INTRODUCTION
Apathy, defined as the impairment in motivation and goal-directed behaviour, is a pervasive syndrome in neurological disorders (R. Levy, Reference Levy2012; Marin, Reference Marin1991). People with brain-related disorders commonly show apathy because of disruptions to brain circuits associated with reward-effort processing (Husain & Roiser, Reference Husain and Roiser2018; Le Heron, Apps, & Husain, Reference Le Heron, Apps and Husain2018). According to the Dimensional Apathy Framework (R. Levy & Dubois, Reference Levy and Dubois2006; Radakovic & Abrahams, Reference Radakovic and Abrahams2018), one of the most widely used theoretical models for this behavioural syndrome, symptoms of apathy can be classified into three dimensions: 1) executive apathy: the decline of organisation and planning; 2) emotional apathy: affective indifference and 3) initiation apathy: the reduction of behaviour execution and spontaneity. These divergent clinical features of apathy are linked to disturbances in different neural regions: executive apathy is associated with disruptions to the dorsolateral prefrontal cortex, emotional apathy with disruptions to the orbito-medial prefrontal cortex, and initiation apathy with disruptions to subcortical areas such as the basal ganglia (Kumfor, Zhen, Hodges, Piguet, & Irish, Reference Kumfor, Zhen, Hodges, Piguet and Irish2018; Wei, Irish, Hodges, Piguet, & Kumfor, Reference Wei, Irish, Hodges, Piguet and Kumfor2020). The presence of apathy is linked to detrimental outcomes for both the patients (e.g., higher mortality rates, poor commitment to interventions and lower levels of daily independence) (Ciurli, Formisano, Bivona, Cantagallo, & Angelelli, Reference Ciurli, Formisano, Bivona, Cantagallo and Angelelli2011; Lansdall et al., Reference Lansdall, Coyle-Gilchrist, Vázquez Rodríguez, Wilcox, Wehmann, Robbins and Rowe2019; Zahodne & Tremont, Reference Zahodne and Tremont2013) and their family members (e.g., elevated levels of burden and distress) (Dauphinot et al., Reference Dauphinot, Delphin-Combe, Mouchoux, Dorey, Bathsavanis, Makaroff and Krolak-Salmon2015; Leroi et al., Reference Leroi, Harbishettar, Andrews, McDonald, Byrne and Burns2012; Wong et al., Reference Wong, Irish, Husain, Hodges, Piguet and Kumfor2020), highlighting the importance of clinical and research approaches to apathy.
Despite the well characterised neural substrate of apathy, mounting research has suggested that the manifestation of apathy may not be universal across cultural contexts (Caracuel et al., Reference Caracuel, Verdejogarcia, Vilarlopez, Perezgarcia, Salinas, Cuberos and Puente2008; Chow et al., Reference Chow, Liu, Fuh, Leung, Tai, Chen and Cummings2002). For example, individuals from the USA with Alzheimer’s disease were reported to have a greater frequency of apathy than their Chinese counterparts (Chow et al., Reference Chow, Liu, Fuh, Leung, Tai, Chen and Cummings2002). The pattern of more severe apathy was also observed in healthy participants in the US compared to those in Spain (Caracuel et al., Reference Caracuel, Verdejogarcia, Vilarlopez, Perezgarcia, Salinas, Cuberos and Puente2008). Cultural differences in family expectations of illness behaviour, or family accommodations of disability, are just two of many possible factors that may affect reporting of apathy symptoms. However, to date, there has been little to no systematic examination of such cross-cultural differences or their causes. Notwithstanding this lack of research, such discrepancies point to a major issue in over-generalisation of results from one culture to another. Particularly, the bias attributed to research on Western, educated, industrialised, rich, and democratic groups is increasingly recognised (Henrich, Heine, & Norenzayan, Reference Henrich, Heine and Norenzayan2010). Variations in the prevalence of apathy between cultures emphasise the importance of understanding this behavioural syndrome from a specific cultural setting so as to enhance the effectiveness of therapeutic interventions.
Vietnam is a developing country with increasing prevalence and high catastrophic impacts of brain-related disorders (Carr et al., Reference Carr, Kahn, Mathkour, Biro, Bui and Dumont2018). While the developmental trend of neurological conditions is reported to mimic the current picture in more industrialised countries (Carr et al., Reference Carr, Kahn, Mathkour, Biro, Bui and Dumont2018), healthcare systems and cultural values in Vietnam are distinct. For example, the responsibility for rehabilitation and recovery after hospital discharge is often placed on patients and families due to the limited availability or high costs of secondary care services (Pekerti, Vuong, Ho, & Vuong, Reference Pekerti, Vuong, Ho and Vuong2017). Furthermore, the dominant Confucian culture in Vietnam, which highly values filial piety and familism, may increase the burden of care duties for caregivers on the one hand, and impede patients’ independence on the other (Meyer et al., Reference Meyer, Nguyen, Dao, Vu, Arean and Hinton2015; T. Nguyen & Levkoff, Reference Nguyen and Levkoff2020). These factors might result in a different profile of apathy in Vietnamese patients relative to those in more individualistic cultures. However, lack of well-validated assessment tools has limited the development of this research area. To our knowledge, no study on apathy has been conducted in either clinical or healthy Vietnamese populations. Based on previously used procedures (e.g., Radakovic & Abrahams, Reference Radakovic and Abrahams2014; Santangelo et al., Reference Santangelo, Raimo, Siciliano, D’Iorio, Piscopo, Cuoco and Trojano2017) and as a first step in paving the way for the cultural exploration of apathy in neurological disorders, the current study aimed to translate and validate the self-report and informant version of two widely used, psychometrically sound measures of apathy, the Frontal Systems Behavioural Scale – Apathy subscale (FrSBe-A) and the Dimensional Apathy Scale (DAS), in a healthy Vietnamese population.
The study had three overarching aims. Firstly, we aimed to use translation and adaption to develop a culturally appropriate version of the FrSBe-A and DAS for the Vietnamese community (V-FrSBe and V-DAS) entailing both self-report and informant versions. The second aim was to evaluate the basic psychometric adequacy of the adapted Vietnamese scales (both informant and self-report), including their reliability (internal consistency) and construct validity (i.e., factor structure, convergent and divergent validity). It was hypothesised that the Vietnamese scales would have adequate internal consistency and that items of the V-DAS would load onto three factors as proposed by the Dimensional Apathy Framework. Regarding convergent validity, the V-FrSBe-A and V-DAS were expected to positively correlate with each other. Within each scale, higher apathy ratings on the self-report version were hypothesised to correlate with higher apathy ratings on its informant-report equivalent. For divergent validity, previous studies have demonstrated that although apathy may co-exist with depression and disinhibition, they are distinct behavioural deficits (M. Levy et al., Reference Levy, Cummings, Fairbanks, Masterman, Miller, Craig and Litvan1998; Zamboni, Huey, Krueger, Nichelli, & Grafman, Reference Zamboni, Huey, Krueger, Nichelli and Grafman2008). Therefore, apathy without depression (assessed with the Vietnamese Depression Anxiety Stress Scale-21 [V-DASS-21]) and without disinhibition (measured by the V-FrSBe–Disinhibition subscale [V-FrSBe-D]) were hypothesised to occur in exclusive proportions of people, confirming the conceptual distinction of these constructs. The third aim was to identify cut-offs for V-FrSBe-A and V-DAS based on the investigation of demographic effects on apathy and preliminary comparisons between the Vietnamese samples and the sample used in the original English validations. Different profiles of apathy between the two samples were expected.
METHOD
Participants
A convenience sample of 112 healthy subjects and 64 close relatives/informants participated in the study. The sample of main subjects included 58 males and 54 females, from 18 to 65 years of age with average 13.3 years of education (see Table 1 for more detail). All participants (main subjects and their informants) were recruited from the community in Ho Chi Minh City and its adjacent metropolises in Vietnam. Information about the study was advertised via social media. Potential participants with interest in the study were contacted and pre-screened. Inclusion criteria included (a) 18 to 65 years of age, (b) normal-to-corrected vision and hearing and (c) being able to give consent to participation. Participants were excluded if they had any significant history and/or current diagnosis of neurological and/or psychiatric disorders. Having met the research criteria, participants either visited the clinic to complete the questionnaires or received an online link to the scales. Most participants filled out the questionnaires without the researcher’s presence, with the exception that those with limited literacy (e.g., education < 5) had assistance (i.e., the researcher read each item for them to answer). Participants received either course credits or VND200,000 (˜USD10) for their participation.
Table 1. Demographic information

Note: the majority of close relatives/informants are immediate family members such as spouses, parents, siblings and adult children.
All participants provided written informed consent. The UNSW Human Ethics Committee and Cho Ray Hospital’s Ethics Committee for Biomedical research approved the study.
Adaptation Procedure for the FrSBe-A and DAS
The FrSBe-A
The FrSBe-A (Grace, Stout, & Malloy, Reference Grace, Stout and Malloy1999) has 14 items to quantify apathy in clinical syndromes including traumatic brain injury, dementia, Parkinson’s disease, multiple sclerosis and schizophrenia (Cahn-Weiner, Grace, Ott, Fernandez, & Friedman, Reference Cahn-Weiner, Grace, Ott, Fernandez and Friedman2002; Goverover, Chiaravalloti, & Deluca, Reference Goverover, Chiaravalloti and Deluca2005; Lane-Brown & Tate, Reference Lane-Brown and Tate2009; Niemeier, Perrin, Holcomb, Nersessova, & Rolston, Reference Niemeier, Perrin, Holcomb, Nersessova and Rolston2013; Velligan, Ritch, Sui, Dicocco, & Huntzinger, Reference Velligan, Ritch, Sui, Dicocco and Huntzinger2002). To determine the clinical significance of apathy, a t score is calculated for all apathy items, adjusted for age, sex and education. The FrSBe-A is a reliable apathy measure (alpha = .88) (Carvalho, Ready, Malloy, & Grace, Reference Carvalho, Ready, Malloy and Grace2013) and correlates with daily functional outcome measures (Smith, Smith, & Juengst, Reference Smith, Smith and Juengst2020; Velligan et al., Reference Velligan, Ritch, Sui, Dicocco and Huntzinger2002). Despite a number of translations available (e.g., Chinese, Dutch, Spanish and German) (Carvalho, Buelow, Ready, & Grace, Reference Carvalho, Buelow, Ready and Grace2016), the scale has only been officially validated in Spanish (Caracuel et al., Reference Caracuel, Verdejo-García, Fernández-Serrano, Moreno-López, Santago-Ramajo, Salinas-Sánchez and Pérez-García2012). In the present study, main subjects and their informants rated each item on a 5-point scale: “almost never” = 1, “seldom” = 2, “sometimes” = 3, “frequently” = 4 and “almost always” = 5. The total score ranges from 14 to 70 with higher scores indicating greater apathy.
The DAS
The 24-item DAS (Radakovic & Abrahams, Reference Radakovic and Abrahams2014) has been established to characterise executive (e.g., “I find it difficult to keep my mind on things”), emotional (e.g., “I express my emotions”) and initiation (e.g., “I contact my friends”) apathy based on the Dimensional Apathy Framework (8 items in each subscale). The scale is used widely in English-speaking countries across clinical groups such as dementia, Parkinson’s disease and amyotrophic lateral sclerosis (Radakovic et al., Reference Radakovic, Colville, Cranley, Starr, Pal and Abrahams2020; Radakovic et al., Reference Radakovic, Stephenson, Colville, Swingler, Chandran and Abrahams2016; Wei et al., Reference Wei, Irish, Hodges, Piguet and Kumfor2020), as well as validated in Italian (Santangelo et al., Reference Santangelo, Raimo, Siciliano, D’Iorio, Piscopo, Cuoco and Trojano2017), French (M’Barek, Radakovic, Noquet, Laurent, & Allain, Reference M’Barek, Radakovic, Noquet, Laurent and Allain2018) and Japanese (Kawagoe, Onoda, Yamaguchi, & Radakovic, Reference Kawagoe, Onoda, Yamaguchi and Radakovic2020). The DAS is reliable for apathy assessment (internal consistent coefficients ≥.77, test–retest reliability coefficients ≥.72), divergent from depression, and convergent with other apathy measures, as well as functioning and disability (Radakovic & Abrahams, Reference Radakovic and Abrahams2014; Radakovic, Davenport, Starr, & Abrahams, Reference Radakovic, Davenport, Starr and Abrahams2018). Main subjects and informants in the current study rated each item on a 4-point scale: “rarely” = 0, “sometimes” = 1, “often” = 2, “almost always” = 3. The highest score for each subscale is 24 and for the total scale is 72. Higher scores indicate greater apathy.
Adaptation procedure
The process for adapting the self-reported and informant-reported DAS and FrSBe-A followed the recommended guidelines (Guillemin, Bombardier, & Beaton, Reference Guillemin, Bombardier and Beaton1993; Sousa & Rojjanasrirat, Reference Sousa and Rojjanasrirat2011):
-
1. The English scales were translated into Vietnamese by a Vietnamese-born, psychology graduate in an Australian university who is cognizant of both language and the theoretical background on apathy (H.Q.).
-
2. The Vietnamese versions were translated back into English by two independent professionals who were bilingual, knowledgeable of both cultures and blind to the original scales. Any disagreements from the translated and back-translated versions were revised and resolved by translators and an expert neuropsychologist (S.M.).
-
3. Five Vietnamese volunteers were invited to explain what they thought each item meant. Where the explanation did not reflect the original content, further refinement was conducted.
-
4. A pre-final draft was read by 20 other volunteers. Follow-up interviews were conducted to gather information about readability and cultural appropriateness of the scales. Any comments raised by the respondents were considered for the final version. For the V-FrSBe-A, upon reproduction permission, a draft of the translated and back-translated version was sent to the publisher for further revision and approval. The DAS is publicly available (Radakovic & Abrahams, Reference Radakovic and Abrahams2014).
-
5. The final sample of 112 main subjects and 64 informants completed the final V-FrSBe-AFootnote 1 and V-DAS (see Supplementary Material 1 for the V-DAS).
Additional Clinical Measures
The V-DASS-21 is reliable and sensitive to mood problems in adolescents and rural women in Vietnam (Le et al., Reference Le, Tran, Holton, Nguyen, Wolfe and Fisher2017; Tran, Tran, & Fisher, Reference Tran, Tran and Fisher2013). Since all 21 items of the V-DASS-21 loaded onto one factor and the combined subscales had stronger detectability for depression (Tran et al., Reference Tran, Tran and Fisher2013), the total scale of V-DASS-21, rather than the depression subscale only, was used to investigate divergent validity of apathy and depression. Main subjects rated each item on a 4-point scale from “did not apply to me at all” = 0 to “applied to me very much, or most of the time” = 3. The total score ranges from 0 to 63 with a cut-off of 36 for the presence of depression (Tran et al., Reference Tran, Tran and Fisher2013).
The V-FrSBe-D, as a measure of disinhibition, was translated and adapted from the original FrSBe (Grace et al., Reference Grace, Stout and Malloy1999) based on the same above procedure of the V-FrSBe-A. The V-FrSBe-D was used to assess divergent validity of apathy and disinhibition. Each of 15 V-FrSBe-D items were rated on a 5-point scale: “almost never” = 1, “seldom” = 2, “sometimes” = 3, “frequently” = 4 and “almost always” = 5. The total score is from 15 to 75 with higher scores indicating greater disinhibition. A cut-off of 43 was established for the presence of disinhibition based on 2 SD of the mean in the current sample.
Statistical Analyses
SPSS-version 26 and R-version 4.0.2 were used for statistical analyses. Normality of distribution and homogeneity of variances were explored using Kolmogorov–Smirnov tests and Levene’s tests, respectively. Internal consistency of the V-FrSBe-A and V-DAS were examined using McDonald’s omega total values, with ω t > .70 indicating adequate internal consistency (McDonald, Reference McDonald2013).
To examine whether the factor structure of V-DAS was consistent with the Dimensional Apathy Framework, a confirmatory factor analysis (CFA) and an exploratory structural equation model (ESEM) was conducted for the self-report V-DAS with the weighted least square estimator for ordinal variables (Hu & Bentler, Reference Hu and Bentler1999; Li, Reference Li2016; Yu, Reference Yu2002). Statistical power for the ESEM was identified as adequate with the Kaiser–Meyer–Olkin measure = .665 and Bartlett’s test of sphericity showing sufficient correlations between items (p < .001). The ESEM, which tolerates the flexibility of cross-loadings, is considered as more advantageous for construct confirmation compared to the traditional CFA (Asparouhov & Muthén, Reference Asparouhov and Muthén2009; Marsh et al., Reference Marsh, Muthén, Asparouhov, Lüdtke, Robitzsch, Morin and Trautwein2009; Perry, Nicholls, Clough, & Crust, Reference Perry, Nicholls, Clough and Crust2015). Based on the previously published procedure (Arens & Morin, Reference Arens and Morin2016; Fischer & Karl, Reference Fischer and Karl2019), both the CFA and ESEM were completed and compared for their goodness-of-fit indices for the solution decision. On the theoretical basis of the DAS, three latent factors for executive, emotional and initiation apathy were entered into each model with an oblimin Geomin rotation being identified in the ESEM. The model fit was assessed using robust estimates of the comparative fit index (CFI), the Tucker–Lewis Index (TLI) and the root mean square error of approximation (RMSEA), with CFI and TLI >.90 and RMSEA <.08 indicating a good model fit (Arens & Morin, Reference Arens and Morin2016; Beauducel & Herzberg, Reference Beauducel and Herzberg2006; Tóth-Király, Bõthe, Rigó, & Orosz, Reference Tóth-Király, Bõthe, Rigó and Orosz2017).
To examine convergent and divergent validity, one-tailed Pearson’s or Spearman’s correlation coefficients were applied depending on the data normality, as the hypotheses were proposed with specific directions. The strength of correlational relationships was identified as follows: r < .30: weak, r = .30–.69: moderate and r ≥ .70: strong. P values were set at .01 for multiple correlation corrections. To examine the distribution of apathy, depression and disinhibition, normative cut-offs, as identified below, were applied to determine people with apathy only, depression/disinhibition only, apathy co-existing with depression/disinhibition and none of apathy or depression/disinhibition.
To identify cut-offs for self-report V-FrSBe-A and V-DAS, we established a procedure based on the methods used in the original validations (Grace & Malloy, Reference Grace and Malloy2001; Radakovic et al., Reference Radakovic, Stephenson, Colville, Swingler, Chandran and Abrahams2016). In the English FrSBe (Grace & Malloy, Reference Grace and Malloy2001), demographic effects on apathy were investigated in an American sample. Then, means and SD were calculated for each group taking these demographic factors into account. For the English DAS (Radakovic et al., Reference Radakovic, Stephenson, Colville, Swingler, Chandran and Abrahams2016), cut-offs for total score and subscales were defined as 2 SD away from the mean in the healthy subject sample. Here, multiple regression models were used to examine the general effect of age, education and gender on V-FrSBe-A and V-DAS total scores for the entire Vietnamese sample. Power calculation showed that, to detect a medium effect (f 2 = .15) of these three demographic factors on apathy with alpha = .05 and power of 90%, a sample of 99 participants was needed. To compare the American and Vietnamese samples, t tests were conducted for each group based on age, education and gender. Analyses were performed for the age group of 18–39 years only (N Vietnamese = 93 and N American = 147 (Grace & Malloy, Reference Grace and Malloy2001)), due to the small sample size for other age groups (see Supplementary Material 2). If the results from the Vietnamese sample showed demographic effects on apathy as found in the US sample, cut-offs were calculated stratified for age, education and gender. If such effects did not exist, cut-offs of both the V-FrSBe-A and V-DAS were determined based on 2 SD away from the mean for the entire sample. P values were set at .05, and effect sizes were reported using Cohen’s f 2 and Hedges’ g.
RESULTS
Internal Consistency of the V-FrSBe and V-DAS
The V-FrSBe-A had ω t = .74 for the self-report version and ω t = .86 for the informant version. McDonald’s omega values for the self-report V-DAS range from .76 to .82 (see Table 2). The V-DAS informant version had ω t = .78 for executive apathy, .83 for emotional apathy, .74 for initiation apathy and .84 for total scores.
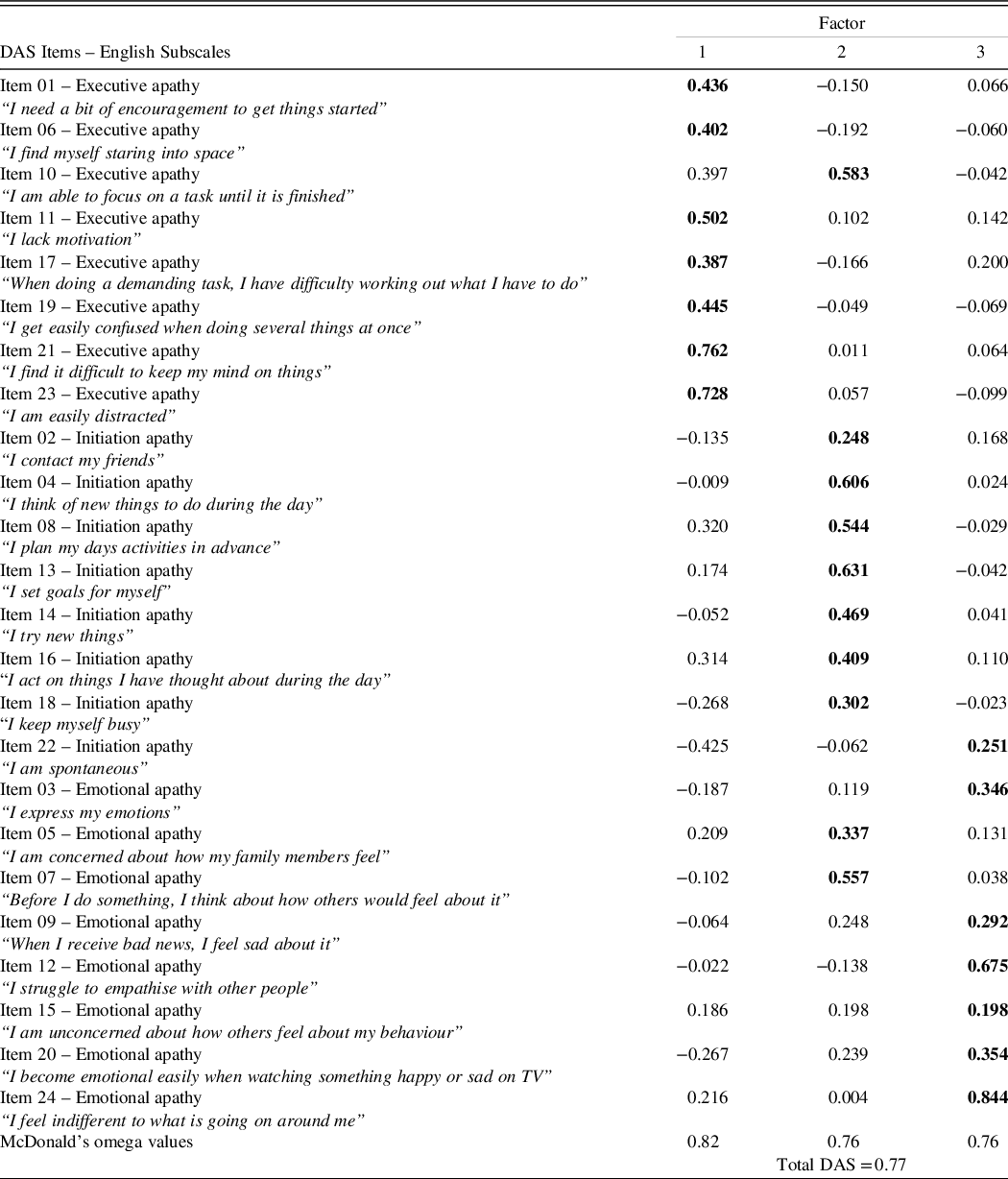
Table 2. Factor loadings for items from the Dimensional Apathy Scale (DAS) – self-report version

Note: Figures in bold indicate main factor loadings of items.
Factor Structure of the Self-report V-DAS
The goodness-of-fit indexes for the CFA provided a poor model fit to the data (CFI = .643, TLI = .605 and RMSEA = .100), while the estimates were significantly better for the ESEM (CFI = .996, TLI = .996 and RMSEA = .010). Given that this result was consistent with previous studies for the superiority of ESEM over CFA (Marsh et al., Reference Marsh, Muthén, Asparouhov, Lüdtke, Robitzsch, Morin and Trautwein2009; Perry et al., Reference Perry, Nicholls, Clough and Crust2015; Tóth-Király et al., Reference Tóth-Király, Bõthe, Rigó and Orosz2017), the ESEM solution was retained for the V-DAS.
Table 2 presents parameter estimates from the ESEM model with substantial factor loadings for most of the items on the DAS subscales whereby factor 1 stands for executive apathy, factor 2 for initiation apathy and factor 3 for emotional apathy. For the executive subscale, seven out of eight items showed main loadings on factor 1 (loading = .387 to .762). The remainder, item 10 (“I am able to focus on a task until it is finished”), although displaying main loadings on factor 2, had cross-loadings on factor 1 (cross-loading = .397). A similar pattern of results was observed for the initiation apathy subscale with all items loading onto factor 2 (loading = .248 to .631), except for item 22 (“I am spontaneous”) which loaded onto factor 3 (loading = .251). Cross-loadings of some items on the initiation apathy subscale were also present on factor 1 (e.g., item 8 (“I plan my days activities in advance”, cross-loading = .320) and item 16 (“I act on things I have thought about during the day”, cross-loading = .314)). For the emotional apathy subscale, six out of eight items loaded mainly on factor 3 (loading = .191 to .844) with item 15 (“I am unconcerned about how others feel about my behaviour”) having equal loadings on factor 2 and 3 (loading = .198 each). The remaining item 5 (“I am concerned about how my family members feel”) and item 7 (“Before I do something, I think about how others would feel about it”) revealed main loadings on factor 2 (loadings = .337 and .557). Interestingly, compared to other items on the emotional apathy subscale, items 15, 5 and 7 all have the content of affective concerns and human-to-human social interactions. Following previously published guidelines (Perry et al., Reference Perry, Nicholls, Clough and Crust2015), we retained the original internal structure of executive, emotional and initiation apathy for the V-DAS as the majority of items (87.5%) loaded onto their intended factors.
Convergent Validity
Significant positive relationships were identified between self- and informant-ratings for the V-FrSBe-A score (r(110) = .25, p < .001), V-DAS total score (r(110) = .450, p < .001), and each V-DAS subscale (r(110) = .405 for executive apathy, r(110) = .367 for emotional apathy and r(110) = .348 for initiation apathy, all p < .003).
For the self-report versions, a significant moderate relationship between higher self-reported V-FrSBe-A and higher self-reported V-DAS total score was observed, r(110) = .589, p < .001 (Figure 1.A). Self-reported V-FrSBe-A was also moderately correlated with all subscales of the self-reported V-DAS, with r(110) = .438 for executive apathy, r(110) = .353 for emotional apathy and r(110) = .375 for initiation apathy, all p values <.001.

Figure 1. Pearson’s correlation for the significant relationship between higher V-FrSBe-A and V-DAS total scores (A). Distributions of people who had apathy only, depression/disinhibition only, coexisting apathy and depression/disinhibition and none of these symptoms (B). Note: Determination for the presence of apathy: ≥ 43 on the V-DAS total or on the V-FrSBe-A, determination for the presence of depression: ≥ 36 on the V-DASS-21 (Tran et al., Reference Tran, Tran and Fisher2013) and determination for the presence of disinhibition: ≥ 43 on the V-FrSBe-D. V-DAS = the Vietnamese version of Dimensional Apathy Scale, V-FrSBe-A = the Vietnamese version of Frontal Systems Behaviour Scale – Apathy Subscale, V-DASS-21 = the Vietnamese version of Depression Anxiety Stress Scale – 21, V-FrSBe-D = the Vietnamese version of Frontal Systems Behaviour Scale – Disinhibition Subscale.
Identification of Cut-offs
Across the total Vietnamese sample (N = 112), the multiple regression analyses did not show significant models for the influence of age, education and gender on self-reported apathy on the V-FrSBe-A (F(3,108) = 1.545, p = .207, f 2 = .043) and V-DAS total scale (F(3,108) = 1.547, p = .207, f 2 = .043). Figure 2 reveals that, within the age group of 18-39, the Vietnamese reported significant higher levels of apathy on the FrSBe-A compared to the Americans across gender and education groups (t(13) = 2.23 p = .044, g = .69) for men with ≤12 years of education, t(29) = 4.183, p < .001, g = .81 for men with >12 years of education, t(8) = 5.905, p < .001, g = 1.37 for women with ≤12 years of education and t(39) = 8.16, p < .001, g = 1.43 for women with >12 years of education).
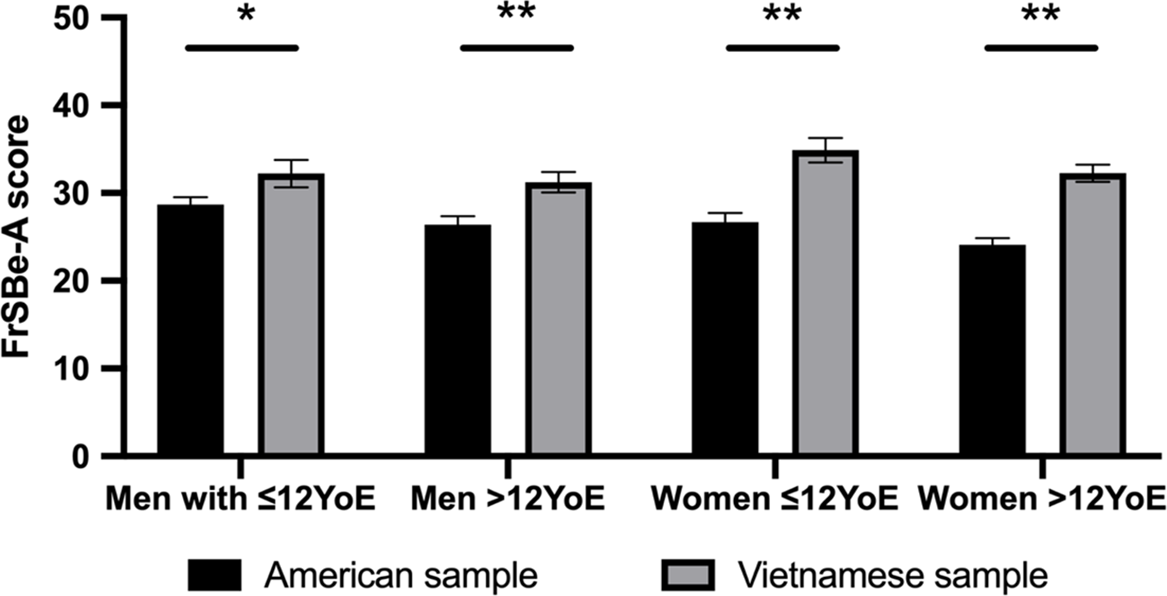
Figure 2. Comparison of self-rated apathy on the Frontal Systems Behaviour Scale – Apathy subscale (FrSBe-A) between American (Grace & Malloy, Reference Grace and Malloy2001) and Vietnamese people,with age range from 18–39 years according to gender and years of education. Note: YoE = Years of education. * p < .05, ** p < .001.
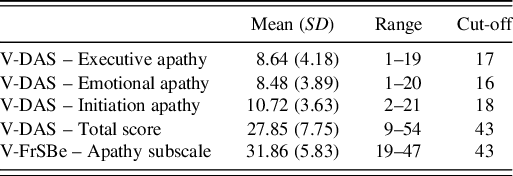
Because no significant effects of age, education and gender on the self-report V-FrSBe or V-DAS were observed, cut-offs were calculated based on 2SD from the mean. Table 3 shows cut-offs for the presence of apathy (V-FrSBe-A = 43, V-DAS-total = 43, V-DAS-executive apathy = 17, V-DAS-emotional apathy = 16 and V-DAS-initiation apathy = 18).
Table 3. Cut-off scores for the presence of apathy assessed with the self-report version of Vietnamese dimensional apathy scale and the frontal systems Behaviour scale – apathy subscale based on 2 standard deviations of the mean (N = 112 healthy subjects, years of age = 28.77 (11.82), years of education = 13.3.(3.45), 51.8% males)

Note: Minimum and maximum scores: FrSBe-A: 14–70; DAS Total: 0–72. V-DAS = the Vietnamese version of Dimensional Apathy Scale, V-FrSBe = the Vietnamese version of Frontal Systems Behaviour Scale.
Divergent Validity
Greater self-reported apathy on the V-FrSBe-A and DAS total score were significantly correlated with greater self-reported depression on the DASS-21 (r(110) = .425 p < .001 for V-FrSBe-A and r(110) = .519, p < .001 for V-DAS) and elevated self-reported disinhibition on the V-FrSBe-D (r(110) = .507, p < .001 for V-FrSBe-A and r(110) = .219, p = .01 for V-DAS). When applying cut-offs for the V-FrSBe-A (i.e., 43), V-DAS (i.e., 43), the V-DASS (i.e., 36) and the V-FrSBe-D (i.e., 43), results revealed that people who had apathy (as determined as having either V-FrSBe-A or V-DAS above the cut-offs) did not necessarily have depression or disinhibition (Figure 1B). Particularly, only 1.8% main subjects had both apathy and depression, whereas 5.4% had depression only and 4.5% had apathy only. The majority of main subjects (88.4%) did not have either apathy or depression. Regarding the distribution of apathy and disinhibition, no people had apathy co-existing with disinhibition. Disinhibition only was present in 1.8% main subjects and 6.3% had apathy only. In total, 92% did not have either apathy or disinhibition. Equivalent analyses for the informant versions of the V-FrSBe-A and V-DAS showed similar patterns of results (see Supplementary Material 3).
DISCUSSION
To our knowledge, this study is the first to adapt, validate and identify cut-offs for the self-reported and informant-reported V-FrSBe-A and V-DAS in a Vietnamese healthy sample. A rigorous adaptation procedure was employed to ensure both the translated content and cultural appropriateness of the scales. Our results confirmed that the adapted scales had good internal reliability and also demonstrated good construct validity in terms of factor structure, convergent and divergent validity. For the first time, divergent profiles of apathy were also uncovered between individuals in Vietnam and in the USA.
Consistent with validations in French, Italian and Japanese, our ESEM findings of the excellent model fit with most of the V-DAS items loading onto their intended factors confirm the robust loadings of DAS items as well as the theoretical basis of the Dimensional Apathy Framework (Kawagoe et al., Reference Kawagoe, Onoda, Yamaguchi and Radakovic2020; M’Barek et al., Reference M’Barek, Radakovic, Noquet, Laurent and Allain2018; Santangelo et al., Reference Santangelo, Raimo, Siciliano, D’Iorio, Piscopo, Cuoco and Trojano2017). While the previous versions of DAS were validated using traditional CFA, we incorporated advantageous features of ESEM and found important insights. The strict CFA model did not fit our Vietnamese data, indicating that DAS items do not uniquely reflect any of the executive, emotional and initiation components. By using the ESEM, we found sizeable cross-loadings of executive and emotional apathy items onto initiation apathy and vice versa. Conversely, executive and emotional apathy items were quite distinct with minimum cross-loadings found. These findings are in line with the Dimensional Apathy Framework, such that executive apathy and emotional apathy are separate dimensions while their presence or interaction may reflect initiation apathy (R. Levy, Reference Levy2012). In fact, most of the items, which had joint components between initiation apathy and the other dimensions, have cross-content (e.g., “I act on things I have thought about during the day”). Neural explanations for this are proposed as arising from connections between the dorsolateral prefrontal system (which underpins executive apathy), the medial prefrontal system (which underpins emotional apathy) and subcortical structures for initiating behaviour (R. Levy & Dubois, Reference Levy and Dubois2006). Clinically, the ability to distinguish between these different apathy manifestations while understanding their interactions, may facilitate the detailed characterisation of patients’ strengths and weaknesses in order to better target interventions and treatment plans.
Interestingly, items 5, 7 and 15 (“I am concerned about how my family members feel”, “Before I do something, I think about how others would feel about it” and “I am unconcerned about how others feel about my behaviour”) had equal or higher loadings onto initiation apathy rather than their expected dimension of emotional apathy. These items shared common content regarding a reduction in affective concerns and social interactions. This finding is convergent with results from the French validation of DAS showing two potentially distinct aspects of emotional apathy including “Individual Emotional” (i.e., items 3, 9, 20 and 24) and “Social Emotional” (i.e., items 5, 7, 12 and 15) apathy (M’Barek et al., Reference M’Barek, Radakovic, Noquet, Laurent and Allain2018). The social component of motivation has increasingly been recognised and assessed via varying measures, such as the Apathy-Motivation Index or behavioural paradigms quantifying willingness for prosocial acts (Ang, Lockwood, Apps, Muhammed, & Husain, Reference Ang, Lockwood, Apps, Muhammed and Husain2017; Lockwood et al., Reference Lockwood, Hamonet, Zhang, Ratnavel, Salmony, Husain and Apps2017). Our data suggested an interaction between emotional indifference and reductions of social engagement in social apathy, which can be measured by the DAS. The development and utility of these assessment tools may have potential for future research to advance current knowledge of motivation and its association with clinical conditions of social cognition.
Both the V-DAS and V-FrSBe-A also demonstrated good convergent and divergent validity in healthy subjects. In both scales, expected concordance between self-report and informant versions was observed, confirming that the self-reflection of healthy subjects is largely consistent with how others view them. Further, while apathy was associated with depression and disinhibition, as expected, distribution of these behavioural and mood disturbances did not always overlap. Although reductions of engagement in activities are key outcomes of both apathy and depression, depression refers to negative moods while apathy reflects flattening in emotion (R. Levy, Reference Levy2012). Apathy and disinhibition also appeared to be distinct. Although both are related to disrupted frontal lobe function, apathy reflects a decline whereas disinhibition reflects an excess (i.e., lack of control) in behaviour. These divergent concepts of apathy, depression and disinhibition, which have been identified in both the general population and a variety of neurological conditions in English-speaking countries (Kirsch-Darrow, Fernandez, Marsiske, Okun, & Bowers, Reference Kirsch-Darrow, Fernandez, Marsiske, Okun and Bowers2006; Lane-Brown & Tate, Reference Lane-Brown and Tate2009; Radakovic & Abrahams, Reference Radakovic and Abrahams2014; Wei et al., Reference Wei, Irish, Hodges, Piguet and Kumfor2020; Zamboni et al., Reference Zamboni, Huey, Krueger, Nichelli and Grafman2008), have now been demonstrated in a Vietnamese healthy population. Taken together, these findings of the good psychometric properties facilitate the utility of V-FrSBe-A and V-DAS for clinical practice and research in Vietnamese cohorts.
Our study also offered initial evidence for cultural variations in apathy between individuals from the USA and Vietnam. First, demographic factors were not predictive of apathy in the Vietnamese as they were in the US sample, indicating the individual differences in motivation that occur irrespective of gender, age and education in the Vietnamese. In older Australians, apathy is associated with more difficulty executing activities of daily living and lower quality of life (Tierney, Woods, Weinborn, & Bucks, Reference Tierney, Woods, Weinborn and Bucks2018). Future investigations of apathy and its potential impact on functioning and quality of life in both younger and older Vietnamese adults are warranted. Second, the Vietnamese sample rated their apathy levels on the FrSBe-A higher compared to the Americans. A similar pattern was seen on the DAS, as our data suggested higher overall apathy scores relative to an older aged group of healthy people in the UK (age mean = 63.7, SD = 13.0; DAS-total mean = 24.1, SD = 7.3) (Radakovic et al., Reference Radakovic, Stephenson, Colville, Swingler, Chandran and Abrahams2016). As a result, higher cut-offs were required for the V-DAS than the DAS (i.e., 17, 16, 18 and 43 compared to 14, 15, 16 and 39 for executive, emotional, initiation and global apathy, respectively) (Radakovic et al., Reference Radakovic, Stephenson, Colville, Swingler, Chandran and Abrahams2016). The discrepancies in levels of behavioural difficulties and cut-offs are not unusual for Vietnamese questionnaires (P. Nguyen et al., Reference Nguyen, Ocansey, Miller, Le, Schmidt and Prado2019; Tran et al., Reference Tran, Tran and Fisher2013) and may reflect higher expectations or self-criticism in the Vietnamese. Future work which incorporates self-report questionnaires and informant ratings with in-depth interviews is needed to provide more detailed explanations. Moreover, emerging evidence has demonstrated different profiles of apathy for clinical groups in English-speaking countries. For example, after traumatic brain injury, some people showed high emotional apathy only while some exhibited co-existing high executive and initiation apathy (Arnould, Rochat, Azouvi, & Van der Linden, Reference Arnould, Rochat, Azouvi and Van der Linden2015). Similarly, existing research in English-speaking countries that directly compared self-ratings and informant-ratings has frequently reported insight impairment of apathy in patients with neurological conditions. Whether this pattern is consistent across cultures, especially in Vietnam, requires further examinations.
Some methodological limitations warrant considerations. Comparisons between the Vietnamese and the US sample in the FrSBe-A were only conducted for the 18–39 age group because of the small participant numbers in the other age groups. Similarly, the small number of participants with education ≤12 years in our Vietnamese sample should be noted. The total sample size met power requirement for the multiple regression model analysis. Further, the effect size associated with demographic factors was small, suggesting that age and education were not salient influences on apathy scores. Nonetheless, studies which employ larger samples of older adults and those with lower education will be important to verify the effects of age and education on apathy in Vietnamese individuals. Moreover, the DAS’s factor structure was confirmed using the self-report version, whereas the informant version is widely used with clinical populations. The self-report version for validation analyses was selected based on adequate statistic values from a priori power analysis. Healthy participants typically have sufficient awareness of their behaviour, and our informant-rated results showed a similar pattern to the self-reported results. This indirect evidence suggests that the structure confirmation is likely also applicable for the informant-rated DAS.
In conclusion, the V-FrSBe-A and V-DAS are reliable and valid for apathy assessment in Vietnamese populations. Common and distinct features of apathy between Vietnamese and Americans were also revealed. Given that apathy is a well-recognised but inadequately understood behavioural syndrome, our findings provide initial grounds for assessing apathy in Vietnamese populations, contributing to the existing evidence for cultural examinations and mechanistic investigations of motivation and its related disorders. This will potentially offer more effective care and interventions for apathy in Vietnam and other countries that share common cultural backgrounds.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S135561772100031X
ACKNOWLEDGEMENTS
The authors thank all the participants and their families for the involvement in this research. We are also grateful for the support of Nguyen Thanh Truc Quynh and Nguyen Mo My Ngan in data collection as well as of Yin Li and Nguyen Ky Mai Anh in questionnaire translation.
FINANCIAL SUPPORT
H.Q. is supported by an Australian Government Research Training Program Scholarship and a UNSW Scientia PhD Scholarship. F.K. is supported by a National Health and Medical Research Council Career Development Fellowship (GNT1158762) and Project Grant (GNT1121791).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest in this research.