Introduction
Inflammatory bowel disease (IBD) [i.e., Crohn’s disease (CD) and ulcerative colitis (UC)] is a complex remitting and relapsing, immune-mediated disease of the gastrointestinal tract that affects approximately 1·5 million Americans, 2·2 million Europeans and a growing number of people in the Middle East and Asia where incidence is increasing.Reference Ananthakrishnan1 Incidence rates for both CD and UC are believed to be rising with no singular identifiable cause since IBD develops at the crossroads of genetics, immunology, environment and the gut microbiome.Reference Ananthakrishnan1
In the fields of radiation oncology, urology and gastroenterology, there still exists a pervasive notion that the presence of IBD is a relative/absolute contraindication for radiation therapy (RT) to the abdomen or pelvis. Large-scale studies aimed at elucidating the toxicities of abdominopelvic irradiation in patients with IBD are essentially non-existent due to relative low incidence rates for IBD (North American incidence rates of 0–19·2 per million for UC and 0–20·2 per million for CD).Reference Ananthakrishnan1 Most of the available literature consists of case reports or single-institutional retrospective studies with relatively small sample sizes. The purpose of this study is to compile and evaluate the currently available literature to better characterise the toxicities of RT in this small but important subset of patients.
Literature Review
A literature search was conducted using PubMed/MEDLINE with the following keywords for filtering of results: RT, brachytherapy, inflammatory bowel disease, Crohn’s disease, ulcerative colitis and toxicity. The PubMed search yielded 1,206 results which were screened with the addition of 8 studies which were identified through hand searching. Case reports, and case series with a population less than 5 were excluded, and the number of full-text articles assessed for eligibility was 25. Among the full-text articles assessed for eligibility, 5 review articles and 1 non-English publication were excluded for a total of 19 studies meeting inclusion criteria to be selected for quantitative analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) conventions as shown in Figure 1. Three abstracts reported in the International Journal of Radiation Oncology, Biology, Physics (IJROBP) were included in the 19 studies reported in this review, those studies are denoted with an asterisk when appropriate in Tables 1–5.

Figure 1. Flow diagram for data collection following PRISMA convention
Table 1. Study Characteristics—Patient Factors
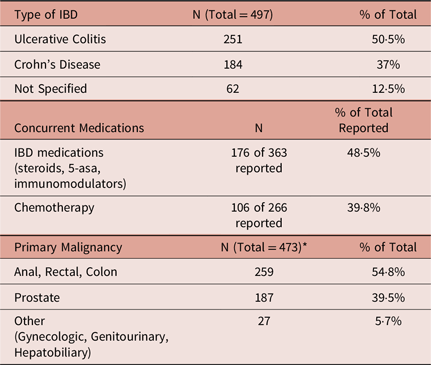
* Song et al. patients (N = 24) excluded from total because primary malignancy not specified.
Table 2. Study Characteristics—Radiation Therapy Modality and Techniques

Table 3A. Individual Study Characteristics

Table 3B. Reported Acute and Late Toxicities

Table 4. Summary of Results for those studies with matched controls
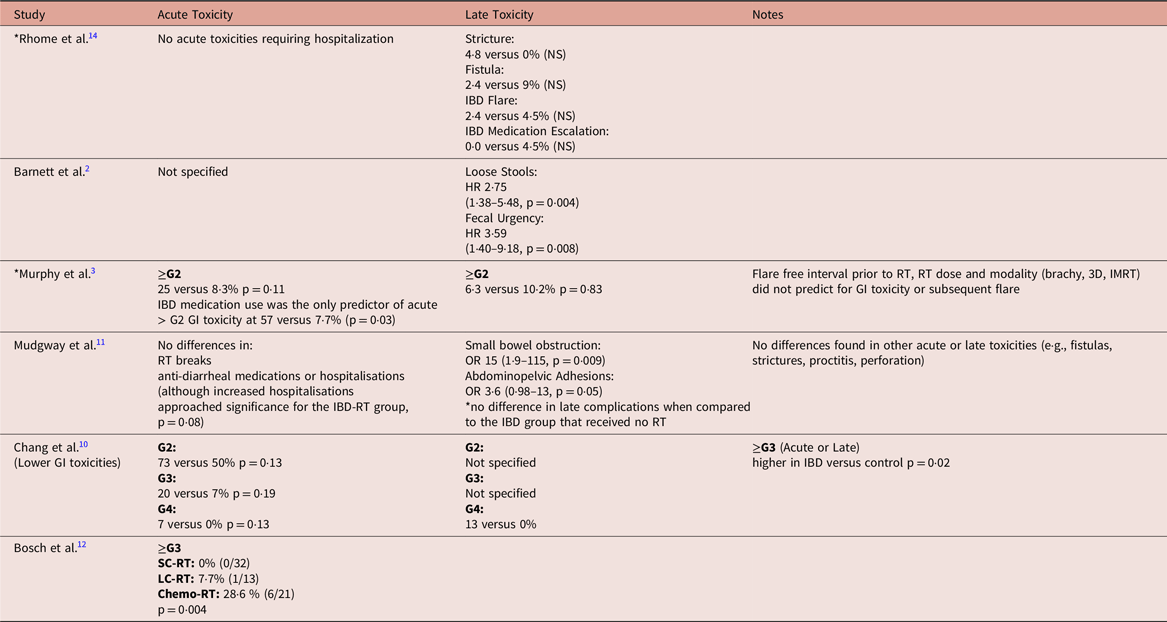
Table 5. Multivariate logistic regression model of IBD flare predictors in 1 year following prostate cancer treatment (taken from Refs.Reference Kirk, Govani and Borza16,Reference Feagins, Kim and Chandrakumaran18 )
Results
Overall, 19 studies were included in the review, and 17 of those studies are summarised in Table 3 with the remaining two studies summarised in Table 5. The total population of the studies summarised in Table 3 was 497 patients with 50·5% composed of UC and 37% composed of CD with an additional 12·5% carrying the diagnosis of IBD without a specific type indicated (Table 1). Roughly, 55% of the population was composed of patients with a gastrointestinal primary malignancy followed by prostate cancer which composed 40% of reported primary malignancies (Table 1). Of the studies that reported the modality of radiation, 70% of the population received external beam RT (EBRT) alone, 12% received brachytherapy alone, 3% received a combination of EBRT and brachytherapy and the remaining 15% was not specified (Table 2). Of the studies that reported treatment with EBRT alone, approximately 12% of patients were treated with intensity-modulated RT (IMRT), 18% with 3D-conformal techniques, 19% with IMRT or 3D-conformal radiotherapy (3D-CRT) without specification, 13% with 3 or 4 field technique, 4% with opposed anterior and posterior fields (AP/PA) and the remaining 34% without a specific technique documented (Table 2). Roughly, 49% of the population was taking some form of IBD medication at the time of therapy, and approximately 40% received concurrent chemotherapy (Table 1).
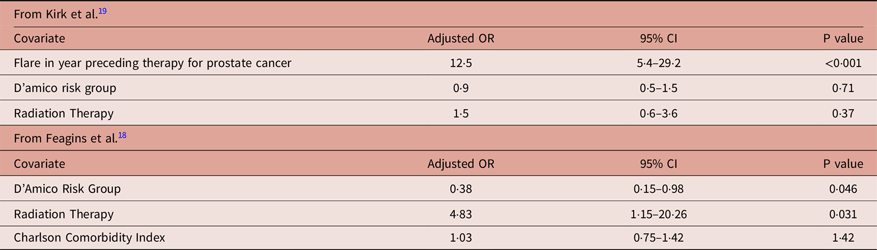
Tables 3A and 3B list individual study characteristics of the 17 included studies reported from 1999–2019. Of those 17 studies, 11 were retrospective studies without case–control and 6 studies compared those patients with IBD to matched or unmatched control patients. These case–control or matched studies are also summarised separately in Table 4. The mean radiation dose for each individual study is listed in Table 3A and varies based on site of primary malignancy. Acute and late radiation toxicities were reported using Radiation Therapy Oncology Group (RTOG) grading in 7 of 17 studies and Common Terminology Criteria for Adverse Events (CTCAE) grading in 7 of 17 studies with 3 of 17 studies using their own criteria. When including all primary malignancies, the reported range for acute toxicity ≥ grade 2 was 0–47% and acute toxicity ≥ grade 3 was 0–28% (Table 3B). When including all primary malignancies, the reported range for late toxicity ≥ grade 2 was 0–39% and late toxicity ≥ grade 3 was 0–29% (Table 3B).
Looking at trials specific to prostate cancerReference Barnett, De Meerleer and Gulliford2–Reference Mohammed, Hoskin and Henry8, the reported range for acute toxicity ≥ grade 2 was 25–33% for EBRT (2 studies reporting) and 0–38% for brachytherapy (4 studies reporting), and acute toxicity ≥ grade 3 was 0% (1 study reporting) for EBRT and 0–23% for brachytherapy (4 studies reporting) (Table 3B). Reported late toxicities in prostate cancer studies are also reported in Table 3B, with a range for late toxicity ≥ grade 2 of 6·3–17% for EBRT (2 studies reporting) and 0–38% for brachytherapy (4 studies reporting), and late toxicity ≥ grade 3 of 0% (1 study reporting) for EBRT and 0–15% for brachytherapy (3 studies reporting). When looking at those studies that only included gastrointestinal (GI) primary malignanciesReference Green, Stock and Greenstein9–Reference Bosch, van Rooijen and Bokkerink12, namely rectal and anal cancers, the reported range for acute toxicity ≥ grade 3 was 0–28% and the late toxicity ≥ grade 3 was 13% (Table 3B).
Table 3A describes the individual study characteristics of the identified articles. Crohn’s disease and ulcerative colitis are abbreviated as CD and UC, respectively. For brevity, common RT abbreviations were used: EBRT for external beam radiotherapy, IMRT for intensity modulated radiotherapy, 3D-CRT for 3D conformal radiotherapy, AP/PA for opposed anterior and posterior fields. In the case of brachytherapy, the commonly used abbreviations for low-dose rate (LDR) and for high-dose rate (HDR) have been employed. If a particular definition was used within the article of origin, that term was also used in the table for example, Willett et al.Reference Willett, Ooi and Zietman13 specified ‘conventional’ as 1·8–2 Gy fraction size photon irradiation and ‘specialised’ as specific RT techniques (small fields, decubitus position, proton beam irradiation or scheduled rest periods during treatment) or surgical procedures (clips to delineate tumor bed, omentoplasty or Dexon mesh) to minimise or avoid small and large bowel irradiation. For the data reported from Bosch et al.,Reference Bosch, van Rooijen and Bokkerink12 short-course RT (SC-RT) is defined as pre-operative 5 Gy × 5 fractions given over a 5- to 7-day period, long-course RT (LC-RT) is defined as pre-operative radiation with 45–50 Gy given in 25–28 fractions of 1·8–2·0 Gy, and combined-modality chemoradiation (chemo-RT) is defined as fluoropyrimidine-based chemotherapy concurrent with long-course radiation schedule. The number of patients receiving a defined RT modality was not listed in chart if not specified in source article. As mentioned earlier, the asterisk indicates data published in abstract form in IJROBP. Table 3B displays the reported acute and late toxicities from the articles outlined in Table 3A. The following abbreviations were employed for statistically significant (SS), small bowel obstruction (SBO), side-effects (SE) and bowel movement (BM).
Several studies also compared toxicities with matched controls at their individual institutionsReference Barnett, De Meerleer and Gulliford2,Reference Murphy, Ruth and Buyyounouski3,Reference Chang, Kumar and Koyfman10–Reference Bosch, van Rooijen and Bokkerink12,Reference Rhome, Axelrad and Itzkowitz14 and are highlighted in Table 4. In an abstract published in IJROBP, Rhome et al. reported on 64 patients with IBD and a primary malignancy (mostly comprised of GI and prostate malignancies), with 42 of 64 patients treated with RT for their cancer and 22 of 64 treated without radiation.Reference Rhome, Axelrad and Itzkowitz14 There were no acute toxicities requiring hospitalisation and no significant differences in stricture formation, fistula formation, IBD flares or IBD medication escalation among the groups.Reference Rhome, Axelrad and Itzkowitz14
Barnett et al. reported on prostate cancer patients with and without IBD treated with EBRT and found SS hazard ratios of 2·75 (1·38–5·48, p = 0·004) for loose stools and 3·59 (1·40–9·18, p = 0·008) for fecal urgency in those patients with IBD.Reference Barnett, De Meerleer and Gulliford2 There was no statistical difference with regard to rectal bleeding, proctitis, sphincter control, stool frequency or urinary SE when comparing patients with IBD versus those without IBD.Reference Barnett, De Meerleer and Gulliford2
In another abstract published in IJROBP, Murphy et al. reported on 21 patients with IBD and prostate cancer treated with either brachytherapy or EBRT. When compared to those matched patients without IBD, there was no statistical difference in the acute or late toxicity ≥ grade 2.Reference Murphy, Ruth and Buyyounouski3 They also reported that flare-free interval prior to RT, RT dose and modality (brachytherapy, 3D, IMRT) did not predict for GI toxicity or subsequent flareReference Murphy, Ruth and Buyyounouski3. IBD medication use was the only predictor of acute > grade 2 GI toxicity at 57% for those requiring medication versus 7·7% for those not requiring medication (p = 0·03).Reference Murphy, Ruth and Buyyounouski3
Mudgway et al. reported on 71 patients with IBD and rectal cancer treated with EBRT compared with 71 matched controls with rectal cancer without IBD treated with EBRT, and 44 non-matched controls with IBD and rectal cancer treated without EBRT.Reference Mudgway, Bryant and Heide11 In the acute setting, there were no differences in breaks in RT, anti-diarrheal medication use or hospitalisations when compared to matched controls [although hospitalisations approached significance (adjusted OR 2·69, 95% CI, 0·88–8·22, p = 0·08)].Reference Mudgway, Bryant and Heide11 There was a SS higher rate of SBO (OR 15, 95% CI 1·9–115, p = 0·009) and higher rates of abdominopelvic adhesions that approached statistical significance (OR 3·6, 95% CI 0·98–13, p = 0·05) in the IBD + RT cohort.Reference Mudgway, Bryant and Heide11 When compared to the non-matched cohort with IBD treated without RT, there was no difference in long-term complications, suggesting that these adhesions or obstructions may be unrelated to RT.Reference Mudgway, Bryant and Heide11
Chang et al. reported on 15 anal/rectal cancer patients with IBD treated with EBRT.Reference Chang, Kumar and Koyfman10 When compared with matched-control patients without IBD, there was no significant difference in rates of grade 2, 3 or 4 acute toxicity; however, when grouping acute and late toxicities grade 3 or greater, there was a SS higher rate versus the control group (p = 0·02)Reference Chang, Kumar and Koyfman10. Another study, by Bosch et al., looked at 161 patients with rectal cancer and IBD. Sixty-six of these patients were treated with some form of RT and the remaining patients served as matched control subjects.Reference Bosch, van Rooijen and Bokkerink12 Of the patients treated with RT, 32 received SC-RT (2500 cGy in 5 fractions over 5–7 days), 13 received LC-RT (4500–5000 cGy given in 25–28 fractions of 180–200 cGy), and 21 received LC-RT with concurrent chemotherapy.Reference Bosch, van Rooijen and Bokkerink12 In total, 0% of patients receiving SC-RT, 7·7% (n = 1) of patients receiving LC-RT, and 28·6% of patients receiving chemo-RT experienced acute grade 3 or greater toxicity (p = 0·004).
IBD activity after therapy
There is scarce information regarding the effect of RT on IBD activity following completion of treatment. Willett et al. found no SS difference in toxicity frequency related to IBD activity.Reference Willett, Ooi and Zietman13 There were two studies that reported on IBD flares in the year following RT.Reference Annede, Seisen and Klotz15,Reference Kirk, Govani and Borza16 Glick et al. reported in abstract that IBD type, active use of IBD medications and previous IBD-related surgery were not predictive for acute toxicity on univariate analysis.Reference Glick, Warde and Su17 As mentioned previously and outlined in Table 4, Murphy et al. found IBD medication use to be a predictor for acute > grade 2 toxicity (57·1% versus 7·7% p = 0·03) but found the flare-free interval prior to RT to not be predictive for subsequent flare.Reference Murphy, Ruth and Buyyounouski3
There were two larger studies that reported on IBD flares in the year following RT.Reference Kirk, Govani and Borza16,Reference Feagins, Kim and Chandrakumaran18 Kirk et al. analysed a population of 240 men with prostate cancer and a diagnosis of IBD at the time of cancer diagnosis treated in the United States Department of Veterans Affairs (VA) system.Reference Kirk, Govani and Borza16 In the VA population, it was found that patients with IBD and prostate cancer were much more likely to receive surgery than RT compared to those patients without a diagnosis of IBD treated in the same system, 41 versus 28% (p < 0·001).Reference Kirk, Govani and Borza16 Among the patients with IBD and prostate cancer, there was no SS difference in flares occurring in the year following treatment between the surgery alone, RT alone and active surveillance/androgen deprivation therapy cohorts, with a flare rate of roughly 18%.Reference Kirk, Govani and Borza16 The only predictor for flare following therapy was the presence of a flare in the year preceding treatment (OR 12·5, 95% CI 5·4–29·2), p < 0·001).Reference Kirk, Govani and Borza16 There was no increased risk of flare on multivariate analysis based on D’Amico risk group or RT status (Table 5).Reference Kirk, Govani and Borza16
Another group also looked at a similar population within four VA hospitals in Texas and Virginia.Reference Feagins, Kim and Chandrakumaran18 In this study, 100 patients with IBD (29% CD, 66% UC and remainder with undefined colitis) with prostate cancer were identified. In total, 47% of patients underwent RT (of these 72% EBRT and 28% brachytherapy). Of the 53 patients who did not undergo RT, 77% underwent surgery with the remainder receiving androgen deprivation therapy or chemotherapy.Reference Feagins, Kim and Chandrakumaran18 There was no statistical difference in age, race, IBD type or cancer stage between the RT group and those treated with other modalities. On univariate analysis, there was no statistical significance in IBD flares, IBD hospitalisations or IBD-related surgeries following therapy, as well as no differences seen in overall survival.Reference Feagins, Kim and Chandrakumaran18 However, on multivariate analysis (as shown in Table 5), RT was identified as a risk factor for subsequent IBD flare. Interestingly, D’Amico risk group was also associated with flare on multivariate analysis.
Annede et al. reported on 28 patients with various malignancies treated in France.Reference Annede, Seisen and Klotz15 In this study, the severity of IBD was assessed with the Harvey Bradshaw Index (HBI) and the Mayo Index for CD and UC, respectively, with severe IBD being classified as HBI > 16 or Mayo > 7, moderate IBD as HBI 8–15 or Mayo 5–6, mild IBD as HBI 5–7 or Mayo 2–4 and remission if less than the aforementioned thresholds. During follow-up after completion of RT, the median scores for patients within 6 months of treatment was Mayo of 4 and HBI of 2 for UC and CD, respectively, and after 6 months those scores improved to 3 and 1 respectively. Within the first 6 months following treatment, 50% of patients were classified as ‘in remission’, and this improved by 61% after 6 months. Only two patients experienced severe IBD symptoms in follow-up, both of which were patients with prostate cancer (one treated with EBRT to a dose of 7400 cGy, and one treated with EBRT, 4600 cGy to prostate and seminal vesicles with 1400 cGy HDR brachytherapy boost).
Discussion
Historically, IBD has been considered a contraindication for RT largely related to concerns of increased acute and late toxicities. Further compounding the issue is the fact that most of the existing studies have small patient populations, employ different toxicity-reporting conventions, involve numerous disease sites and were largely performed in an era of RT predating the widespread adoption of advanced techniques such as IMRT. Elucidating the improvement of SE profile with the widespread adoption of advanced radiation techniques (such as IMRT) is difficult with the current literature available since most series describe patients treated over a wide range of time.
White et al. reported lower acute ≥ grade 2 toxicity in the IMRT treated patients compared to those treated w/out 3D-CRT (14 versus 100% p = 0·002).Reference White, Murphy and Chang21 Feagins et al. attempted to demonstrate this effect.Reference Feagins, Kim and Chandrakumaran18 In their study, when analysing 100 VA patients with IBD and prostate cancer from 1996 to 2015, there was no statistical difference in the rate of subsequent IBD flares in those treated in the 1st decade (1996–2005) versus the 2nd decade (2006–15), nor was there a difference in flares between those getting treated with and without radiation in the 1st versus 2nd decade.Reference Feagins, Kim and Chandrakumaran18 Although this study does not show a difference when looking specifically at IBD flares, it is assumed that other GI/GU grade 3 toxicities would have improved over that time period due to the adoption of more advance delivery modalities.
Only one meta-analysis has been performed regarding this topic and was published in 2019 by Lin et al.Reference Lin, Lehrer and Rosenberg19 Using data from 8 of the 17 studies included in this report,Reference Peters, Cesaretti and Stone6,Reference Pai, Keyes and Morris7,Reference Green, Stock and Greenstein9,Reference Chang, Kumar and Koyfman10,Reference Bosch, van Rooijen and Bokkerink12,Reference Willett, Ooi and Zietman13,Reference Song, Lawrie and Abrams20,Reference White, Murphy and Chang21 the rate of acute toxicity grade 3–5 was calculated to be 14% (7–22·4) and late toxicity grade 3–5 was calculated at 10·2% (3·2–19·7).Reference Lin, Lehrer and Rosenberg19 These figures are within the range of the values reported in Table 3B (up to 27% for acute ≥ grade 3 toxicities and up to 29% for late ≥ grade 3 toxicities).
It is instructive to compare the above rates of toxicity to those seen in modern studies of patients without IBD treated with RT. RTOG 04-15 randomised patients with prostate cancer to receive either conventionally fractionated EBRT versus hypofractionated EBRT, reporting rates of acute ≥ grade 3 GI and GU toxicities of 0·6 and 2·4% for the hypofractionated arm and 0·8 and 3·3% for the conventional arm. Rates of late ≥ grade 3 GI and GU toxicities were 2·6% and 2·3% for hypofractionated RT and 4·1% and 3·5% for conventional RT for patient treated with IMRT or 3D-CRT.Reference Lee, Dignam and Amin22
The RTOG 98-11 trial which randomised patient with anal cancer to RT plus 5-FU and mitomycin or RT plus 5-FU and cisplatin reported rates of acute ≥ grade 3 GI toxicity of 36·6% and 46·6% between trial arms, respectively. Late ≥ grade 3 GI toxicity for RTOG 98-11 occurred in 3% and 2·5% of patients repectively.Reference Gunderson, Winter and Ajani23 The RTOG 05-29 trial which utilised dose-painted IMRT to treat anal cancer with 5FU and mitomycin reported grade ≥ 3 GI toxicity in 11 of 52 (21·1%) patients—decreased compared to RTOG 98-11.Reference Kachnic, Winter and Myerson24
Late effects, strictures and fistulas
One of the primary concerns for patients with IBD receiving RT is the development of late toxicities such as fistulas or strictures. These are difficult toxicities to approximate since either can be the result of the radiation or the IBD itself. Mudgway et al. found no difference in stricture or fistula formation when compared to matched controls receiving RT or unmatched patients with IBD receiving no RT.Reference Mudgway, Bryant and Heide11 Rhome et al. reported similar results, finding that there was no SS difference in the formation of fistula or stricture, IBD flares or IBD medication escalation between groups of patients with IBD receiving radiation and matched controls with IBD treated without RT.Reference Rhome, Axelrad and Itzkowitz14
IBD activity
Although there is limited supporting evidence, there seems to be little effect of RT on the severity or activity of IBD. One study,Reference Kirk, Govani and Borza16 found that the activity of disease in the year preceding therapy was the best predictor of disease activity after therapy; however, another studyReference Murphy, Ruth and Buyyounouski3 reported that the flare-free interval prior to RT was not predictive of GI toxicity or subsequent IBD flare. Yet, another studyReference Annede, Seisen and Klotz15 found that most patients remained in remission in the year following RT, improving from 50% at 6 months to 60% at a year, with only two patients experiencing severe IBD symptoms following radiation. Feagins et al. did find RT to be a SS risk factor for subsequent IBD flare on multivariate analysis.Reference Feagins, Kim and Chandrakumaran18 Understanding how active or well-controlled a patient’s IBD is prior to therapy is likely the best way to estimate risk of flare following RT. When available the number of patients on IBD medications at the time of treatment was listed for each study in Table 3A. However, information on which IBD medications were being used was limited and most studies either grouped all IBD medications together or did not compare outcomes based on specific IBD medication use. In clinical practice, however, the class of medication used can indicate how severe or active the IBD actually is (e.g., use of biologic as a surrogate for severe disease). There was a significant number, roughly 50%, of patients across all studies examined who were not taking IBD medications, but it is unknown if these patients were in remission or if maintenance therapies were stopped specifically for cancer therapy as is sometimes done, especially in the case when concurrent chemotherapy is used.
Concurrent chemotherapy
It is well established that the addition of chemotherapy to radiation or vice-versa will result in increased toxicity; however, it is not known to what extent this effect will manifest in IBD patients. In some cases, systemic therapy with its immunomodulation can improve IBD symptoms even while increasing toxicity in other areas. Further complicating this point within the literature are the rather small sample sizes and differing disease sites. For example, it is exceedingly rare for a prostate cancer patient, unless metastatic, to receive chemotherapy, whereas most protocols for GI malignancies include chemotherapy, see Table 3A. In the only study to report the toxicity outcomes for those receiving RT or RT plus chemotherapy, the addition of chemotherapy to LC-RT increased acute ≥ grade toxicity by roughly 20%.Reference Bosch, van Rooijen and Bokkerink12 This increase in toxicity was not different from the toxicity seen in the matched control group.
Proton therapy
Unsurprisingly, there is a lack of information regarding proton therapy in this patient population. Willett et al. reported on 28 patients with IBD who received RT.Reference Willett, Ooi and Zietman13 Of these patients, 12 were treated with conventional radiation and 16 were treated with a combination of specialised techniques (e.g., proton therapy, small fields, decubitus position or scheduled breaks).Reference Willett, Ooi and Zietman13 There was a SS difference in severe late effects between patients receiving conventional treatment and those receiving specialised radiation techniques (50 versus 13%, p = 0·02).Reference Willett, Ooi and Zietman13
Conclusions
Although limited, current literature demonstrates that definitive radiation can be delivered effectively and safely to patients with IBD with acceptable toxicity profiles. Though not consistently reported, patient characteristics including IBD distribution relative the irradiated field (e.g., isolated small bowel disease versus colon and rectum involvement), inflammatory activity at time of radiation, overall disease severity and finally disease phenotype in case of CD (fistulising versus stricturing versus inflammatory only) are likely to impact the incidence of acute and late radiation toxicity, and should be taken into account on each individual basis when evaluating potential patients. When possible, advanced techniques with strict organ-at-risk tolerances should be employed to limit toxicity in this patient population.
Acknowledgements
None.










