INTRODUCTION
Essential tremor (ET) is a neurological movement disorder characterized by uncontrollable shaking or tremor that is commonly expressed during the execution of voluntary upper extremity movements (Heldman et al., Reference Heldman, Jankovic, Vaillancourt, Prodoehl, Elble and Giuffrida2011; Louis, Reference Louis2009; Pauletti et al., Reference Pauletti, Mannarelli, De Lucia, Locuratolo, Currà, Missori and Fattapposta2015). Efforts to control the tremor generally exacerbate the tremor, suggesting that ET may interfere with cognitive control mechanisms involved in regulating motor performance in addition to the clear impact on motor execution processes.
We investigated the hypothesis that ET disrupts the proficiency of inhibiting motor actions independent of any effects on response execution. The ability to inhibit or stop actions is critical for navigating many dynamic, action-oriented daily life environments, and deficits in inhibitory motor control can be just as detrimental to quality of life and everyday functioning as deficits initiating and executing actions (Leroi, McDonald, Pantula, & Harbishettar, Reference Leroi, McDonald, Pantula and Harbishettar2012).
Key brain networks involved in inhibitory action control include circuitries linking the inferior frontal cortex (IFC, often right lateralized), pre-supplementary motor area (preSMA), anterior insula, striatum, and subthalamic nucleus (STN) (Aron, Herz, Brown, Forstmann, & Zaghloul, Reference Aron, Herz, Brown, Forstmann and Zaghloul2016; Cai, Ryali, Chen, Li, & Menon, Reference Cai, Ryali, Chen, Li and Menon2014; Swick, Ashley, & Turken, Reference Swick, Ashley and Turken2011). However, what aspect of stop-signal performance is reflected by each of those nodes in the circuitry in inhibitory action control (actual inhibitory process versus processing salient cues or updating of action plans), their temporal activation profile related to action cancelation, and whether the inhibition network is right-lateralized is still under debate (Aron et al., 2006; Bartoli, Aron, & Tandon, Reference Bartoli, Aron and Tandon2018; Hampshire, Chamberlain, Monti, Duncan, & Owen, Reference Hampshire, Chamberlain, Monti, Duncan and Owen2010; Hung, Yamak, Gaillard, & Arsalidou, Reference Hung, Gaillard, Yarmak and Arsalidou2018).
Inhibitory action control deficits in diseases that affect those cortico-striatal circuitries (such as Parkinson’s disease, Tourette’s, and attention deficit hyperactivity disorder [ADHD]) have received a lot of attention (Alderson, Rapport, & Kofler, Reference Alderson, Rapport and Kofler2007; Manza, Amandola, Tatineni, Li, & Leung, Reference Manza, Amandola, Tatineni, Li and Leung2017; Wylie, Claassen, Kanoff, van Wouwe, & van den Wildenberg, Reference Wylie, Claassen, Kanoff, van Wouwe and van den Wildenberg2016). ET, in contrast, is more commonly associated with disruption to cerebellar-thalamo-cortical circuitries (Bagepally et al., Reference Bagepally, Bhatt, Chandran, Saini, Bharath, Vasudev and Pal2012; Cerasa & Quattrone, Reference Cerasa and Quattrone2016). Since the thalamus has widespread cortical connections, including areas crucial for inhibition such as IFC, insula, and preSMA (O’Muircheartaigh, Keller, Barker, & Richardson, Reference O’Muircheartaigh, Keller, Barker and Richardson2015; Sun et al., Reference Sun, Wang, Cui, Wu, Li and Bao2018), disruptions along cerebellar-thalamo-cortical circuits as found in ET could hamper inhibitory control.
Behavioral investigations of inhibitory action control in ET are relatively sparse though; most cognitive studies have focused on performance on neuropsychological tasks of working memory, language, attention, and visuospatial function (Bhalsing et al., Reference Bhalsing, Upadhyay, Kumar, Saini, Yadav, Gupta and Pal2014; Troster et al., Reference Tröster, Woods, Fields, Lyons, Pahwa, Higginson and Koller2002). Of interest, recent functional magnetic resonance imaging (fMRI) and patient lesion studies (cerebellar lesions) have linked cerebellar-thalamic-cortical circuitries to the proficiency of response inhibition (measured by the stop task and Go/No-Go task; Brunamonti et al., Reference Brunamonti, Chiricozzi, Clausi, Olivito, Giusti, Molinari and Leggio2014; Hirose et al., Reference Hirose, Jimura, Kunimatsu, Abe, Ohtomo, Miyashita and Konishi2014). These putative linkages between cerebellar-thalamic-cortical circuitries and inhibitory action control further bolster the hypothesis that ET may interfere with these circuitries and the proficiency of inhibitory control directly.
The current study investigated the effects of ET on response going and inhibition by combining two well-established experimental cognitive paradigms. A basic two-choice reaction task quantified latencies (i.e., reaction times [RT]) of go reactions in contexts free of the need to inhibit or control these reactions. Participants also completed a stop-signal task, which required similar go reactions, but included occasional and unpredictable stop signals that required the inhibition or stopping of go reactions. The stop-signal task provides an estimate of an individual’s stopping latency (i.e., stop-signal RT [SSRT]) as well as strategic slowing of go reactions (i.e., proactive slowing) induced by the expectation of having to stop on occasion. Slower stopping latencies indicate difficulties inhibiting actions, and pronounced proactive slowing is considered an adaptive consequence of difficulties inhibiting actions (i.e., go reactions are slowed to compensate for difficulties inhibiting them) (Bisset & Logan, 2011).
Notably, both forms of action control have been linked to frontal-basal ganglia and cerebellar-thalamo-cortical circuitries (Aron et al., Reference Aron, Herz, Brown, Forstmann and Zaghloul2016; Hubner et al., Reference Hübner, Sprenger, Klein, Hagenah, Rambold, Zühlke and Helmchen2007; Kunimatsu, Suzuki, & Tanaka Reference Kunimatsu, Suzuki and Tanaka2016). Given the presumed cerebellar-thalamo-cortical dysfunction caused by ET, we predicted that ET patients would show prolonged stopping latencies and exaggerated proactive slowing independent of any effects of ET on response going latencies.
METHOD
Participants
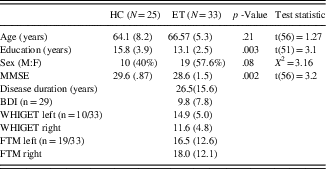
We recruited two groups of participants: healthy controls (HCs, n = 25) and ET patients (n = 33). All ET participants for this study were recruited from the Movement Disorders clinic in the Neurology department at Vanderbilt University Medical Center. An age-matched HC group was recruited by community advertisement (see Table 1 for demographic information). All patients were diagnosed with ET by a board-certified neurologist. Motor severity was evaluated in 29 ET patients using the Washington Heights-Inwood Genetic Study of ET (Louis et al., Reference Louis, Ottman, Ford, Pullman, Martinez, Fahn and Hauser1997; WHIGET; n = 10) or the Fahn-Tolosa-Marin (FTM, Fahn, Tolosa, & Marín, Reference Fahn, Tolosa and Marín1988) rating scale (n = 19). The deep brain stimulation (DBS) movement disorders clinic switched from using the WHIGET to FTM for ET motor symptom assessment because it provides a more comprehensive evaluation of the tremor (covers midline tremor, voice, face). Thus, depending on the time tested (before or after the FTM implementation), patients either have a WHIGHET or an FTM score.
Table 1 Characteristics of HC and ET subjects (age matched), mean and SD
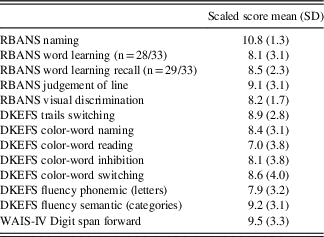
As part of the routine pre- and post-DBS neuropsychological evaluations, patients completed a battery of measures spanning several cognitive domains, including: Mini Mental-State Examination (MMSE, Folstein, Folstein, & McHugh, Reference Folstein, Folstein and McHugh1975); Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Randolph, Tierney, Mohr, & Chase, Reference Randolph, Tierney, Mohr and Chase1998): Line Orientation, Picture Naming, Word List; Delis-Kaplan Executive Function System (DKEFS, Delis, Kaplan, & Kramer, Reference Delis, Kaplan and Kramer2001): Trail Switching, Color Word, Verbal Fluency; Beck Depression Inventory (BDI; Beck, Steer, & Brown, Reference Beck, Steer and Brown1996) and Digit Span Forward. See Table 2 for an overview of neuropsychological information.
Table 2 Neuropsychological characteristics of ET subjects (scaled scores, mean and SD)
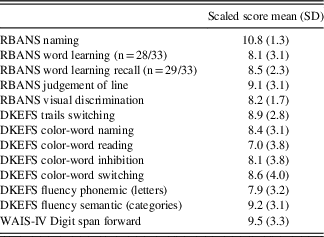
Note. Across neuropsychological functions, subject’s performance ranged from low average (6–8) to average (8 and up).
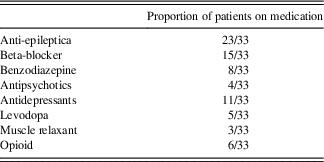
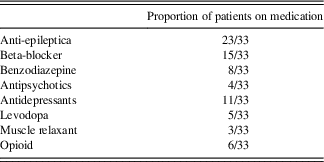
Both ET and HC subjects were excluded if they had a medical history of (i) a neurological condition besides ET; (ii) bipolar affective disorder, schizophrenia, or other psychiatric condition known to compromise executive cognitive functions; or (iii) a medical condition known to interfere with cognition (e.g., diabetes, pulmonary disease). They scored 25 or higher on the Mini-Mental Status Examination to rule out severe gross cognitive deficits. ET patients completed the study on their regular medication (beta blockers, anti-epileptic drugs, and occasionally benzodiazepines, see Table 3 for medication information in the ET group).
Table 3 Frequency of medication use in the ET subjects
All participants had corrected-to-normal vision. The participants provided informed consent before participation in any study procedures in full compliance with the standards of ethical conduct in human investigation as regulated by the Vanderbilt University Institutional Review Board.
Experimental Tasks and Procedures
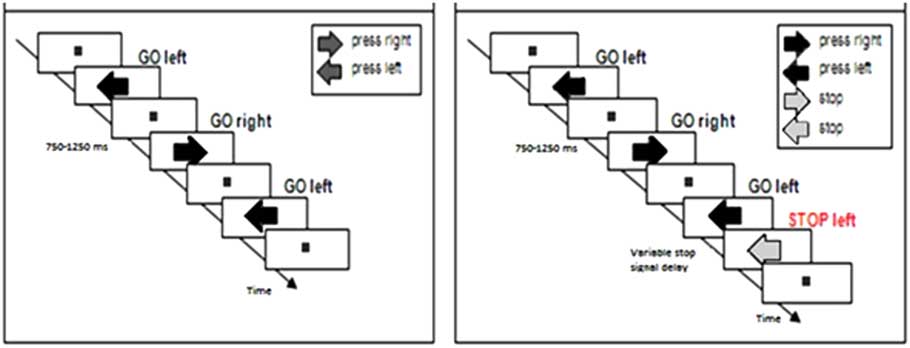
Participants completed two tasks: A choice reaction task (Figure 1a) and a stop-signal task (Figure 1b). All subjects completed the choice reaction task first, and subsequently performed the stop-signal task. Figure 1 presents trial examples of each task.
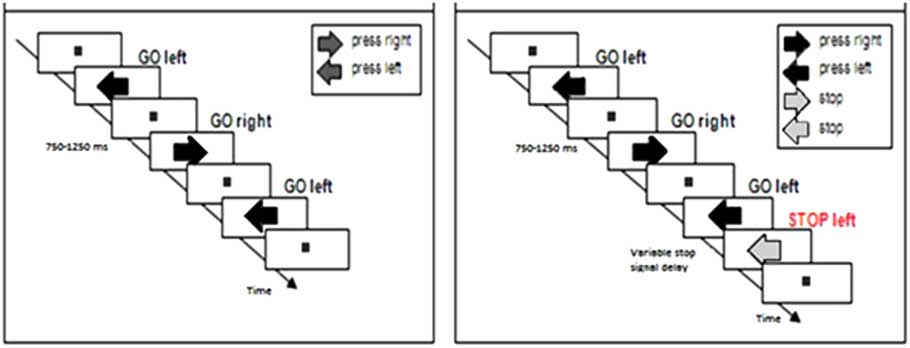
Fig. 1 Trial examples of (a) the choice reaction task where participants manually respond to the direction of dark-gray arrows presented on the screen with a left or right button press, and (b) the stop-signal task in which subjects were instructed to respond to the direction of the dark-gray arrow with a left or right button press and to stop their response when the arrow turned purple (30% of the trials).
Choice reaction task
Participants first saw a fixation square in the center of the computer screen. They were instructed to focus their attention on this fixation point. Next, a series of dark gray-colored arrows appeared, one at a time, in the same central location. An arrow pointed in the rightward or leftward direction. Participants were instructed to respond to the direction of the arrow using handheld grips in their left and right hands, with left pointing arrows calling for a left hand button press and right pointing arrows calling for a right hand button press. An arrow appeared and remained on the screen until a participant pressed a button.
Next, an intertrial interval randomly selected between 750 and 1250 ms in increments of 50 ms transpired before the next arrow appeared. The fixation point was visible at all times except when an arrow was being presented on the screen. Participants were instructed to respond as quickly and accurately as possible to each arrow. The task was counterbalanced so that participants were exposed to an equal number of left and right pointing arrows (randomly presented). A practice session of 16 arrows was completed before participants completed 168 experimental trials across two blocks of 84 arrows.
Stop-signal task
The stop-signal task was identical to the choice reaction task with one key difference. On 30% of trials, a gray-colored arrow first appeared, but after a brief delay, the arrow changed color from gray to purple. This color change served as a stop signal that instructed participants to try to stop or inhibit their button response to the arrow’s direction. Participants were instructed to respond fast to the direction of gray arrows and not to delay responding in anticipation of the presentation of stop signals. On these stop trials, the delay between the onset of the gray arrow and the color change (i.e., stop-signal delay, or SSD) adjusted dynamically using a 50 ms staircase-tracking procedure that was based on the participant’s success or failure to stop on the previous stop trial (Levitt, Reference Levitt1971). If the participant successfully stopped on a stop trial, the SSD on the next stop trial would increase by 50 ms to increase the difficulty of stopping, while a failure to stop a reaction on a stop trial led to a shortening of the SSD on the next stop trial to make stopping easier.
The dynamic tracking procedure converged on an overall 50% response stopping rate on stop trials, which is optimal for obtaining a reliable estimate of SSRT (Band, van der Molen, & Logan, Reference Band, van der Molen and Logan2003). For the stop-signal task, participants completed a practice session of 40 trials followed by three blocks of 90 experimental trials comprised of 189 go trials and 81 stop trials.
Data Analyses
Our first set of analyses compared go RT (GoRT) and accuracy rates from the choice reaction task with GoRT times and accuracy rates from the stop-signal task. RT slowing in the latter compared to the former provided a measure of proactive slowing in situations when the need to inhibit action may occur. We performed repeated-measures analyses of variance (ANOVAs) with Group (ET and HC) as a between-subject factor and Task (Choice RT task, stop-signal task) as a within-subjects factor.
The second set of analyses used ANOVAs to compare response inhibition latencies (SSRT) as a function of Group (ET and HC). SSRT was estimated using the well-established horse-race model and integration method (Logan, Reference Logan1994). The horse-race model assumes an independent race between the go and stop processes. The onset of the stop process is experimentally controlled by the length of the SSD. The finishing time of the stop process is inferred from the time point at which the internal response to the stop-signal occurs and subtracting the SSD from this point. The time point in the GoRT distribution that corresponds to P (failure-to-stop) is assumed to be equal (SSD + SSRT). Individual GoRTs were rank ordered and the nth GoRT was selected, where n is point P (failure-to-stop). The mean SSD was then subtracted from this finishing time to obtain an estimate of SSRT.
We first verified a few critical assumptions of the method requiring that the probability of stopping responses on stop-signal trials approximated 50% and that failed inhibition trials were associated with shorter RT than average go trials (Band et al., Reference Band, van der Molen and Logan2003; Logan, Reference Logan1994).
Additionally, in the patient group, we correlated ratings of clinical motor impairments, as measured by the FTM, with measures of going and action control (SSRT and proactive slowing), corrected for age (by using partial correlation analyses). Patients with WHIGET scores were not included in the correlation due to the small sample size (n = 10) and the absence of a valid transformation between the motor scales to allow combining the scores.
RESULTS
Go Trials and Proactive Slowing
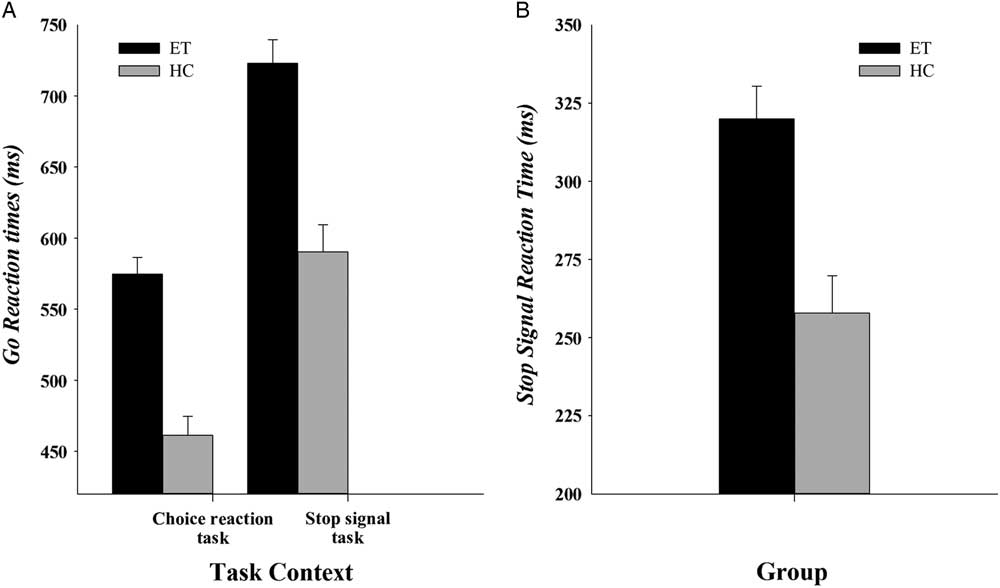
Mean GoRT in ET patients (649 ms) was significantly slower than in HC (526 ms), (Group: F(1,56) = 42.37; p <.001; η 2 = .43)Footnote 1 and patients made more omission errors (5%) on Go trials compared to HC (0.8%, F(1,56) = 7.17; p = .01, η 2 = .11); see Figure 2a for GoRTs. Proactive slowing was revealed by reactions to go stimuli that slowed significantly in a context that included the unpredictable need to stop reactions abruptly (stop-signal task, 657 ms) compared to a context that did not require stopping (choice reaction task, 518 ms, Task, F(1,56) = 152.30; p <.001; η 2 = .73). The magnitude of proactive slowing did not differ between ET patients and controls (Group x Task, F(1,56) = .74; p = .39; η 2 = .01). No effects of Group, Task or an interaction between Group and Task were found for mean accuracy rates (all Fs <1.04; ps >.31).
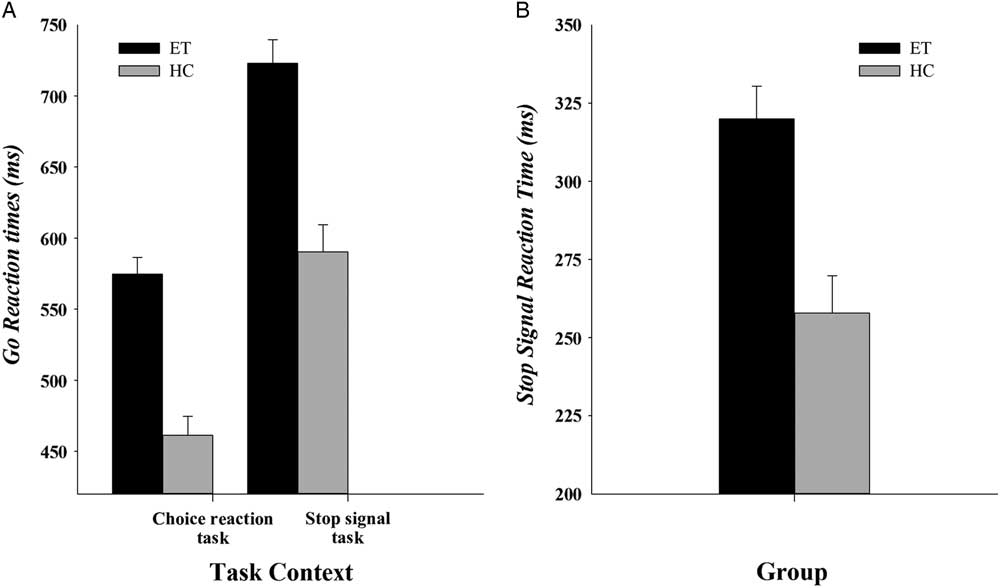
Fig. 2 (a) Mean GoRT for the choice reaction task and for the stop-signal task for ET patients and HC. (b) Mean SSRT for ET patients and HC. Error bars represent standard errors.
Stop-Signal Dynamics
The tracking algorithm controlling stop-signal onset successfully converged to overall stop percentages near 50% (min 43% and max 56% for both groups) and was similar across groups (ET: 48%, HC: 48%; F(1,56) = .02; p = .89; η 2 <.001). The SSD was shorter for HC (304 ms) compared to ET (383 ms, F(1,56) = 6.0; p = .02; η 2 = .10).
Also consistent with a key assumption of the horse-race model (Logan, Reference Logan1994), mean RT for failed stop trials (i.e., those that escaped inhibition) was shorter than the overall mean GoRT in all subjects (570 vs. 657 ms; F(1,56) = 458.45; p <.001; η 2 = .89). The difference between GoRT and SSRT was significant in both groups: in ET patients it was 94 ms (t(32) = 18.9; p <.001; Cohen’s d = 1.03, min 49 ms–max 162 ms) and in HC = 81 ms (t(24) = 12.05; p <.001; Cohen’s d = .97, min 17 ms–max 150 ms). These patterns satisfy key assumptions needed to reliably estimate SSRT.
Mean SSRTFootnote 2 , shown in Figure 2b, differed between the groups (F(1,56) = 15.3; p <.001; η 2 = .22). Specifically, SSRT was significantly longer among ET patients (320 ms) compared to HC (258 ms). In line with the horse-race model, predictions that going and stopping processes are independent, GoRT did not correlate with SSRT across all subjects (Spearman’s rho = 0.08; p = .56), nor did GoRT correlate with SSRT within each group separately (Spearman’s rho = <.3; ps >.13)
Stop Task Performance and Relation With Clinical Variables
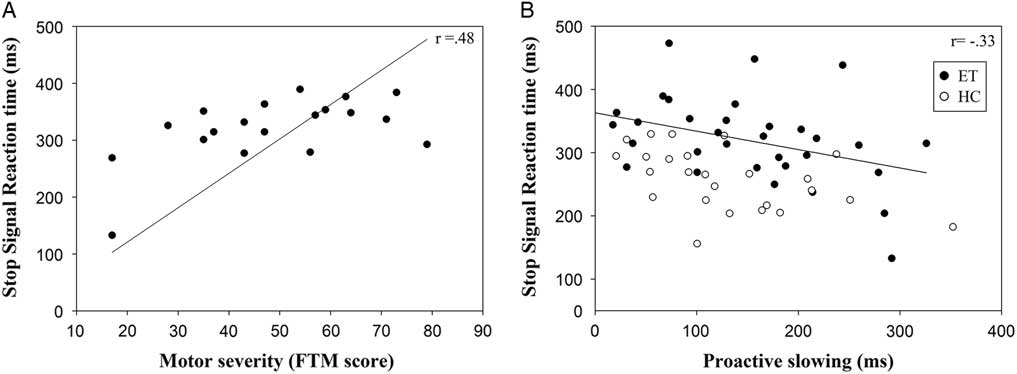
For a subset of ET patients (patients with a motor symptom severity score, the FTM score, n = 19) we correlated GoRT, proactive slowing, and SSRT with motor severity, corrected for age. FTM scores were not related to GoRT (Spearman’s rho = −.04; p = .87) or proactive slowing (Spearman’s rho = −.38; p = .11). Increased severity of motor impairment (higher FTM scores) was associated with longer SSRT (Spearman’s rho = .48; p <.05), see Figure 3a. Additionally, across all 58 participants (both patients and HC), less proactive slowing was associated with impaired stopping, that is, prolonged SSRT (corrected for age, Spearman’s rho = −.33; p <.05), see Figure 3b. When looking at the groups separately, we found similar correlations between proactive slowing and SSRT (HC Spearman’s rho = −.50; p <.05; ET Spearman’s rho = −.42; p <.05)
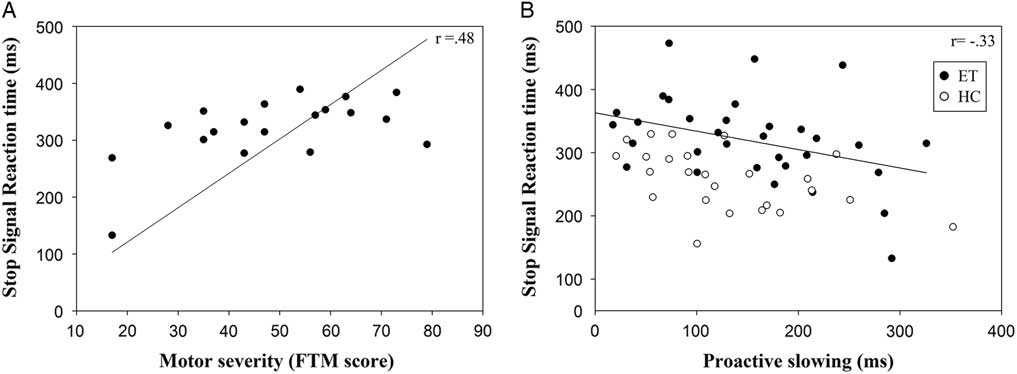
Fig. 3 (a) Correlation of SSRT in ET patients and motor severity as measured by the FTM rating scale (b). Correlation of SSRT and proactive slowing in both ET patients and HC. Note that, for clarity and interpretation of the scales, the values represented in the graph are the original scales (not corrected for age).
DISCUSSION
ET is a neurological movement disorder characterized by action tremor that interferes with the execution of voluntary movements. We hypothesized that cerebellar-thalamo-cortical circuitry dysfunction produced by ET would also impair the ability to inhibit or stop voluntary movements. The current study demonstrated that ET independently slows going and stopping latencies, revealing that ET compromises both the execution and the inhibition of movement.
ET slowed responses to go stimuli across both reaction contexts. In the choice reaction task, reactions to go stimuli were on average 123 ms slower among ET patients compared to controls, revealing a clear impact of ET on response speed. When occasional stop signals were introduced in the stop-signal task, both ET and controls slowed their reactions to go stimuli, consistent with a strategic, proactive slowing of go reactions to accommodate the expected but unpredictable need to stop reactions. While this form of proactive slowing has been linked to cerebellar dysfunction, we did not observe differential magnitudes of proactive slowing in ET compared to controls. Both groups slowed go reactions by approximately 140 ms on average. This suggests that ET patients were able to make normal strategic adjustments in response speed to accommodate demands on inhibitory action control.
Independent of the slowing of go reactions, ET selectively impaired stopping latency. Moreover, ET patients with more severe tremor ratings (i.e., higher FTM motor scores) showed the most pronounced slowing of stopping latencies. Notably, tremor severity was unrelated to the magnitude of slowing of go reactions and of proactive slowing, suggesting a more direct linkage between tremor severity and inhibitory action control. As an interesting side note, given that ET patients show intact proactive slowing, but within the ET group larger proactive slowing was associated with faster stopping latencies, one practical strategy in ET would be to promote greater proactive slowing in functional situations that place greater demands on motor control. This certainly represents an intriguing area for future investigation. Additionally, future studies that systematically manipulate going speeds or inhibitory demands would inform our understanding of how strategic adjustments in control impact motor performance in ET (Bissett & Logan, Reference Bissett and Logan2011; van Wouwe et al., Reference van Wouwe, van den Wildenberg, Claassen, Kanoff, Bashore and Wylie2014; Wylie et al., Reference Wylie, van den Wildenberg, Ridderinkhof, Bashore, Powell, Manning and Wooten2009).
Potential Neural Mechanism of Inhibitory Control Changes in ET
Inhibitory control impairments and exacerbated tradeoffs between going and stopping mechanisms have been well reported in diseases characterized by cortico-striatal network dysfunction (e.g., Parkinson’s disease, Tourette’s, and ADHD; Alderson, Rapport, & Kofler, Reference Alderson, Rapport and Kofler2007; Manza et al., Reference Manza, Amandola, Tatineni, Li and Leung2017; Wylie et al., Reference Wylie, Claassen, Kanoff, van Wouwe and van den Wildenberg2016). The underlying neuropathology in ET is less clearly understood; it includes degradation of cerebellar gray and white matter along with abnormal signaling in cerebellar-thalamic-cortical circuitries (Cerasa & Quattrone, Reference Cerasa and Quattrone2016). Although inhibitory control has repeatedly been linked to activity in frontal-striatal networks, including the preSMA, IFC, striatum, premotor cortex, anterior insula, and STN (Aron et al., Reference Aron, Herz, Brown, Forstmann and Zaghloul2016; Mirabella, Pani, Ferraina, Reference Mirabella, Pani and Ferraina2011), more recent imaging work with healthy adults has suggested that the cerebellum might be critically involved in inhibitory control as well (Hirose et al., Reference Hirose, Jimura, Kunimatsu, Abe, Ohtomo, Miyashita and Konishi2014). ET patients in the current study showed prolonged inhibition latencies, which is in line with the presumed cerebellar-thalamo-cortical dysfunction caused by ET. Future imaging studies in ET should investigate whether structural or functional changes in the frontal-striatal circuitries or the cerebellar-thalamo-cortical circuitries are linked to impaired inhibitory control in ET.
How might we understand the link between tremor severity in ET and dysfunctional inhibitory control? Bhalsing et al. (Reference Bhalsing, Upadhyay, Kumar, Saini, Yadav, Gupta and Pal2014) found that hampered performance on Wisconsin Card Sorting Test and Stroop interference correlated with gray matter volume loss of anterior cingulate, right inferior frontal gyrus, and right inferior parietal lobe in ET patients. Bagepally et al. (Reference Bagepally, Bhatt, Chandran, Saini, Bharath, Vasudev and Pal2012) also found a positive relation between the amount of frontal cortical and striatal gray matter atrophy and disease severity in ET patients (FTM tremor severity).
Cerebellar degeneration might additionally affect functional connectivity in ET patients. For example, Schnitzler and colleagues (Schnitzler, Münks, Butz, Timmermann, & Gross, Reference Schnitzler, Münks, Butz, Timmermann and Gross2009) identified a network of brain areas (including M1, premotor cortex, thalamus, cerebellum, and brainstem) that show coupled oscillatory activity at the frequency of the tremor in ET patients, suggesting a pathological alteration of communication between areas that are also important to inhibitory control (Mirabella et al., Reference Mirabella, Pani and Ferraina2011).
Benito-León et al. (Reference Benito-León, Louis, Romero, Hernández-Tamames, Manzanedo, Álvarez-Linera and Onofrj.2015) showed that resting state activity changes across a range of functional networks (both increased and decreased activity), suggesting dysfunctional networks in ET patients compared to HC. More specifically, enhanced activity in the resting state networks (the default mode network and frontoparietal network) correlated with increased disease severity, disease duration, and reduced cognitive ability; that is, reduced performance on tasks measuring executive (Stroop interference and frontal assessment battery), attention (WAIS digit span), verbal memory (word list learning and recall), and visuo-spatial ability (line orientation). Consistent with the above-described imaging studies, the relationship between cognitive impairment and disease severity in ET in our study could be mediated by gray matter volume reductions in cerebellum and fronto-cortical areas, and enhanced activity in resting state networks (Bagepally et al., Reference Bagepally, Bhatt, Chandran, Saini, Bharath, Vasudev and Pal2012; Benito-León et al., Reference Benito-León, Louis, Romero, Hernández-Tamames, Manzanedo, Álvarez-Linera and Onofrj.2015; Bhalsing et al., Reference Bhalsing, Upadhyay, Kumar, Saini, Yadav, Gupta and Pal2014).
In contrast to inhibitory control deficits, proactive slowing was similar for ET patients and HC. Previous work suggests that the cerebellum, an area crucially impacted by ET, might play a role in proactive control processes (Brunamonti et al., Reference Brunamonti, Chiricozzi, Clausi, Olivito, Giusti, Molinari and Leggio2014). Olivito et al. (Reference Olivito, Brunamonti, Clausi, Pani, Chiricozzi, Giamundo and Ferraina2017) found that patients with focal cerebellar atrophy showed increased post error slowing (indicative of proactive trial-by-trial adjustments) and increased stop latencies when compared to HCs, thus implicating the cerebellum in both proactive control and reactive motor inhibition. Future fMRI studies could provide insight in which cerebellar-thalamo-cortical loops are critically involved in proactive control and how these networks are recruited in ET.
Limitations and Future Studies
In the current study, data collection in ET was performed in participants who remained on medications for tremor. We did not test cognitive performance OFF medication; thus, we cannot account for possible medication effects on action control. However, a post hoc comparison between ET patients using anti-epileptics (n = 23), which may alter cognitive performance (Kay, Schwartz, Wingertzahn, Jayawardena, & Rosenberg, Reference Kay, Schwartz, Wingertzahn, Jayawardena and Rosenberg2016), versus patients that did not use this medication (n = 10), did not reveal a difference in SSRT (F(1,32) = .90; p = .35) or proactive slowing (F(1,32) = .73; p = .40).
Additionally, we acknowledge that the use of different motor scales in our sample is a limitation and reduced the sample size for the correlational analysis between motor symptoms and cognitive measures. Future studies should replicate the current findings in a larger sample to improve generalizability.
Another limitation with respect to generalizability of the current study was the age range of the patients. Based on a meta-analysis of 51 studies on the role of the cerebellum in ET neuropathology, Cerasa et al. (2016) concluded that the age of ET patients in most studies ranged from 53 to 70 years with the exception of a few studies including younger patients. Future studies should replicate ET effects on inhibitory control in a wider age range (including more younger patients) to improve generalizability.
Moreover, the patient group tended to have a slightly different sex distribution compared to the control group (more males in patient group), although there is no evidence that this should strongly impact stop signal performance (for a meta-analysis on sex effects on the SSRT see Lipszyc & Schachar, Reference Lipszyc and Schachar2010).
Finally, it is unlikely that physical manipulation of the response device impacted performance. Responding to the computer task required a simple button press while the button grips were resting comfortably in the participant’s lap; no overt limb movement was needed. Additionally, tremor severity did not correlate with going speed in either left or right hand.
Given the results of the current study, and previous studies that examined functional and structural changes in ET, there are multiple directions for further study. Functional imaging studies investigating the involvement of the cerebellar-thalamo-cortical loops in ET during performance of an inhibitory control paradigm such as a stop-signal task could provide insight into the underlying neural mechanism that explains changes in inhibitory control in ET. Longitudinal studies could also help advance our understanding of ET’s disease pathology (disease severity) in relation to changes in going and stopping latencies. Future studies might compare the effects of medical and surgical interventions (i.e., deep-brain stimulation of the ventral intermediate nucleus of the thalamus) on inhibitory action control in ET (see also van den Wildenberg et al., Reference van den Wildenberg, van Boxtel, van der Molen, Bosch, Speelman and Brunia2006).
CONCLUSION
The current study demonstrates that ET patients show a selective impairment in their ability to inhibit, or stop, movements. The ability to stop was also linked to tremor severity and independent of generalized response slowing. Clinically, an improved understanding of how ET impacts action control processes might enable optimization of therapeutic strategies and interventions.
ACKNOWLEDGMENTS
We thank Bert van Beek for programming the computer task. The authors acknowledge support with patient testing from Maxim Turchan. Dr. Phibbs has done consulting for Boston Scientific, Medtronic, and Teva. There are no other conflicts of interest to report. This work was supported by the National Institutes of Health RO1NS097783-01 to D.O.C.