INTRODUCTION AND BACKGROUND
Lung cancer is the leading cause of cancer mortality worldwide. 1 For early stage lung cancer, surgical lobectomy is generally believed to offer the best survival rates in appropriately staged patients. However when the cancer is located in the superior sulcus or in proximity to the critical structure, radiotherapy is a preferred choice. Conventional radiation therapy does not approach surgical cure rates because it has not been practically possible to achieve ablative radiation dose tolerably using such techniques. Over the past decade, development of stereotactic ablative radiotherapy (SABR) has revolutionised radiation therapy for early stage lung cancer. Advances in onboard imaging and highly conformal, and precise radiation delivery have made possible the safe and sound administration of ablative radiation doses, achieving tumour control rates similar to surgery, 2 SABR demonstrates high rates (>90%) of primary tumour control within the irradiated target volume.Reference Billy 3 With these rapidly evolving sophisticated radiation treatment technologies, it is important to ensure that an improved local tumour control does not compromise the protection of patients against long-term effects like radiation-induced second cancer. A concern of advanced treatment delivery techniques, such as intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT), has been the potential large whole-body integral dose due to radiation scattering and head leakage, such that a broad volume of at-risk normal tissue may receive a carcinogenic radiation dose. Moreover, IMRT and VMAT compared with conventional radiotherapy requires longer beam-on time and uses a larger number of treatment fields, thus delivering a larger number of monitor units, which is associated with greater integral whole body dose.
This study is aimed to evaluate the risk of radiation-induced second cancer following ablative radiotherapy for lung cancer. The secondary malignancy can occur either within the high-dose region (inside the treated volume) or within the medium–low dose region (inside the beam path). Hence, the main goal of the treatment planning is to find the right balance between reducing low dose spillage and providing adequate, homogeneous target coverage. The increased complexity of treatment techniques makes it critical to consider factors such as second cancer risk (SCR) while comparing and analysing different planning methodologies. The relationship between low-level peripheral organ dose in radiation therapy to secondary induced cancers, especially for patients with long-term survival rates has been the subject of many studies. Chaturvedi et al. showed that even 40 years after radiotherapy for cervical cancer, survivors remain at an increased risk of second cancers.Reference Chaturvedi, Ahamad and Iyer 4 Travis et al. discussed secondary breast cancer in patients 30 years after the initial treatment.Reference Travis, Hill, Dores and Gospaodarowicz 5 Hall et al. presented the increased risk moving from three-dimensional conformal radiotherapy (3DCRT) to intensity-modulated radiotherapy (IMRT), and reported IMRT almost doubled the second cancer risk compared to 3DCRT.Reference Hall and Wuu 6 Brenner et al. reported a 4–6% increase in second lung malignancies after prostate radiotherapy as compared to surgery.Reference Brenner, Curtis and Hall 7 Movas et al. observed that 5·7% treated with radiation developed second tumours.Reference Movas, Hanlon and Pinover 8
In this study, we compared the SCR from SABR for five treatment planning and delivery techniques using the concept of organ equivalent dose (OED).
MATERIALS AND METHODS
Patient data and treatment planning
Five randomly selected patients with T1, T2 (≤4 cm), N0, M0 medically inoperable non-small cell lung cancer were used for treatment planning. Patient selection criteria was based on RTOG 0236. 9 The patient’s age ranged from 39 to 57 years old with an average age of 45. All patients had undergone four-dimensional computed tomographic image scans using a Somatom CT scanner (Siemens Medical Solutions, Erlangen, Germany) of the chest for identification of the target and normal critical structures. Targets were defined in accordance with the report of the International Commission on Radiation Units and Measurement (ICRU50). 10 The gross tumour volume (GTV), internal tumour volume (ITV) and organs at risk (OARs) were contoured on the planning CT scan. Planning volumes for PTV were delineated with circumferential 3 mm margins for the ITV to allow for setup uncertainty. Critical structures like chest wall, heart, lungs, small and large airways, spinal cord, oesophagus, ribs, skin and all remaining soft tissue were delineated for risk analysis. Table 1 lists the patient characteristics, patient age and size of the target volume.
Table 1 Patient information
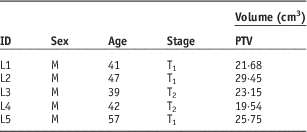
Abbreviations: M, male; PTV, planning target volume.
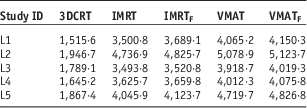
An Infinity linear accelerator with agility multi leaf collimator (MLC) (Elekta, Stockholm, Sweden) and Monte Carlo-based planning system Monaco v5.11 was used for IMRT and VMAT planning. Both IMRT and VMAT plans used dynamic MLC, 2 mm grid size and 1% variance for calculation. A 13-field 3DCRT plan, two IMRT plans, one with standard 6 MV beams and the other with energy-matched 6 MV flattening filter free (FFF) beams (IMRT, IMRTF), and two VMAT plans using 225° arc, one with standard 6 MV beams and the other with energy-matched 6 MV FFF beams (VMAT, VMATF) were produced. All plans were prescribed to 60 Gy in five fractions to primary PTV volume so that ≥98% of the PTV received ≥98% of the prescription dose and ITV received 100% of the prescription dose. For 3DCRT, IMRT and VMAT plans, the dose was accurately calculated using Monte Carlo simulations. All treatment planning was undertaken using 6 MV beams, since low photon beam energy is always recommended when irradiating lung tumours because of the smaller penumbra widening. This recommendation is also suggested by the smaller difference found between the experimental and the predicted percentage depth doses inside the lung.Reference Weiss, Siebers and Keall 11 , Reference Marco, Stefano and Vitaliania 12 All plans were created by a physicist, approved by a radiation oncologist, and satisfied all clinical protocols and constraints. Treatment plan details are listed in Table 2. A side-by-side comparison of the relative dosimetry between all the plans for patient L3 is shown in Figure 1 and the dose volume histogram (DVH) comparison between plans for patient L5 is shown in Figure 2.
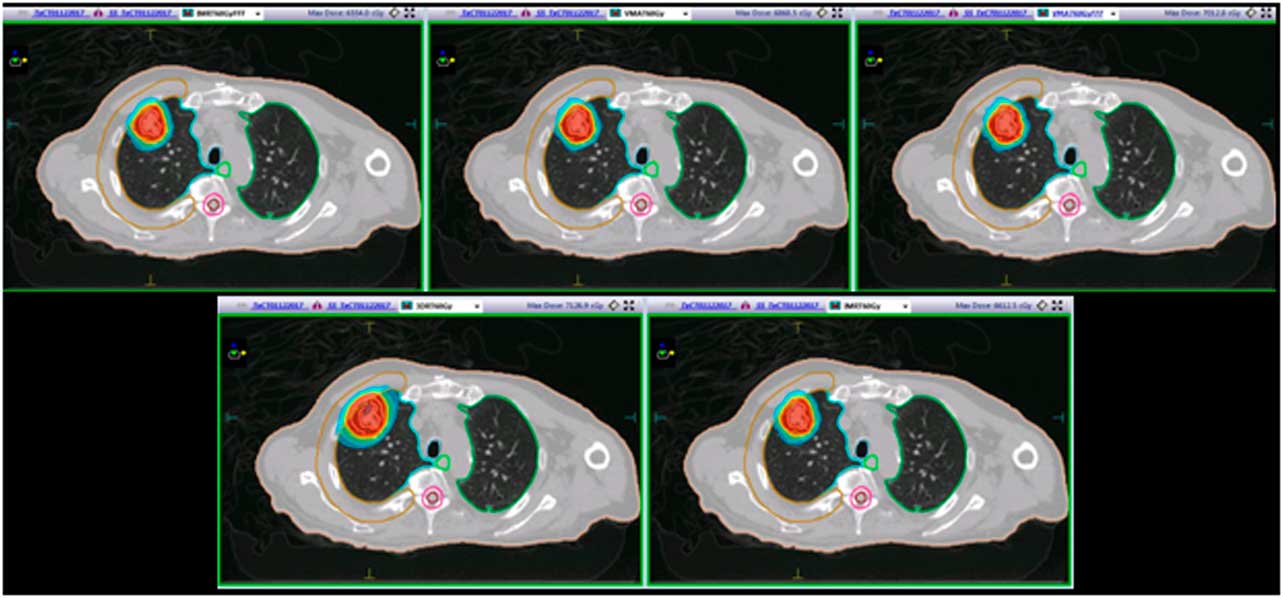
Figure 1 Patient L3: a side-by-side comparison of the relative dosimetries between an IMRTF, VMAT, VMATF, 3DCRT and an IMRT plan. Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; IMRTF, intensity-modulated radiotherapy with flattening filter free beam; VMAT, volumetric modulated radiotherapy; VMATF, volumetric modulated radiotherapy with flattening filter free arcs
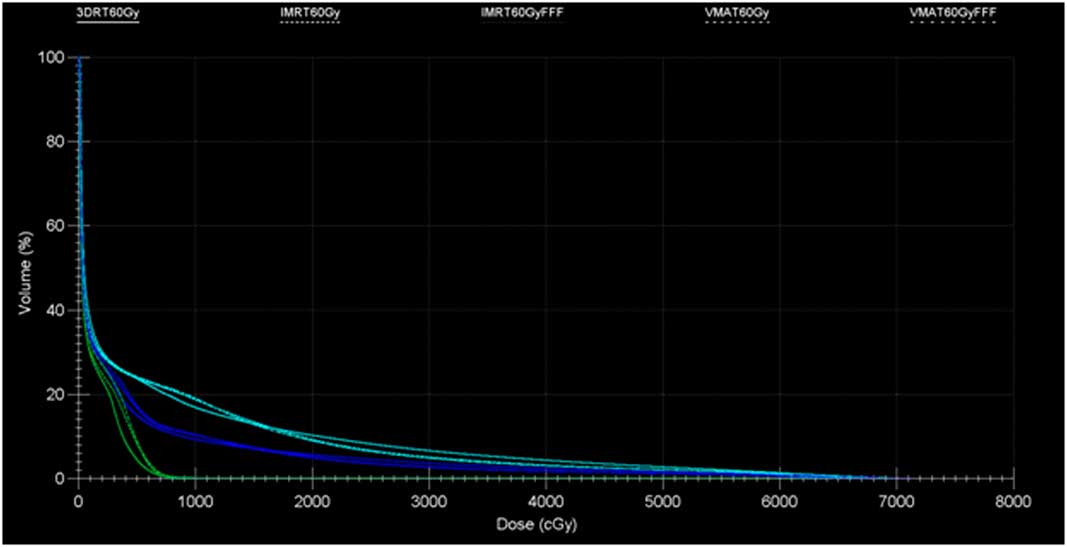
Figure 2 Study L5: dose volume histogram comparison between five plan: cord (green), soft tissue (blue) and lung (cyan).
Table 2 Treatment plan details: monitor unit (MU) per fraction
Risk modelling
To calculate the risk of second malignancies, all corresponding DVH using 0·01 Gy bin widths were extracted from Monaco planning system and exported to the software developed for risk modeling. This formulation used in this study had been previously used in several studies to estimate the in-field organ dose. According to this concept, dose distributions that cause the same radiation-induced cancer incidence have the same OED.Reference Schneider and Walsh 13 It takes into account the effects of cell sterilisation and repopulation at higher dose levels. The OED was calculated according to Equation 1.
where V T is the total volume of the organ of interest, V i is the volume and RED i are the risk-equivalent dose in the i th DVH bin.
 $${\rm RED}_{{{\rm mechanistic }}} ={{{\rm exp}\left( {{\minus}\alpha ^{{\rm '}} D} \right)} \over {\alpha ^{{\rm '}} R}}{\rm }\Bigg[ \left( {1{\minus}2R{\plus}R^{2} {\rm exp}(\alpha ^{{\rm '}} D)} \right){\rm }-\left( {1{\minus}R} \right)^{2} {\rm exp}\left( {{\minus}\left( {{{\alpha ^{{\rm '}} R} \over {\left( {1{\minus}R} \right)}}} \right)D} \right)\Bigg] $$
$${\rm RED}_{{{\rm mechanistic }}} ={{{\rm exp}\left( {{\minus}\alpha ^{{\rm '}} D} \right)} \over {\alpha ^{{\rm '}} R}}{\rm }\Bigg[ \left( {1{\minus}2R{\plus}R^{2} {\rm exp}(\alpha ^{{\rm '}} D)} \right){\rm }-\left( {1{\minus}R} \right)^{2} {\rm exp}\left( {{\minus}\left( {{{\alpha ^{{\rm '}} R} \over {\left( {1{\minus}R} \right)}}} \right)D} \right)\Bigg] $$
The OED for carcinoma and sarcoma induction was used to approximate the risk for a radiation-induced second cancer was written as follows.Reference Schneider and Walsh 13 , Reference Schneider 14
 $$\eqalignno{ &{\rm OED}_{{{\rm carcinoma}}} ={1 \over {N{\rm }}}\mathop{\sum}\limits_i {{\rm exp}\left( {{\minus}\alpha ^{{\rm '}} _{i} D_{i} } \right)} {{{\rm exp}\left( {{\minus}\alpha _{{i }}^{{\rm '}} D_{i} } \right)} \over {{\minus}\alpha _{{i }}^{{\rm '}} R} }\cr &{\left( {1{\minus}2R{\plus}R^{2} {\rm exp}\left( {{\minus}\alpha _{{i{\rm }}}^{{\rm '}} D_{i} } \right){\minus}\left[ {1{\minus}{\rm }R} \right]^{2} {\rm exp}\left[ {{\minus}{{\left. {(\alpha _{{i }}^{{\rm '}} R} \right)} \over {1{\minus}R}}D_{i} } \right]} \right){\rm }\,\,\,$$
$$\eqalignno{ &{\rm OED}_{{{\rm carcinoma}}} ={1 \over {N{\rm }}}\mathop{\sum}\limits_i {{\rm exp}\left( {{\minus}\alpha ^{{\rm '}} _{i} D_{i} } \right)} {{{\rm exp}\left( {{\minus}\alpha _{{i }}^{{\rm '}} D_{i} } \right)} \over {{\minus}\alpha _{{i }}^{{\rm '}} R} }\cr &{\left( {1{\minus}2R{\plus}R^{2} {\rm exp}\left( {{\minus}\alpha _{{i{\rm }}}^{{\rm '}} D_{i} } \right){\minus}\left[ {1{\minus}{\rm }R} \right]^{2} {\rm exp}\left[ {{\minus}{{\left. {(\alpha _{{i }}^{{\rm '}} R} \right)} \over {1{\minus}R}}D_{i} } \right]} \right){\rm }\,\,\,$$
 $$\eqalignno{ {\rm OED}_{{{\rm sarcoma}}}\,=\,&{1 \over {N{\rm }}}\mathop{\sum}\limits_i {{\rm exp}\big( {{\minus}\alpha ^{{\rm '}} _{i} D_{i} } \big)} {{{\rm exp}\left( {{\minus}\alpha _{{i{\rm }}}^{{\rm '}} D_{i} } \right)} \over {{\minus}\alpha _{{i }}^{{\rm '}} R}} \cr &{\big( {1{\minus}2R{\plus}R^{2} {\rm exp}\big( {{\minus}\alpha _{{i{\rm }}}^{{\rm '}} D_{i} } \big) }\big }\cr &{\left {\minus}[1{\minus}R]^{2} {\rm exp}\Big[ {{\minus}{{\left. {(\alpha _{{i{\rm }}}^{{\rm '}} R} \right)} \over {1{\minus}R}}D_{i} } \Big]{\minus}\alpha _{{i{\rm }}}^{{\rm '}} RD_{i} } \Big)\,\,\qquad $$
$$\eqalignno{ {\rm OED}_{{{\rm sarcoma}}}\,=\,&{1 \over {N{\rm }}}\mathop{\sum}\limits_i {{\rm exp}\big( {{\minus}\alpha ^{{\rm '}} _{i} D_{i} } \big)} {{{\rm exp}\left( {{\minus}\alpha _{{i{\rm }}}^{{\rm '}} D_{i} } \right)} \over {{\minus}\alpha _{{i }}^{{\rm '}} R}} \cr &{\big( {1{\minus}2R{\plus}R^{2} {\rm exp}\big( {{\minus}\alpha _{{i{\rm }}}^{{\rm '}} D_{i} } \big) }\big }\cr &{\left {\minus}[1{\minus}R]^{2} {\rm exp}\Big[ {{\minus}{{\left. {(\alpha _{{i{\rm }}}^{{\rm '}} R} \right)} \over {1{\minus}R}}D_{i} } \Big]{\minus}\alpha _{{i{\rm }}}^{{\rm '}} RD_{i} } \Big)\,\,\qquad $$
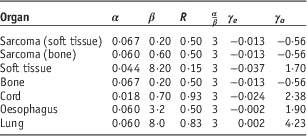
where R is the repopulation parameter, d F is dose per fraction, D the total dose, α′ is the cell kill parameter, α and β varies for each organ and were derived from data based on atomic bomb survivors.
For each organ of interest, the OED derived from DVH of all five study patients was used to find the mean OED. The mean OED values were then combined with organ-dependent parameters to estimate the EAR attributable to breast cancer irradiation. Equation 3 was used for EAR assessment.
The EAR is factorised into a function of dose ρ(D), γ e , γ a are model parameters and the attained age (a) at exposure (e). β is the slope of the dose-response curve in low dose region, s is used to include gender specificity and is set to −0·17 (male). The model parameters used in the calculation model are listed in Table 3.
Table 3 Organ equivalent dose (OED) and excess absolute risk (EAR) calculation parameters for the mechanistic model
The LAR, which gives the percentage likelihood in excess of the baseline risk of second malignancy happening to one’s lifetime, was calculated using EAR (per 10,000 persons-year) as a function of point dose.Reference Schneider 14 It can be considered an effective means of calculating the risk because it takes the patient age at the time of treatment and predicted lifespan into account.Reference Maryam, Torunn and Harlad 15
The integration was performed over an attained age from a latent period of solid cancer induction after the exposure (L=5 years) to 70 years of age. The ratio S(a)/S(e) defines the probability of surviving from age at exposure to the attained age, which was obtained from life table for the US population. 16
RESULTS
Treatment plans, target coverage and conformity
For all treatment plans, the DVH showed clinically acceptable values; it met adequate clinical target coverage and dose constraints for all OARs. Dosimetric planning cohort was met with V98%=98%±1·2% for PTV. The data onto the target coverage and conformity showed that FFF beam plans, in most cases were dosimetrically equivalent. For larger PTV volumes (L2 & L5) the standard beams managed better plans using a lower number of monitor units.
The VMAT plan had 0·91±0·037 times fewer monitor units (MU) than VMATF. The VMAT plan resulted in a statistically significant better PTV homogeneity index (HI) compared with all other plans. The mean HI difference was 1·1 and 1·4% for IMRT and IMRTF. The dose homogeneity within the PTV was slightly improved by the VMAT technique when compared with all 3DCRT, IMRT and VMATF plans, although the difference was not statistically significant between IMRT plan and VMAT. The volume of 5 Gy (V5 Gy) was statistically significantly lower for 3DCRT (35·2±2·4%) than VMAT (44·7±4·1%). V5 Gy for VMAT was higher than VMATF.
Risk analysis
For all patients, the mean V20 volumes of the SABR plan were 4·1% (3DRT), 11·8% (IMRT), and 12·7% (VMAT), respectively (p<0·05 for each pair wise comparison, two-tailed paired t-test). The mean lung doses were 2·85 Gy (3DCRT), 4·90 Gy (IMRT), and 5·71 Gy (VMAT) (p>0·1 for each pair wise comparison). The relative OED with respect to the 3DCRT plan for all OARs based on DVH study are shown (Table 4; Figure 3). The VMATF plan had the best conformal dose distribution out of all plans studied, thus resulting in a significant risk reduction to close to field organs, such as the oesophagus, heart, airways, chest wall and spinal cord.
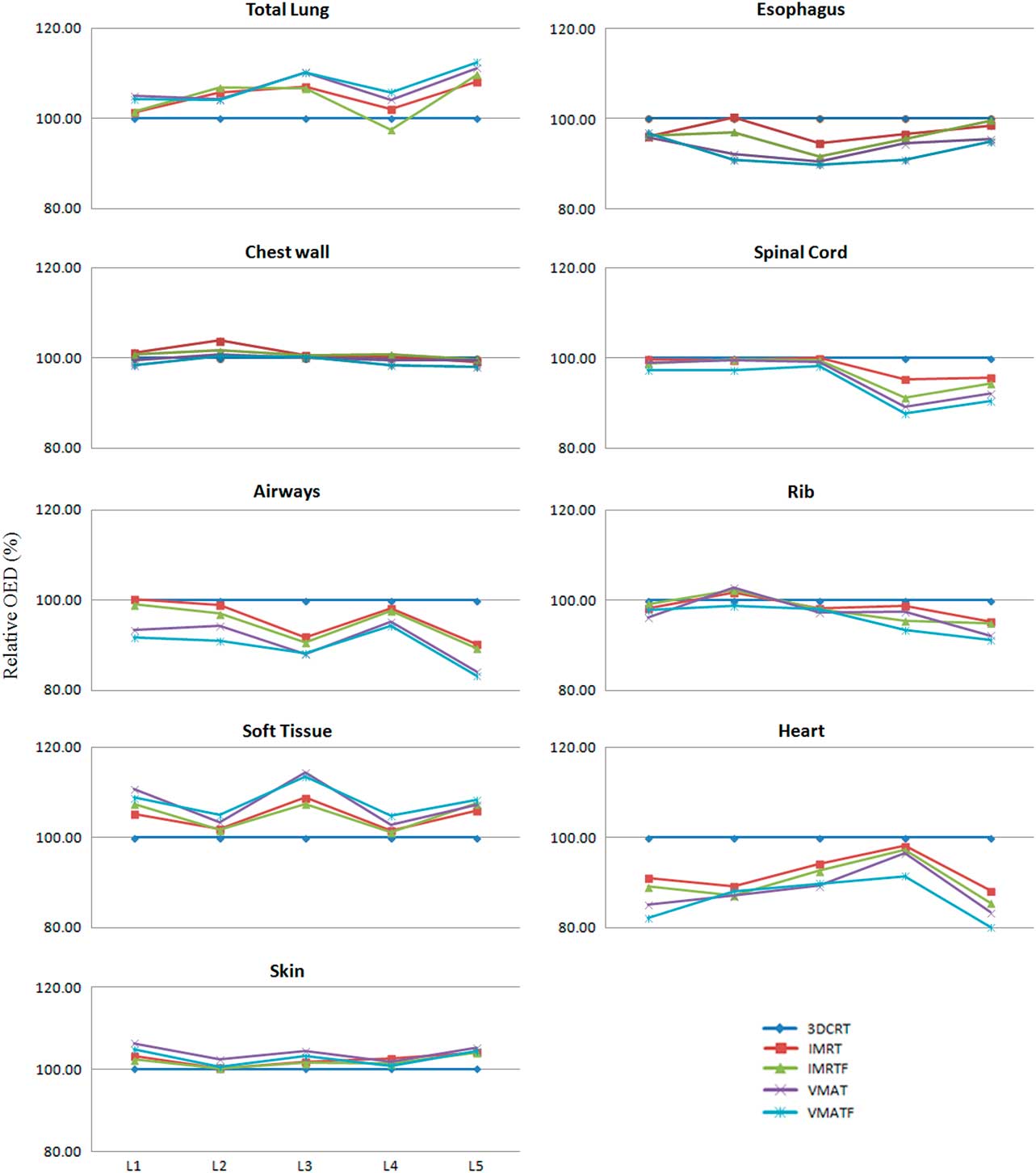
Figure 3 Relative percentage organ equivalent dose of organs for different plans.
Table 4 Relative percentage organ equivalent dose (OED) normalised to three-dimensional conformal radiotherapy (3DCRT)
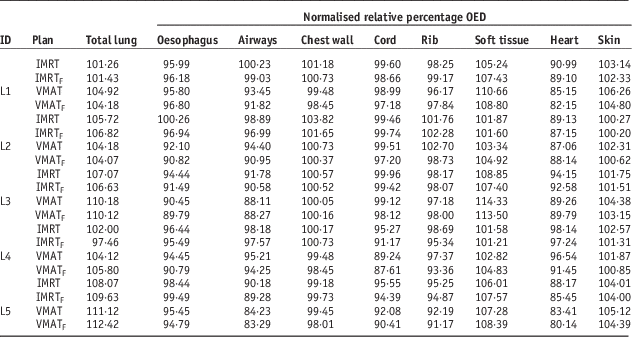
Abbreviations: IMRT, intensity-modulated radiotherapy; IMRTF, intensity-modulated radiotherapy with flattening filter free beam; VMAT, volumetric modulated radiotherapy; VMATF, volumetric modulated radiotherapy with flattening filter free arcs.
The EAR for each OAR for complete treatment course estimated with the mechanistic model is shown in Figure 4. The EAR (combining all organs EAR) for all the organs studied, ranged from 8·5 to 10·6/10,000 persons/year for VMATF and 3DCRT, respectively (Table 5; Figure 4). The absolute EAR difference between IMRT and IMRTF were low ranging from 0·2 to 0·4/10,000 persons-year, whereas delivery difference (IMRT and VMAT) had a significant impact on EAR with absolute difference between IMRT, VMAT and IMRTF, VMATF ranged from 0·5 to 1·0/10,000 persons-year and 1·1 to 1·5/10,000 persons-year, respectively.
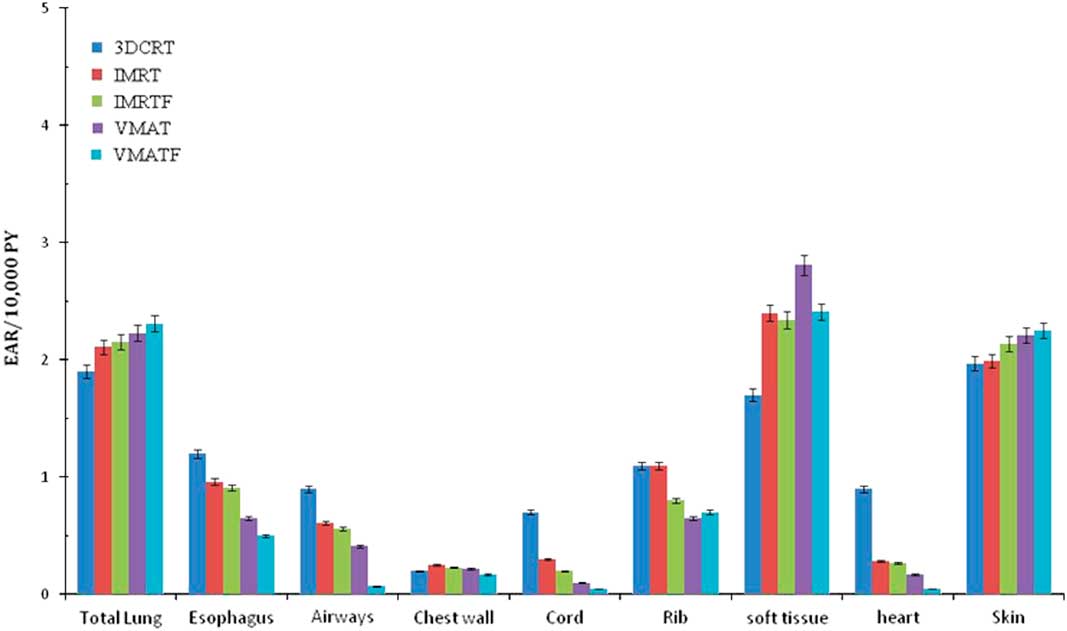
Figure 4 Excess absolute risks of second cancer (Mechanistic model) for complete treatment course based on patients being irradiated and attaining age 70 years 3% error bars are shown for dosimetric uncertainity.
Table 5 Summed excess absolute risk (EAR) for all evaluated organs

Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; IMRTF, intensity-modulated radiotherapy with flattening filter free beam; VMAT, volumetric modulated radiotherapy; VMATF, volumetric modulated radiotherapy with flattening filter free arcs.
The absolute risks (LAR based on EAR) for all considered cases are given in Table 6. It provides LAR normalised per MU (%/MU) for OAR for the five different treatment plans considered. The LAR data showed a strong dependence on age at exposure and decreased as a function of age at exposure. The absolute attributable risk for bone sarcoma was lower with the VMAT plan and was significantly higher with the 3DCRT plan (Table 7).
Table 6 Average lifetime attributable risk (LAR) as a function of organ and age at exposure (year) for the five patients considered

Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; IMRTF, intensity-modulated radiotherapy with flattening filter free beam; VMAT, volumetric modulated radiotherapy; VMATF, volumetric modulated radiotherapy with flattening filter free arcs.
Table 7 Average absolute lifetime attributable risk (LAR) (%) integrated up to an age of 70 years
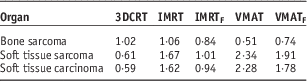
Abbreviations: 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; IMRTF, intensity-modulated radiotherapy with flattening filter free beam; VMAT, volumetric modulated radiotherapy; VMATF, volumetric modulated radiotherapy with flattening filter free arcs.
DISCUSSION
Many epidemiological studies have reported elevated second cancer risk in radiotherapy compared to surgery. Yu et al. reported on second cancer development after high dose, high conformal, single-fraction irradiation.Reference Yu, Wilson and Keith 17 Kim et al. presented the secondary radiation doses of IMRT and proton therapy in patients with lung and liver cancer.Reference Kim, Chung and Dongoh 18 Dasu et al. quantified risks of second rectal and bladder cancer following 42·7 Gy in seven fractions.Reference Dasu, Toma-Dasu, Olofsson and Karlsson 19 Murray et al. analysed the impact of the unflattened beam on second cancer risk.Reference Murray, Thompson and Lilley 20 But the impact of linear accelerator based SABR techniques using unflattened beams on second malignancy risk has not been widely examined. This study examined radiation-induced second cancer risk following advanced, modern, clinically relevant lung SABR techniques.
The advancements in external beam radiation delivery, characterized by a transition from rectangular portals to irregular shapes with rigid collimation, to computer-controlled multi-leaf collimators, have enabled precise dose distribution to target volumes. Advancements with beam collimation and delivery techniques, like IMRT and VMAT, led to large integral whole body dose caused by radiation scattering associated with beam delivery, therefore exposing an extensive volume of susceptible normal tissue to carcinogenic low-dose radiation. It has been widely assumed that VMAT increases second cancer risk compared with conventional radiotherapy techniques, however our results show that this was not always necessarily true. In VMAT treatments, a higher proportion of soft tissue received doses in the 15 to 25 Gy range compared with IMRT and 3DCRT.Our data shows that a larger volume of the contra lateral lung, for most of the patients, received a dose higher than 5 Gy in the IMRT and VMAT plans, with VMAT plan being the highest. In order to correctly account for the dose to the patient outside the treatment field, the calculated peripheral dose was compared against measurements in solid water at various depths and at various distances from the central axis using an ion chamber, and was found to be in close agreement with Monte Carlo-based simulations from the planning system.
To precisely account for the high-dose region in the primary beam, this study adopted a mechanistic model and used it for risk evaluation. This model has an all-inclusive approach to the problem by accounting for cell killing at high doses, as well proliferation and repopulation effects. It should be noted that this study was based on IMRT and VMAT versus a comparable conventional plan delivered on an Elekta Infinity linear accelerator with Agility MLC head. Our results indicate that beam modulation resulting in higher delivered monitor units is a principal contributing factor in the overall risk of second malignancy. Neutron contributions were not considered in this work because photon energies greater than 6 MV were not used in this study.
We found that the treatment plan quality, in terms of target coverage and doses to OARs, was similar for VMATF and VMAT across all patient cohorts investigated. However, the target coverage was significantly better for VMAT for larger targets. In addition, we found that the number of MU was significantly larger for VMATF. Further, the treatment time was substantially reduced and statistically significant for SABR treatment with unflattened beams. For all techniques, based on detailed Monte Carlo simulations, we did not observe a significantly greater risk of second cancer developing among patients treated with unflattened beam compared with standard radiation. Overall, we observed 9·5 cancers/1,000 person-years for VMAT and 8·5 cancers per 1,000 person-years for the VMATF. Overall, VMATF conferred a lower second cancer risk in most of the organs. Most second cancers occur in organs adjacent to or near the target volume.Reference Rehman, Tailor and Isa 21 Our study confirms this observation as higher LAR was found for organs close to the PTV including oesophagus, rib and soft tissue irrespective of radiotherapy (RT) technique. Comparing the RT techniques used in this study, organ-specific LAR was significantly lower using VMAT than 3DCRT. This is in line with the study done by Mok et al., reporting on lower doses to OAR close to the PTV using either 6 MV VMAT or 6 MV IMRT.Reference Mok, Carane and Palmer 22
There are several limitations that are important to note with respect to the current study. This study was performed on a limited number of patients (five patients) hence, the risk factor may not be representative of those of the general population. In addition, there are uncertainties associated with radiation-induced second cancer models used and its parameters. We did not include the impact of image-guided radiotherapy on second cancer risk as this will expose larger volume of normal tissues with radiation dose. In the future, we intend to apply the model to additional second cancer data sets. To extend our framework to include scatter doses, imaging doses and to validate this on existing second cancer data sets. It is also intended to extend this work to compare more contemporary protocols for various radiotherapy treatment techniques to predict the risk of second cancers for individual patients.
CONCLUSION
Radiotherapy continues to be a vital component of oncologic care. Radiation-induced second malignancies are an uncommon, late effect of cancer treatment. As cancer survival improves, the late effects of radiotherapy can impact long-term patient health because SABR is utilised more in younger, medically operable patients, the long-term late toxicities and optimal radiation technique need to be determined. With a greater understanding of radiotherapy techniques and side effects, secondary cancer incidence can be limited. For clinically comparable treatment plans, the risk of second cancer should be an important factor in the selection of the treatment. The current study provides the model and organ-dependent excess risk for organs attributable to SABR treatment for lung cancer.
The radiation-induced LAR was significantly lower when using VMAT than when using IMRTF. Organ-specific LAR was higher with VMAT compared with 3DCRT for the skin. The absolute attributable risk of bone sarcoma was significantly higher with IMRT plan (Table 6). VMATF resulted in reduced relative second cancer risk in all organs except skin and soft tissue close to PTV. In-depth Monte Carlo simulations showed VMATF and VMAT had the lowest associated risk, followed by the IMRTF and IMRT plan. There was a solid relationship between patient risk and age at the time of radiotherapy. In terms of overall SCR, 6 MV VMAT is an acceptable alternative to IMRT for lung SABR and offers advantages in terms of sparing adjacent OAR. In addition, the relatively low levels of absolute lifetime risks support the use of VMAT with 6 MV photons as a viable treatment modality. However, improvements in estimation and long-term validation of risk models are required before affirming these outcomes. Treatment planning for modern radiotherapy can probably do no more at the present than limit the doses to critical organs outside the target volume to avoid stochastic effects. Despite the importance of radiation-induced second cancer, which is a late effect, the primary goal of cancer control should never be compromised.
From a clinical perspective, it should be concluded that all five solutions investigated in the study can offer high quality of patient treatments and only estimates of radiation-induced malignancies can truly differentiate among them. In conclusion, the LAR of radiation-induced secondary cancer was significantly lower when using VMATF than when using IMRT for SABR lung patients. The results suggested that it would be reasonable to use the cumulative LAR difference when needed to select between treatment techniques and this study strongly recommends that finding. VMATF would be the right choice for the treatment of SABR lung patients in terms of LAR. However, more work is required for the specific estimation and long-term validation and updating of the models behind LAR estimation.
Acknowledgement
The authors are thankful to their colleagues at Cancer Specialists of North Florida who provided expertise that greatly assisted the research. They are also grateful to Shelley Smith, CMD for assistance with proofreading, and Dr. Christine Bang who moderated this paper, gave comments on an earlier version of the manuscript and in that line improved the manuscript significantly.
Conflicts of Interest
The authors declare that they have no competing interests.













