Introduction
With the advent of volumetric modulated arc therapy (VMAT), the treatment of head-and-neck cancer with radiation has considerably improved the quality of life. Reference Nutting, Morden and Harrington1 Precise target delineation and its spatial relation to the surrounding critical organs are of major concern in the treatment planning of head-and-neck tumours as it involves multiple planning target volumes (PTV) such as high-risk PTV, intermediate-risk PTV and low-risk PTV with differing dose prescriptions. Reference Katsochi2,Reference Mohan, Wu, Manning and Schmidt-Ullrich3 Radiation dose is being delivered to these different targets sequentially by shrinking the fields gradually depending on the dose required for each. Reference Katsochi2 The simultaneous integrated boost (SIB) technique enables the delivery of different doses to different targets simultaneously in a single session by modulating the intensity of the radiation beam. Hence, SIB improves planning efficiency and allows the clinician to escalate the dose to the gross target volume per fraction without increasing the dose significantly to neighbouring critical normal structures. Reference Mohan, Wu, Manning and Schmidt-Ullrich3
Though SIB technique is preferred in VMAT for head-and-neck cancers, the dose optimisation approaches differ between institutions. As inverse planning and optimisation algorithms improve continually, clinicians have developed interests on different planning strategies. One such interest is regarding the choice of segmentation of low/intermediate-risk target volume for optimisation. In the segmented low/intermediate-risk volume as shown in Figure 1, high-risk target volume is excluded from the low/intermediate-risk PTV, whereas the non-segmented low/intermediate-risk volume encompasses the high-risk volume within it. According to AAPM TG 263, Reference Mayo, Moran and Bosch4 both segmented and non-segmented volumes can be valuable for dose evaluation. Typically, the cumulative dose volume histogram (DVH) of non-segmented low/intermediate dose PTVs shows a ‘foot’ of the overlap with the high-dose PTV, whereas in segmented low/intermediate-dose PTVs a long high-dose tail is observed (see Figure 2). As non-segmented PTVs retain information about the overlap, many institutions prefer to use non-segmented volumes for plan evaluation. However, studies have reported the use of segmented volumes for planning and evaluation as well. Reference Fogliata, Bolsi, Cozzi and Bernier5,Reference Chakraborty, Ghoshal, Patil, Oinam and Sharma6
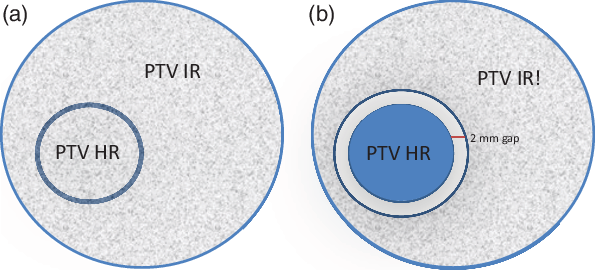
Figure 1. Showing the two types of segmentation for intermediate- and low-risk volumes used for optimisation. (a) Non-segmented volume (PTV IR = PTV IR + PTV HR) and (b) segmented volume (PTV IR! = PTV IR - PTV HR with a 2 mm gap).

Figure 2. A representative cumulative DVH showing the dosimetry of segmented (PTV_IR!) (red) and non-segmented (PTV_IR) (blue) volumes. The elephant foot noted in the non-segmented volumes is due to overlap with high-risk PTV.
Studies have evaluated the dosimetric advantages between the plans using the aforementioned strategies. Reference Fogliata, Bolsi, Cozzi and Bernier5 However, due to the paucity of evidence with respect to the pros and cons of using these two planning approaches, some ambiguity still remains and there is a lack of consensus on the best method for better plan optimisation. In this study, we have evaluated and compared the dose volume parameters with relation to the two types of segmentation in head-and-neck cancer.
Materials and Methods
Volume delineation
CT data of 20 patients with locally advanced head-and-neck cancer treated with radical chemoradiation with SIB-VMAT were retrospectively selected for this study. The study was approved by the Institutional Review Board. All the patients selected for the study had laryngeal primaries. The delineation of the different targets and organs-at-risk (OAR) were performed as per the RTOG head-and-neck primary and nodal delineation guidelines. Reference Brouwer, Steenbakkers and Bourhis7–Reference Grégoire, Ang and Budach9 Eclipse planning system v13·7 (Varian Medical Systems, Palo Alto, CA, USA) was used for contouring and planning. All patients’ CT data were planned to receive a total dose of 70 Gy in 35 fractions to high-risk planning target volume (PTV HR) and 63 Gy in 35 fractions to intermediate-risk target volume (PTV IR) simultaneously. PTV HR encompasses the primary and nodal gross tumour volume with (5–10 mm) clinical target volume (CTV) margins and a setup margin of 3 mm. PTV IR includes the CTV-IR which are areas of potential microscopic spread of the disease which are primarily the drainage lymph nodal levels of the primary tumour and a setup margin of 3 mm. Segmented and non-segmented PTVs generated for the intermediate-risk volumes are shown in Figure 3. The segmented intermediate-risk planning target volume (PTV IR!) was generated by cropping the PTV HR volume from PTV IR with a margin of 2 mm in this study. The non-segmented PTV IR includes the PTV IR! as well as PTV HR. The nomenclatures are as per AAPM TG 263. Reference Mayo, Moran and Bosch4 Post-processing was performed using the enhancement tool (Eclipse V.13.7) to ensure smooth tumour outline and contours smaller than 0·2 cm3 were removed.

Figure 3. Representative CT images of a case of laryngeal cancer. Both panels represent the same slice. (a) Segmented PTV IR! is shown in red and PTV HR in orange and there is a 2 mm gap between PTV structures. (b) Non-segmented PTV IR is in blue colour with a Boolean addition of PTV HR in orange.
Plan generation
Two VMAT plans, namely segmented and non-segmented, were generated for every CT dataset, the difference between the two being the choice of the intermediate-risk target volume for optimisation. The segmented intermediate-risk target volume (PTV IR!) was used for the segmented plan, whereas PTV IR was used for the non-segmented plan. All plans were generated with two full arcs (181o–179o) using 6 MV photon beams from the linear accelerator (Varian TrueBeam™ STx). To avoid planning bias, similar target and OAR constraints, priorities and normal tissue objectives were used. The target constraints were logically similar for all the plans except that an additional upper constraint corresponding to the percentage of overlap of the two targets was defined for PTV IR in non-segmented plans. The photon optimiser with four levels of progressive sampling and AcurosXB dose calculation algorithm were used in Varian Eclipse treatment planning system. Single optimisation and leaf segmentation calculation was performed for every plan to avoid bias and to verify the time taken for each optimisation and calculation.
Plan evaluation
A detailed plan evaluation included qualitative (visual) slice-by-slice analysis of the dose distribution in all three planes (transverse, sagittal and coronal) of the CT, and a quantitative analysis of the various parameters from the DVHs of each plan was carried out by a single senior radiation oncologist avoiding possible inter-observer variations. Different dose volume parameters signifying the target coverage, hotspots, spillage and quality indices, demonstrating the sculpting of reference isodose around the target and the extent of uniformity of dose within the target, were evaluated. The conformity index Reference Sasidharan, Aljabab and Saini10–Reference Palazzi, Orlandi and Pignoli13 and homogeneity index (HI) Reference Krishna, Srinivas, Ayyangar and Reddy14,Reference Kataria, Sharma, Subramani, Karrthick and Bisht15 were calculated using equations (1) and (2), respectively.
where V95 is the volume of tissue covered by the 95% isodose line of prescription dose, that is, 66.5 Gy and VPTV is the volume of the target in cm3 (VPTV HR and VPTV IR!, respectively). D95 and D5 are the doses received by 95% and 5% of the target volume, respectively, and DMean is the mean target dose. Data collected were statistically analysed using STATA v.16.0 (Statacorp., LLC, College Station, TX, USA). The dosimetry parameters were compared using paired t-test.
Results
VMAT plans generated with segmented and non-segmented target volumes were evaluated in the treatment planning system by a radiation oncologist and a physicist. Various dosimetry parameters obtained from cumulative DVH and the quality indices such as conformity and homogeneity indices were evaluated on both plans and compared. Only segmented volumes (PTV IR!) were used for comparison and reporting. Reference Fogliata, Bolsi, Cozzi and Bernier5 The dose distribution was evaluated in all CT slices for high-risk PTV (PTV HR) and intermediate-risk PTV (PTV IR!) target coverage. Table 1 illustrates the different dose volume parameters of both segmented and non-segmented plans. The median volume of PTV HR was observed to be 166·7 cm3 (interquartile range (IQR) = 86·4 cm3) and the median segmented volume of PTV IR! was 351·75 cm3 (IQR = 173 cm3). The mean volume receiving 105% (V105) of the prescribed dose in PTV HR was 2·9% and 1·55 % in segmented and non-segmented plans, respectively, while V105 of PTV IR! in both the plans was 12·1% and 13·2%, respectively. In the parameters assessed, the maximum dose of PTV IR! volume was higher in non-segmented plans and the difference was statistically significant (7281·45 versus 7075·75 cGy. p = 0·002). It implies a better dose distribution of the 95% of target volume as well as lesser hotspots in the segmented plan. Figure 4 shows the high dose spillage of 66·5 Gy and above into the intermediate-risk volume dose in a representative non-segmented plan.
Table 1. Analysis of dose volume parameters of segmented and non-segmented plans. Level of significance by paired t-test

* Denotes significance.
Abbreviations: IQR, interquartile range; SD, standard deviation.

Figure 4. Variation in dose spillage with the 95% of the prescription dose of PTV HR in non-segmented plans (a) and segmented plans (b). The dose colour wash set here for a minimum dose of 66·5 Gy and above.
The HI of PTV IR! (HI = 0·1 versus 0·12, p = 0·01) in segmented plans fared better compared to the non-segmented counterpart. The median conformity index was found to be 1·20 and 1·19 in the segmented plans and non-segmented plans, respectively, for the high-risk volume, whereas the median conformity index in the segmented plans and non-segmented plans for the intermediate-risk volume was 1·28 and 1·25, respectively. The median monitor units (MU) of the segmented and non-segmented plans were 519·95 MU (IQR = 60·45) and 503·45 MU (IQR = 71·48), respectively. No significant difference was noted in any of the above parameters. The calculation time (233·26 ± 37·94 (seg) versus 234·1 ± 36·85 (non-seg) seconds, p = 0·95) and optimisation time (218·11 ± 32·03 (seg) versus 213 ± 36·25 (non-seg) seconds, p = 0·65) were observed to have no statistically significant difference between plans. Table 2 illustrates the quality indices of both segmented and non-segmented plans.
Table 2. Analysis of the quality indices of both segmented and non-segmented plans

* Denotes significance.
Abbreviations: CI, conformity index; HI, homogeneity index.
Discussion
Conformal radiotherapy is aimed at achieving an optimal plan in which the prescription isodose adequately envelopes and is restricted to the target volume, sparing the adjacent normal tissues and critical organs. Reference Rosenwald, Gaboriaud and Pontvert16 With the advent of intensity modulated radiotherapy (IMRT), differential dose distributions could be achieved within the target volume thereby maximising the therapeutic index. In situations pertaining to multiple targets with differential doses, the debate on selection of sequential versus SIB technique for planning and evaluation needs further consideration. Reference Chakraborty, Ghoshal, Patil, Oinam and Sharma6,Reference Studer, Huguenin, Davis, Kunz, Lütolf and Glanzmann17,Reference Jiang, Zhang, Yang, Liang, Wu and Wang18 The proposed advantages of SIB technique include better conformity of dose around the high-risk PTV and reducing the undesirable high dose spillage in low/intermediate-risk target volumes. The Swiss multi-institutional patterns of care study by Elcin et al. reported that about 80% of centres consented favouring the SIB technique over other techniques for planning. Reference Broglie, Dulguerov and Henke19 Conventionally, sequential IMRT follows a non-segmented way of contouring the target volume in which low/intermediate-risk PTV includes the high-risk PTV, whereas, in SIB, both segmented and non-segmented target volumes with regard to low-risk PTV can be considered for planning. Although there is no clinical evidence on improved outcome from either, the disadvantage of using sequential IMRT includes longer planning time and the need for summation of different treatment plans. Reference Vlacich, Stavas, Pendyala, Chen, Shyr and Cmelak20 Our institutional practice when handling multiple PTVs in SIB technique has been segmenting the target volumes for plan optimisation as well as evaluation. According to AAPM TG 263, both these methodologies are acceptable but need to be named correctly.
In our study, no significant variation in PTV HR coverage and OAR doses could be observed between the plans. However, the analysis of these plans indicated that the doses were highly confined to each high-risk volume in the segmented plan with limited spillage of high doses to the overlapping targets. The plans generated using segmented volumes have shown mild improvement in the target volume coverage (95% isodose covering the target volume) and reduction in maximum dose of the intermediate-risk PTV. A statistical difference in dose homogeneity observed for the intermediate-risk volume implies a better dose coverage with lesser hotspots in the segmented plan.
The strength of this study is in the use of single optimisation and fairly comparable volumes reducing a significant number of interacting variables that might affect the inferences. The impact of segmenting the low/intermediate-risk PTV might become more pronounced with increase in anatomic complexity and variety of dose levels. In the current study, we have used laryngeal tumour volumes which are reasonably similar to patients with complex volumes of other head and neck sub-sites. Even though the results of this study are confined to the discussion in a controlled setting, it does predict the applicability and possible benefits of using the segmented planning technique for targets of higher anatomical complexity. We do not deny the possibility of obtaining similar plans with the non-segmented method, but at the cost of extended optimisation and calculation times and multiple cycles of optimisation. The development of more intelligent and robust planning systems in the future may help in this regard. In routine clinical practice, following a uniform segmentation method universally will help in better reporting of these volumes and pooling of data in multi-centric dosimetry studies and treatment plan comparisons.
From the planner’s perspective, unambiguous and well-defined target constraints could be used in the segmented method, whereas the non-segmented plan required additional constraints to be described, necessitating experience and expertise, in order to achieve a qualitatively comparable plan within the controlled setting in which the study was conducted. Table 3 shows the differences between segmented and non-segmented plans. Though non-segmented planning was practiced in our institution when SIB-based IMRT was introduced, we moved to the segmented technique owing to its uncomplicated nature and easy learning curve for beginners in planning.
Table 3. Comparison of the segmented and non-segmented plans in SIB IMRT based on our study results

a Can vary depending upon the number of optimisations. Valid only in a controlled setting. The non-segmented plans would require more optimisation cycles to achieve similar results.
Conclusion
IMRT techniques have helped in improving the quality of life of patients with head-and-neck cancers. It has helped the clinician to enhance the target dose and even differentially deliver doses to targets simultaneously without allowing undue damage to normal tissue by SIB technique. This study compares dosimetrically two segmentation methodologies that could be followed for SIB planning, one in which the high-risk target is excluded from the low-risk target (segmented) and the other without (non-segmented) and concludes by suggesting use of segmented PTVs considering the possible improvement in dose homogeneity when performed in a controlled setting.
Acknowledgements
The authors thank Dr H.M.T Thomas for her assistance in technical editing, language editing and proofreading this manuscript.
Conflicts of Interest
The authors declare none.
Consent for Publication
All authors have read and approved the final manuscript and give consent to the publication of the manuscript in Journal of Radiotherapy in Practice should the article be accepted by the Editor-in-chief upon completion of the refereeing process.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.










