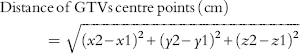Introduction
Radiation therapy plays an important role in the treatment of non-small cell lung cancer (NSCLC), and the target volume delineation is one of the most important factors for adequate tumour coverage and sufficient sparing of critical structuresReference Van de Steene, Linthout and De Mey1 that governs the success of radiotherapy (RT) treatment. Accuracy in tumour delineation improves precision in the radiation therapy process, which leads to more effective treatment and improved prognosis. The majority of tumour delineation at the present time is based on computed tomography (CT) images. CT is known as a diagnostic imaging procedure that uses X-rays in order to present cross-sectional images that show the distribution of structures inside the body.Reference Seeram2 It can demonstrate good anatomical information. However, the information from CT alone may be insufficient in target delineation if the tumour and surrounding normal tissue have similar densities.Reference Zhang, Wang and Wang3 As it is not functional imaging, it is difficult to distinguish the demarcation between the tumour and surrounding normal tissue in some cases, thus leading to insufficient dose coverage of the target volume or too much damage to normal tissue. The invention of positron emission tomography/CT (PET/CT) can help overcome this problem. PET/CT is a fusion of functional information obtained from PET and anatomic information from CT.Reference Zhang4–Reference Kang, JH and Baik6 18F-fluorodeoxyglucose (FDG)-PET has a major role in the selection of patients with NSCLC for treatment with definitive RT as it is able to detect unsuspected distant metastases and to identify very advanced locoregional disease.Reference Vansteenkiste, Fischer and Dooms7
There are many methods of PET target volume definition and it is still unclear which method is the best. The PET visual assessment (PETvis) method is routinely used in clinical practice, however, the different training and experience of nuclear medicine clinicians may lead to a wide deviation in estimation of gross tumour volume (GTV). In order to reduce the inter-observer variability in 18F-FDG-based GTV definition, several target volume definition methods have been proposed to contour the PET volume in an automatic fashion such as absolute standardised uptake value (SUV), which has a fixed threshold value of maximum activity in tumour lesions. Hong et al.Reference Hong, Halama and Bova8 showed that at a SUVmax >7, the regions of 40% SUVmax had a wide variability in volume due to tumour heterogeneity. For regions of SUVmax 4–6 the highest correlation appears in tumours of small volume, close to 100 cc. The optimal technique to incorporate the PET SUV thresholds to contour GTV depends on the maximum tumour SUV and volume.
PET/CT fusion images have the cumulative benefit of providing physiologic data with precise topographic localisation. PET/CT can help identifying the edge of the lung cancer, delineate the radiation target area precisely, avoid unnecessary radiation injury and reduce radiation complications, thereby improving the effectiveness of radiation treatment. Hoseok et al.Reference Hoseok, Kim and Kim9 and Wang et al.Reference Wang, Chen and Li10 showed that based on the PET/CT scan, the outline of the GTV in RT treatment planning could be improved, reducing high doses in the lung and oesophageal illuminated doses, reducing radiation toxicity to the lung and oesophagus and improving the patient’s quality of life as a consequence.
In this study, the intensity-modulated radiation therapy (IMRT) treatment planning between using PET/CT and CT for target volume delineation in patients with NSCLC were retrospectively analysed.
Methods and Materials
Clinical data
Nine consecutive patients with pathologically proven NSCLC who underwent PET/CT at the PET/CT and Cyclotron center of Medical Excellence at Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Thailand, were retrospective studied. PET/CT data were used for target delineation and IMRT plans were performed for dosimetric comparison for the purpose of this study only. Patients with bronchial carcinoid tumour or adenocarcinoma in situ of the lung or distant metastasis were excluded. There were seven males and two females. Of all patients, four had squamous cell carcinoma and five had adenocarcinoma. This study was approved by the research ethics committee of Chiang Mai University Hospital, Faculty of Medicine, Chiang Mai University, Thailand.
Equipment and reagents
The Biograph mCT-X PET/CT system (128 slices; Siemens Medical Solutions USA, Inc., Knoxville, Tennessee, USA) equipped with the Sumitomo HM-20 cyclotron was used in this study. 18F-FDG with a radiochemical purity of >96% was the tracer used in PET imaging.
PET-CT procedure
The standard protocol for PET/CT imaging was followed in all patients, that is, fast for at least 6 hours before the PET/CT procedure, blood glucose levels being determined. 18F-FDG was then intravenously injected at a dose of 5·18 MBq/kg in patients whose blood glucose levels were within the normal range. Examinations started 60 minutes after injection, which consisted of a spiral CT scan followed by a PET scan during quiet breathing in the supine position. CT data were used for attenuation correction of PET images. Images were then reconstructed using iterative methods; OSEM3D+time of flight. PET image reconstruction parameters were as follows: 2 mm slice thickness for PET image reconstruction, 168×168 matrix, 2 mm slice thickness for CT image reconstruction and 512×512 cross-sectional resolution. PET and CT data were on Syngo Acquisition Workspace where the datasets were fused automatically.
Target delineation
PET target delineation was performed using the RT image Suite, Syngo Acquisition Workspace (Siemens healthcare) by an experienced PET scan nuclear medicine physician. The automated SUV thresholds methods (regions>2·5 and 40% maximum SUV based on published literature recommendations)Reference Huang11–Reference Erdi, Rosenzweig and Erdi13 and visual assessment method were evaluated. As a first step, in all patients, an experienced PET scan nuclear medicine physician used the region-of-interest (ROI) standard evaluation tool provided by the manufacturer of the PET system to generate a visual PET GTV, visually adjusted to the part of the malignant primary tumour (PETvis). Then, for all tumours, two more GTVs were defined at the PET work station. ROIs were positioned around the tumours slice by slice in the volume configuration, using first an isocontour of SUV>2·5 (PET2·5) and, second, an isocontour of 40% of the SUVmax of the whole lesion (PET40). Finally, PET, CT images and all PET target delineation method data were transferred to the Oncentra v. 4.3 treatment planning system (TPS) workstation.
CT target delineation was performed on Oncentra TPS by an experienced radiation oncologist. As is normal in cases of lung cancer, the GTV derived from CT (CT based) was generated using the soft-tissue window with respect to the lung window.
Organs at risk (OARs) delineation
All OARs were delineated on CT images on Oncentra TPS.
(1) Lungs: (consisting of ipsilateral lung and contralateral lung) automatically delineated and then manually modified to exclude the trachea and bronchi.
(2) Heart: delineated slice by slice from the bottom of the aortic arch to the bottom of the heart.
(3) Oesophagus: delineated slice by slice from the level of the cricoid cartilage to the area above the oesophagogastric junction.
(4) Spinal cord: delineated slice by slice after adjusting CT window width and level to clearly demonstrate the spinal cord.
RT planning and dosimetry
RT planning was performed with Oncentra TPS, using inhomogeneity corrections based on a convolution algorithm. For PET planning, the GTVs (PET2·5 and PET40) were compared with the GTV by PETvis to find out the SUVmax threshold for using PET2·5 and PET40 methods. This analysis showed that the PET40 method should be applied when SUVmax<7 and the PET2·5 method when SUVmax≥7. In this context, automated PET (‘PETauto’) referred to both PET2·5 and PET40 methods and was used for PET/CT planning. For all patients, the clinical target volume (CTV) was a 6 mm margin extended to the GTVs obtained from the PETauto and CT-based scans and 5 mm to the CTV for the planning target volume (PTV). No assessments of tumour movement by slow CT scans were performed.
A step and shoot IMRT treatment plan was calculated using the CT-based PTV and PETauto, PTV referred to as plan CT and plan PET/CT, respectively, both to deliver 60 Gy in 30 fractions to the PTV, according to the International Commission on Radiation Units and Measurements Report 83 guidelines.Reference Menzel, Wambersie and Jones14 The beam orientation and configuration of two treatment plans were the same in each patient but the optimisation parameters may be adjusted in order to keep the tumour dose in accordance with ICRU 83 guidelines. Dosimetric values were calculated on the basis of dose–volume histograms and dose distributions on each axial CT images for both CT and PET/CT planning. The V95 of the PET/CT PTV derived from CT-based treatment planning was calculated to determine the volumes of PET/CT PTV, receiving 95% (57 Gy) of the prescribed dose. For the OARs, V20 and mean lung dose (MLD) were analysed as predictors of radiation pneumonitis in the lung.Reference Graham, Purdy and Enami15, Reference Kwa, Lebesque and Theuws16 The volume of the ipsilateral lung receiving 20 Gy minus the PTV was used to calculate the ipsilateral lung V20, whereas the volume of the ipsilateral lung minus the PTV and the volume of the contralateral lung were considered for the ipsilateral and contralateral MLD. The constraints used for the ipsilateral lung were a V20<50%, V30 (the volume of ipsilateral lung receiving 30 Gy)<39% and for the contralateral lung was a V20<37%. For the oesophagus, the mean oesophageal dose was analysed as a predictor of early and late oesophageal toxicityReference Hirota, Tsujino and Endo17, Reference Werner-Wasik, Pequignot and Leeper18 and the constraint was a mean oesophageal dose (Dmean)<34 Gy. The constraint for the heart was D33 (the dose received to 33% of the heart volume)<50 Gy and the constraint for the spinal cord was the maximal dose<45 Gy.Reference Rogerio, Michael and Michele19
Other parameters
(1) Distance of the centre points of the GTVs were calculated using the following equation to determine the discrepancy between the centre points of two GTVs. The sagittal plane was used as the difference in x-coordinates, the coronal plane as the difference in y-coordinates and the horizontal plane as the difference in z-coordinates.
(1)where x1, y1, z1 are the coordinates of GTV1 centre point and x2, y2, z2 the coordinates of GTV2 centre point. $$\eqalignno{{\rm \hskip -16pt Distance}\,{\rm of}\,{\rm GTVs}\,{\rm centre}\,{\rm points}\,{\rm (cm)\qquad }\qquad \quad \qquad \cr \ \ \qquad = \sqrt {\left( {x2{\minus}{\rm }x1} \right)^{2} {\plus}{\rm }\left( {y2{\minus}{\rm }y1} \right)^{2} {\plus}{\rm }\left( {z2{\minus}{\rm }z1} \right)^{2} }\quad \ (1)} $$
$$\eqalignno{{\rm \hskip -16pt Distance}\,{\rm of}\,{\rm GTVs}\,{\rm centre}\,{\rm points}\,{\rm (cm)\qquad }\qquad \quad \qquad \cr \ \ \qquad = \sqrt {\left( {x2{\minus}{\rm }x1} \right)^{2} {\plus}{\rm }\left( {y2{\minus}{\rm }y1} \right)^{2} {\plus}{\rm }\left( {z2{\minus}{\rm }z1} \right)^{2} }\quad \ (1)} $$
(2) Conformity index was calculated using the following equation (2) to determine the proportion of overlap volumes per union volume between two GTVs.Reference Kirby, Yarnold and Evans20
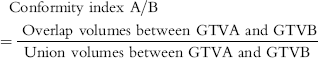 $$\eqalignno{{\rm Conformity\ index\ A/B\qquad\qquad\qquad\qquad\ \ \qquad\quad} \cr\ ={\ {\rm Overlap\ volumes\ between\ GTVA\ and\ GTVB}\over{\rm Union\ volumes\ between\ GTVA\ and\ GTVB}} \ (2)$$
$$\eqalignno{{\rm Conformity\ index\ A/B\qquad\qquad\qquad\qquad\ \ \qquad\quad} \cr\ ={\ {\rm Overlap\ volumes\ between\ GTVA\ and\ GTVB}\over{\rm Union\ volumes\ between\ GTVA\ and\ GTVB}} \ (2)$$
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software. The GTVs comparisons between two groups (PETauto versus PETvis, CT based versus PETauto) were performed using the Wilcoxon’s signed-rank test. Dose–volume histogram parameters data were expressed as mean± SD and analysed using the Wilcoxon’s signed-rank test. p<0·05 was considered statistically significant.
Results
The mean SUVmax of the tumours examined was 7·42 (range 4·18–14·7). The mean GTVs were 15·7 cc (range 4·51–77·01 cc) for PETvis, 6·17 cc (range 2·14–12·25 cc) for PET40 and 14·3 cc (range 1·65–73·58 cc) for PET2·5. The analysis was performed to compare between PETvis and PET40, PETvis and PET2·5. The percentage difference between the GTVs was plotted against their respective SUVmax as shown in Figure 1. The optimal cutpoint SUVmax threshold was found at 7 for PETauto. In a subsequent part of this work, the PETauto referred to PET40 when SUVmax<7 and PET2·5 when SUVmax≥7. The mean GTV of PETauto was 15·04 cc (range 2·14–73·58 cc).

Figure 1 Relationship of the percentage difference between the gross tumour volumes (GTVs) contoured, based on positron emission tomography visual assessment (PETvis) versus PET40 (red triangle), PETvis versus PET2·5 (black dot) compared with maximum standardised uptake value (SUV) of tumour. The vertical axis indicates the percentage GTV difference between the two methods.
Table 1 showed the results of GTV analysis between PETauto and PETvis in all patients. Although the GTVs of PETvis tended to be greater than PETauto, it was not statistically significant (p=0·441). The average absolute GTV difference was 3·62% (range 1·38–6·96%), using 25% as a cutoff,Reference Yin, Yu and Ren21 none of PETauto were significantly different from PETvis. The mean distance of GTV centre points between PETauto and PETvis was 0·06 cm (range 0·02–0·09 cm). Using 0·2 cm as a cutoff, according to uncertainties of isocentre limited criteria,Reference Musolino22 the distance of the GTV centre points between PETauto and PETvis did not exceed the cutoff in all patients. For the conformity index, the GTVs between the two methods are show a high level of conformity to each other if the value approaches 1. In this study, the mean conformity index between PETauto and PETvis was 0·89 (range 0·84–0·97). The results of the GTV analysis between PETauto and CT based was also evaluated as shown in Table 2. The mean GTV of the CT-based study was 15·14 cc (range 2·32–72·54). The GTVs of PETauto were greater than CT based in 44% (4/9) of all patients, although these results were without statistical significant (p=0·594). The average absolute GTV difference was 3·78% (range 1·41–8·68%), by using 25% as a cutoff, none of the PETauto results were different from the CT-based ones. The mean distance of GTV centre points was 0·3 cm (range 0·14–0·66 cm). The distance of GTV centre points between PETauto and CT based exceeded the cutoff in 33% (3/9) of patients using 0·2 cm as a cutoff. The mean conformity index between PETauto and CT based was 0·5 (range 0·39–0·65).
Table 1 Results of gross tumour volume (GTV), distance of GTV centre point and conformity index between positron emission tomography automated (PETauto) and PET visual assessment (PETvis)
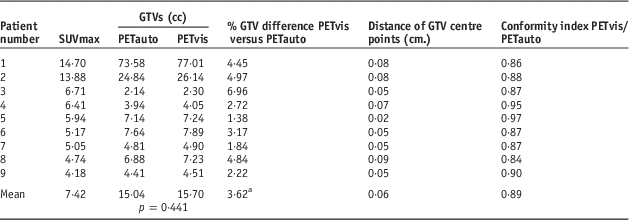
a Note: Calculated from absolute percentage difference of GTV between PETauto and PETvis as the reference.
Abbreviation: SUV, standardised uptake value.
Table 2 Results of gross tumour volume (GTV), distance of GTV centre point and conformity index between positron emission tomography automated (PETauto) and computed tomography (CT) based
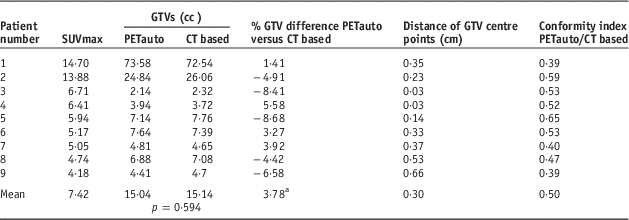
a Note: Calculated from absolute percentage difference of GTV between CT based and PETauto as the reference.
Abbreviation: SUV, standardised uptake value.
After GTV delineation, the radiation therapy treatment planning was performed. The volumes of PET/CT PTV in PET/CT planning receiving 95% of prescribed dose (57 Gy) derived from CT PTV planning was achieved as shown in Figure 2.

Figure 2 Coverage of positron emission tomography/computed tomography (PET/CT) planning target volume (PTV) derived from CT planning.
Transferring CT-based treatment planning onto PET/CT PTV, the mean V95 of PET/CT PTV was 96·72%, maximum at 99·58% in patient no. 3 and minimum at 90·02% in patient no. 7. Using 98% as a cutoff,Reference Menzel, Wambersie and Jones14 66% (6/9) of PET/CT PTVs had insufficient target coverage if the treatment planning was based on CT delineation as shown in Figure 3.

Figure 3 Example computed tomography (CT) slide of insufficient target coverage of positron emission tomography/CT (PET/CT) planning target volume (PTV) derived from CT planning in patient no. 7.
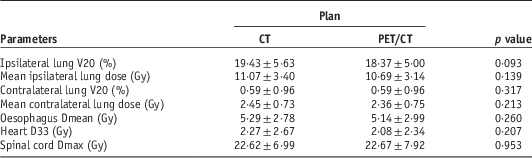
Table 3 shows the dose–volume histogram parameters of the lung, oesophagus, heart and spinal cord. The dose parameters for all OARs were lesser in plan PET/CT except spinal cord Dmax, which was slightly higher than that of plan CT but no statistically significant (p>0.05).
Table 3 Dose–volume histogram parameters for organs at risk of plan computed tomography (CT) and plan positron emission tomography/CT (PET/CT)
Discussion
For PET target delineation, Hong et al.Reference Hong, Halama and Bova8 compared the GTVs obtained by two automated PET methods: isocontour of SUV>2·5 and 40% of the SUVmax of the whole lesion, in 19 NSCLC patients. The results showed that the optimal SUV thresholds to contour GTV depend on maximum tumour SUV and volume. At SUVmax>7, the target volumes of 40% SUVmax were less than the volumes of SUV>2·5, whereas at SUVmax<4, the volumes of SUV>2·5 were much less than the volumes of 40% SUVmax. This study revealed similar results except the volumes of SUV>2·5 were much less than the volumes of 40% SUVmax at SUVmax<5. The optimal cutoff point of the SUVmax threshold in our study was ~7. As a consequence, the 40% SUVmax method was used when SUVmax<7 and the SUV>2·5 method when SUVmax≥7. This was referred to as the ‘PETauto’ method in this study. There was no statistical significance between the GTVs of the PETauto method and PETvis method (p=0·441).
PET/CT target delineation studies have been published. Deniaud-Alexandre et al.Reference Deniaud-Alexandre, Touboul and Lerouge23 studied the delineation of the GTV in 92 NSCLC patients using PET/CT and found that the PET GTV was reduced in 23% of the patients and increased in 26% of cases compared with CT GTV, and 21 patients had a GTV change of ≥25%. Yin et al.Reference Yin, Yu and Ren21 studied the delineation of the GTV in 30 NSCLC patients with atelectasis and found that all 30 patients had varying degrees of changes shown in the PET GTV and CT GTV, including 12 (40%) patients who had a change of over 25%. In this study, we found that the PET GTV was reduced in 55% and increased in 44% of patients, however, none had GTV changes of >25%. The maximum change was 8·68%. These statistics might be due to the diminutive GTV volumes in almost all patients and none in the atelectasis patients. There was no statistical significance between the GTVs of the PETauto and CT (p=0·594).
It was found that the maximum difference of the centre points of a GTV between PETauto and PETvis was 0·09 cm (mean 0·06 cm), which was within the uncertainties of isocentre limited criteria of 0·2 cm according to IAEA TRS398.Reference Musolino22 This implied that the centre points of the GTV of the two PET techniques were relatively the same. In the case of PETauto and CT based, the maximum distance between the centre points of the GTVs was 0·66 cm (mean 0·3 cm) and the distance of the centre points of the GTVs between the two methods exceeded 0·2 cm in 33% (3/9) of patients, indicating that the two GTVs centre points were significantly different in some patients. Regarding the conformity index, the mean conformity index between PETauto and PETvis was 0·89 (range 0·84–0·97). This led to the inference that the GTVs between PETauto and PETvis were slightly different only at the edge of GTVs but the whole shape of GTVs showed high levels of conformity to each other as all conformity indexes were >0·8, whereas the mean conformity index between PETauto and CT based was 0·5 (range 0·39–0·65). PET is molecular imaging that can detect microscopic tumours at the cellular level, whereas CT is only useful for anatomical imaging. In addition, the outline between the tissues of a tumour and surrounding normal tissue were difficult to distinguish in CT images in some cases, so it is possible to be less accurate in the delineation of the GTV. This can affect the coincidence of GTVs between PET and CT, leading to an increase in the distance of the centre points of the GTVs and a decrease in conformity index.
IMRT was performed according to the International Commission on Radiation Units and Measurements Report 83 guidelines.Reference Menzel, Wambersie and Jones14 The volumes of PTV receiving 95% of prescribed dose must be >98% otherwise the patients have a higher risk for cancer recurrence, which means failure of the radiation therapy treatment. As CT-based target delineation is routinely used in radiation therapy treatment planning, the volume of the planned PET/CT PTV receiving 95% of prescribed dose (57 Gy) derived from PTV CT planning in all patients was analysed. It was found that using 98% as a cutoff, 66% (6/9) of planned PET/CT PTVs had insufficient target coverage. Especially in patient no. 7, the PET/CT PTV was greater than the CT PTV and the conformity index of 0·4 was lowest in the studied groups. Using only CT-based target delineation might not be sufficient. The advantage of using PET/CT for target delineation is that tumours can be detected at cellular level and the information obtained can help reducing the risk of cancer recurrence from inadequate target coverage in treatment planning.
Yin et al.Reference Yin, Yu and Ren21 contoured the GTV from CT and PET/CT images in 30 NSCLC patients with atelectasis and found that lung V20, V30, oesophagus V50 and V55 were all statistically significant (p<0·05). The lung V20, V30 as well as oesophagus V50, V55 reduced significantly in plan PET/CT. Bradley et al.Reference Bradley, Thorstad and Mutic24 contoured the GTV from the CT and PET/CT datasets in 26 NSCLC patients and found that in three patients with atelectasis, Lung V20 and MLD decreased in planned PET/CT.
In this study, there were no atelectasis patients. The ipsilateral lung V20, mean ipsilateral lung dose, mean contralateral lung dose, oesophagus Dmean and heart D33 from planned PET/CT insignificantly decreased comparing with that obtained from planned CT (p>0·05). The contralateral lung V20 from planned PET/CT was comparable with planned CT (p=0·317). The spinal cord Dmax slightly increased in planned PET/CT (p=0·953). The dose–volume histogram derived in the study suggested that PET/CT could not significantly help reducing the OAR dose in patients without atelectasis. In addition, the high technology involved in IMRT itself is so efficient regarding tumour dose conformity and OARs dose reduction. As a result, the noticeable reduction of these doses may not be possible due to a small change in GTVs reported in this work. To further verify our findings, the respiratory motion of lung cancer which was not a focus in this study should be taken into account, that is, 4D-PET/CT, with a larger number of patients.
Conclusions
PET/CT does not only detect tumours at the cellular level but also enables a clearer outline of the tumour in NSCLC leading to more accurate application of RT target delineation. V95 from treatment planning based on CT PTV was not sufficiently covered by the PET/CT PTV in most of the cases that may lead to cancer recurrence. However, PET could not significantly help reducing OARs dose in NSCLC patients. Although SUVmax impacted on PET automated target volume delineation in NSCLC patients. Our study suggested using the 40% SUVmax method when SUVmax<7 and using SUV>2·5 method when SUVmax≥7.
Acknowledgements
The authors wish to thank Thailand Nucletron B.V. for providing Oncentra v.4.3 treatment planning system. The technical help of PET/CT and Cyclotron staffs of Medical Excellence at Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Thailand, are gratefully acknowledged.
Financial support
None.
Conflicts of interest
None.
Ethical Standards
This study has been approved by Research Ethics Committee 4, Faculty of Medicine, Chiang Mai University.