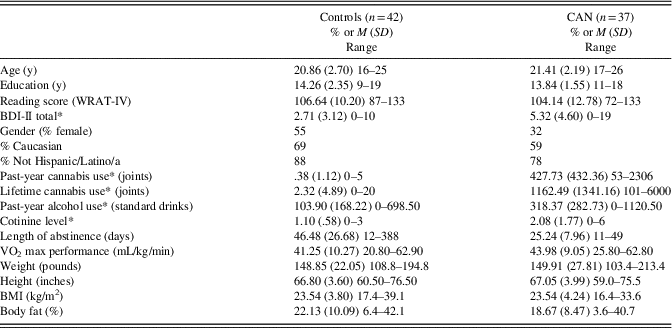
Note. Length of abstinence measures last use of any substance other than tobacco. Length of abstinence data were missing from nine controls as they denied any history of substance use.
* p<.05.
BMI = body mass index; M = mean; SD = standard deviation.
Demographic, Drug Use, and Fitness Differences According to Aerobic Fitness Group
Demographics
Fitness groups did not differ significantly by age [F(1,77) = .30; p = .59], education [F(1,77) = .02; p = .89], reading level (from the WRAT-4) [F(1,77) = 2.84; p = .10], depression symptoms as measured by the BDI-II [F(1,77) = .16; p = .69], race (χ2 = 6.87; p = .33), or ethnicity (χ2 = 3.54; p = .17). They differed by gender (χ2 = 11.67; p = .001), which was included as a covariate for all multiple regression analyses. See Table 2. Drug Use Patterns: Fitness groups differed by cotinine level [F(1,77) = 5.28; p = .02] and past-year alcohol use [F(1,77) = 6.58; p = .01] (high-fit youth used more alcohol and had used nicotine more recently), but not past-year CAN use [F(1,77) = .04; p = .84] or lifetime CAN use [F(1,77) = 3.57; p = .06]. In addition, in individuals who had used CAN, fitness groups did not differ by age of first use (χ2 = 4.23; p = .94) or regular use (χ2 = 6.85; p = .55). Fitness Characteristics: Fitness groups did not differ significantly in weight [F(1,76) = .08; p = .78], but as expected they did differ by fat percentage [F(1,76) = 26.20; p<.001], BMI [F(1,76) = 6.23; p = .02], height [F(1,77) = 19.40; p<.001], and VO2 max performance [F(77) = 136.89; p< .001]. BMI was included as a covariate to control for potential impact of body composition on cognition.
Table 2 Demographics, Substance Use, and Fitness Characteristics by Fitness Group
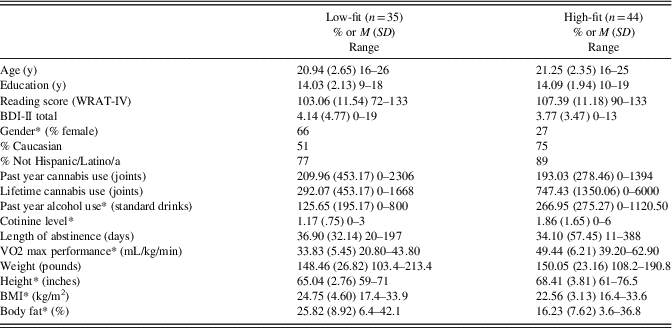
Note. Length of abstinence measures last use of any substance other than tobacco. Length of abstinence data were missing from three high-fit and six low-fit participants as they denied any history of substance use.
* p<.05.
BMI = body mass index; M = mean; SD = standard deviation.
Primary Outcomes
All results are on cognitive performance at a single time point following a 3-week period of abstinence.
CAN results
Greater past-year CAN use was significantly associated with decreased LNS performance (beta = -.39; t = −2.89; p = .005; f2 = .12) and slower time to complete TMT motor sequencing task (beta = .31; t = 2.35; p = .02; f2 = .08).
VO2 max results
Better VO2 max performance significantly related to better verbal fluency (beta = .32; t = 2.16; p = .03; f2 = .07), TMT motor sequencing (beta = -.31; t = −2.26; p = .03; f2 = .07), and facial memory recognition (beta = .45; t = 3.14; p = .003; f2 = .14).
CAN*VO2 results
CAN interacted with VO2 max to predict CVLT Trial 1, TMT switching, TMT motor sequencing, and RCFT delayed recall. CAN interacted with VO2 max in association with CVLT Trial 1 performance (beta = -.25; t = −1.94; p = .05; f2 = .06), with more fit controls having better initial learning, while cannabis users’ performance remained the same regardless of fitness level. On TMT switching (beta = -.29; t = −2.37; p = .02; f2 = .08), individuals with higher past-year CAN use and higher VO2 max and individuals with less (or no) cannabis use and lower VO2 max exhibited faster performance than either low-fit users or high-fit controls (see Figure 1). On TMT motor sequencing (beta = -.32; t = −2.86; p = .006; f2 = .12), individuals who used more CAN in the past year and had better on VO2 max performed more quickly than either individuals with high past-year CAN use and low VO2 max or controls. On RCFT delayed recall (beta = .26; t = 2.02; p = .05; f2 = .06), individuals with more past-year CAN use and with higher VO2 max attained the highest raw score (see Figure 2).

Fig. 1 TMT condition 5 (motor speed) by CAN and VO2 max. Notes: D-KEFS TMT condition 5 psychomotor speed measured in time to complete (seconds), with quicker performance indicating better psychomotor speed. While continuous variables were used in the interaction term for the MJ*VO2 interaction, for the sake of presentation here, VO2 max performance is presented as a categorical variable.

Fig. 2 RCFT delayed recall by CAN and VO2 max. Notes: RCFT Delayed Recall for visual memory, with higher scores indicating better recall. While continuous variables were used in the interaction term for the MJ*VO2 interaction, for the sake of presentation here, VO2 max performance is presented as a categorical variable.
Covariate results
Alcohol: Past-year alcohol was related to increased D-KEFS FAS total correct raw score (beta = .32; t = 2.65; p = .01; f2 = .10), quicker D-KEFS inhibition/switching Stroop completion (beta = -.27; t = −2.13; p = .04; f2 = .07), and fewer commissions in CPT (beta = -.32; t = −2.56; p = .01; f2 = .10). Gender: Females performed better than males on Penn CNP facial memory recognition (beta = .44; t = 3.19; p = .002; f2 = .15).
Discussion
The present study aimed to investigate the impact of adolescent and emerging adult CAN use on neuropsychological functioning following 3 weeks of monitored abstinence, and to assess whether aerobic fitness level moderated the impact of CAN on cognitive function. We found that past-year CAN use, in a dose-dependent manner, was associated with poorer working memory and psychomotor speed following a monitored 3-week abstinence period. In addition, we found that the novel interaction between cannabis use and aerobic fitness level was associated with performance on working memory, sequencing ability, psychomotor speed, and visual memory. In general, CAN users who had higher aerobic fitness level performed better on these cognitive tasks than CAN users who had lower aerobic fitness level.
Consistent with prior findings (Lisdahl & Price, Reference Lisdahl and Price2012; Medina et al., Reference Medina, Hanson, Schweinsburg, Cohen-Zion, Nagel and Tapert2007; Thames et al., Reference Thames, Arbid and Sayegh2014), we found a significant relationship between increased CAN use and poorer attention/working memory and psychomotor speed, even after a monitored abstinence period of 3 weeks. Inconsistent with prior research (Becker, Collins, & Luciana, Reference Becker, Collins and Luciana2014; Fried, Watkinson, & Gray, Reference Fried, Watkinson and Gray2005; Jacobus et al., Reference Jacobus, Squeglia, Infante, Castro, Brumback, Meruelo and Tapert2015; Medina et al., Reference Medina, Hanson, Schweinsburg, Cohen-Zion, Nagel and Tapert2007; Solowij et al., Reference Solowij, Jones, Rozman, Davis, Ciarrochi, Heaven and Yücel2011), we did not find a significant relationship between past-year CAN use and memory in the present study. However, we previously did not find a relationship between verbal memory and CAN in a separate sample (Lisdahl & Price, Reference Lisdahl and Price2012) and attributed this finding to the likely recovery of verbal memory function (Hanson et al., Reference Hanson, Winward, Schweinsburg, Medina, Brown and Tapert2010) and hippocampal volume (Yucel et al., Reference Yucel, Lorenzetti, Suo, Zalesky, Fornito, Takagi and Solowij2016) within the first couple weeks of abstinence from CAN.
We also found that CAN use and aerobic fitness level interacted to predict performance on neuropsychological tasks measuring psychomotor speed, visual memory, and sequencing ability. No other studies to date have examined these relationships. More specifically, when looking at the CAN users, high-fit CAN users performed better than low-fit CAN users on tests of sequencing ability, psychomotor speed, and visual memory. Other groups have found reduced symptoms of CUD and reduced craving in CAN users who exercise (Buchowski et al., Reference Buchowski, Meade, Charboneau, Park, Dietrich, Cowan and Martin2011; Henchoz et al., Reference Henchoz, Dupuis, Deline, Studer, Baggio, N’Goran and Gmel2014). The underlying mechanism for this finding is likely multi-factorial. Engaging in aerobic activity, which improves overall aerobic fitness, may counteract the downregulation of regular CAN use on CB1R (Ferreira-Vieira et al., Reference Ferreira-Vieira, Bastos, Pereira, Moreira and Massensini2014; Hirvonen et al., Reference Hirvonen, Goodwin, Li, Terry, Zoghbi, Morse and Innis2012), especially in frontal and parietal cortical regions. Engaging in AE may also increase circulating eCB levels (Koltyn et al., Reference Koltyn, Brellenthin, Cook, Sehgal and Hillard2014), which are linked with cognitive function (Egerton, Allison, Brett, & Pratt, Reference Egerton, Allison, Brett and Pratt2006; Lee & Gorzalka, Reference Lee and Gorzalka2012). AE also results in release of neurotrophic growth factors such as BDNF, IGF-1, and VEGF (Castrén et al., Reference Castrén, Berninger, Leingärtner and Lindholm1998; Cotman & Berchtold, Reference Cotman and Berchtold2002; Egan et al., Reference Egan, Kojima, Callicott, Goldberg, Kolachana, Bertolino and Weinberger2003; Kim et al., Reference Kim, Lee, Kim, Yoo and Kim2007; Lee et al., Reference Lee, Kim, Lee, Kim, Yang, Chang and Kim2006; Li et al., Reference Li, Jarvis, Alvarez-Borda, Lim and Nottebohm2000; Liu et al., Reference Liu, Diorio, Day, Francis and Meaney2000; Mueller et al., Reference Mueller, Moller, Horstmann, Busse, Lepsien, Bluher and Pleger2015; Whiteman et al., Reference Whiteman, Young, He, Chen, Wagenaar, Stern and Schon2014), improved catecholaminergic (dopamine, norepinephrine, and epinephrine) function (Chaouloff, Reference Chaouloff1989; Dunn et al., Reference Dunn, Reigle, Youngstedt, Armstrong and Dishman1996; Dunn & Dishman, Reference Dunn and Dishman1991; Elam et al., Reference Elam, Svensson and Thorén1987; Heyes et al., Reference Heyes, Garnett and Coates1985; Waters et al., Reference Waters, Emerson, Watt, Forster, Swallow and Summers2005), increased c-FOS expression (Dragunow, Reference Dragunow1996; He et al., Reference He, Yamada and Nabeshima2002; Sim et al., Reference Sim, Kim, Shin, Chang, Shin, Ko and Kim2008; Vann et al., Reference Vann, Brown, Erichsen and Aggleton2000), decreased inflammatory response and oxidative stress (Radak et al., Reference Radak, Kumagai, Taylor, Naito and Goto2007; Sakurai et al., Reference Sakurai, Izawa, Kizaki, Ogasawara, Shirato, Imaizumi and Ohno2009), and increased CB1R activity in the hippocampus (Ferreira-Vieira et al., Reference Ferreira-Vieira, Bastos, Pereira, Moreira and Massensini2014).
Another surprising result was the lack of relationship between alcohol use and neuropsychological outcomes, and occasional positive relationships. One reason for this may be the present study’s assessment of total use, rather than patterns of use that may be more neurotoxic (e.g., frequency, heavy episodic or binge drinking). Others have found age of onset of use (Nguyen-Louie et al., Reference Nguyen-Louie, Matt, Jacobus, Li, Cota, Castro and Tapert2017), frequency (Nguyen-Louie et al., Reference Nguyen-Louie, Castro, Matt, Squeglia, Brumback and Tapert2015), or binge drinking patterns (Nguyen-Louie et al., Reference Nguyen-Louie, Tracas, Squeglia, Matt, Eberson-Shumate and Tapert2016) to be related to decrements in neuropsychological functioning, none of which were specifically investigated here. Alternatively, the socially facilitative nature of initial alcohol experimentation (see Varlinskaya & Spear, Reference Varlinskaya and Spear2015) may lead to better social and, by extension, neuropsychological outcomes, as has been indicated across the lifespan (see Kang, Boss, & Clowtis, Reference Kang, Boss and Clowtis2016).
Given the present results, aerobic fitness may be an exciting and low-cost means of intervening to improve psychological and cognitive functioning in CAN users, as has been suggested by our group (Lisdahl, Gilbart, Shollenbarger & Wright, Reference Lisdahl, Gilbart, Shollenbarger and Wright2013) and others (Brellenthin & Koltyn, Reference Brellenthin and Koltyn2016). Augmentation of existing cognitive behavioral and motivational interviewing interventions for substance using youth with AE may boost youth’s ability to process, manipulate, and retain information, leading to superior outcomes. There is also potential opportunity to use AE as a prevention technique, as improving youth’s executive function, processing speed, and memory may reduce risk for substance use initiation.
There are limitations to consider. While relationships between CAN use, fitness, and neuropsychological functioning were found, causality cannot be established due to the cross-sectional nature of this study; longitudinal studies, such as the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org/), are needed to clarify directionality of relationships. Recent research has indicated that CAN users included in studies such as this are not representative of the “typical” CAN user (e.g., lack of psychiatric co-morbidity, lower frequency of use; Rosen, Sodos, Hirst, Vaughn, & Lorkiewicz, Reference Rosen, Sodos, Hirst, Vaughn and Lorkiewicz2018); therefore, these results may not generalize and/or may not reveal the true extent of relationships between CAN use and cognition in higher-risk groups. Even in domains where deficits were found, these were modest effects well below the typical 1.5 SDs associated with clinical significance; the real-world functional implications about such relative declines are lesser known, although CAN users have reported lower academic achievement (Fergusson & Boden, Reference Fergusson and Boden2008; Horwood et al., Reference Horwood, Fergusson, Hayatbakhsh, Najman, Coffey, Patton and Hutchinson2010; Suerken et al., Reference Suerken, Reboussin, Egan, Sutfin, Wagoner, Spangler and Wolfson2016), reduced salaries (Fergusson & Boden, Reference Fergusson and Boden2008), and reduced life satisfaction (Fergusson & Boden, Reference Fergusson and Boden2008; Grevensteine & Kroninger-Jungaberle, Reference Grevenstein and Kroninger-Jungaberle2015).
Another limitation is that, while participants were encouraged to exercise as long as possible, only 80% reached their true VO2 max as defined by Howley and colleagues (1995); therefore, their predicted performance is used in lieu of maximum performance. In addition, while VO2 max is the gold standard in assessing cardiorespiratory health and is typically highly correlated with recent aerobic activity, it does not indicate exact levels of physical activity or sedentary behaviors. Other factors also influence VO2 maximum performance (gender, genetics, age, body composition, etc.). In this sample, adiposity (body fat composition) was too highly correlated with VO2 max (r’s>.65) to be included in the same regressions due to multicollinearity, although gender and age were controlled for in the regressions. Larger samples may want to consider examining the unique influences of adiposity, levels of physical activity, and aerobic fitness on neurocognition in cannabis users.
Taken together, our results provide further evidence of increased CAN use being related to poorer working memory, sequencing ability, and psychomotor speed, even after 3 weeks of monitored abstinence. Most notably, our results also suggest aerobic fitness may moderate these effects, such that individuals with higher VO2 max and more past-year CAN use may have better neuropsychological performance than low-fit users. AE, therefore, may be a promising ameliorative cognitive intervention for regular CAN users, although future research is needed to assess causality and effectiveness of such an approach.
ACKNOWLEDGMENTS
This study was supported by funding from NIDA (R01 DA030354; P.I.: Lisdahl, K.M.) and manuscript preparation was supported by funding by the National Institutes of Health (U01DA041025; P.I.: Lisdahl, K.M.). The funding sources had no further role in the study design, data collection, analysis, interpretation, the writing of the report, or in the decision to submit the article for publication. The authors have no conflicts of interest to declare.






