Introduction
Heart disease and cancer are the most common causes of death in the United States, and in many cases, cancer-directed therapies can impact the incidence and management of cardiovascular disease.Reference Lee, Aeppli and Nierengarten1–Reference Zagars, Ballo and Lee4 This is most often due to a direct effect of cancer treatment on the heart. For instance, the direct effects of thoracic radiation therapy (RT) on the heart have been well documented, particularly in lymphoma and breast cancer.Reference Lee, Aeppli and Nierengarten1–Reference Huddart, Norman and Shahidi6 However, others have reported an increased risk of cardiovascular sequelae in patients who did not receive thoracic RT. For example, Haugnes et al. demonstrated a 70% increased risk of death from cardiovascular disease (CVD) in patients who received RT to the retroperitoneal lymph nodes for testicular cancer, possibly due to gradual narrowing of the renal arteries, which subsequently induced chronic hypertension.Reference Haugnes, Wethal and Aass5,Reference Huddart, Norman and Shahidi6 Moreover, irradiation of the carotid body baroreceptor during treatment for head-and-neck carcinoma is associated with a decrease in systemic blood pressure (BP).Reference Leibowitz, Grossman and Berkovitch7 Interestingly, such treatments have also been associated with reduced heart rate variability, which is linked with increased risk of cardiovascular disease.Reference Goyal, Shukla and Gupta8
Prostate cancer is the most common malignancy in men, and since it is predominantly a disease of the elderly with many long-term survivors, many affected individuals will either have pre-existing CVD or develop it after treatment.Reference Howlander, Noone and Krapcho9 Interestingly, hypertension itself is a risk factor for developing prostate cancer.Reference Esposito, Chiodini and Capuano10 Androgen deprivation therapy (ADT) has been shown to increase the risk for developing metabolic syndrome, hypertension, myocardial infarction and heart failure.Reference D’Amico, Chen and Renshaw11–Reference Klotz, Boccon-Gibod and Shore13 However, little is known about the long-term effect of prostate RT on the incidence of CVD. Anecdotally, we observed that patients undergoing RT for prostate cancer in our clinic often experienced elevations in BP over the course of therapy, which, if sustained and untreated, could become a significant risk factor for CVD. The goal of this retrospective study was to better evaluate these changes in BP among patients receiving radiation to the prostate or prostate bed.
Methods
Medical records of 76 consecutive patients who underwent external beam RT with or without ADT or a brachytherapy boost for prostate cancer from April 2014 to September 2017 were retrospectively reviewed. BP measurements recorded by automatic cuff were obtained before, during and after competing treatment. Pre-treatment baseline BP was defined as the average of up to five BP measurements within 1 year of RT initiation; for patients with >5 BP measurements during that period, the five measurements that were closest to the RT start date were used. BP measurements were taken weekly while the patient was on treatment, excluding any BP measurements obtained during inpatient hospitalisation for all groups. Radiation-associated hypertension (RAH) was defined based on American Heart Association guidelines, which describe differential risk groups per systolic and diastolic BPs. Per these guidelines, an increase of 10 mmHg in diastolic BP (DBP) from baseline of 80 mmHg is considered hypertension.Reference Whelton, Carey and Aronow14 Furthermore, previous/current recommendations consider elevations of systolic BP (SBP) between 10 and 20 mmHg from baseline of 120 mmHg to be physiologically relevant.Reference Whelton, Carey and Aronow14 As such, RAH was defined as an increase ≥15 mmHg SBP, 10 mmHg DBP, or 5 mmHg mean arterial pressure (MAP). MAP was calculated as (SBP + 2DBP)/3. Post-treatment BP was defined as the average BP measurements within 1 year of RT completion.
Differences in SBP, DBP and MAP during treatment were determined using ANOVA with Dunnett’s test with pre-treatment values as control. Pearson’s and Spearmen’s correlation were used to identify any potential relationship between various patient, tumour and treatment-related factors and development of RAH. Odds ratio between RAH and pre-existing hypertension was determined by binary logistic regression. Of note, for this analysis, prostate cancer risk groups were defined using the National Comprehensive Cancer Network (NCCN) guidelines version 2 (2017).15 Changes in anti-hypertensive regimens were also evaluated by comparing the initial consultation medication lists with subsequent lists at follow-up visits up to 1 year post-treatment. Statistical analysis was performed with IBM SPSS Statistics, version 21.
Results
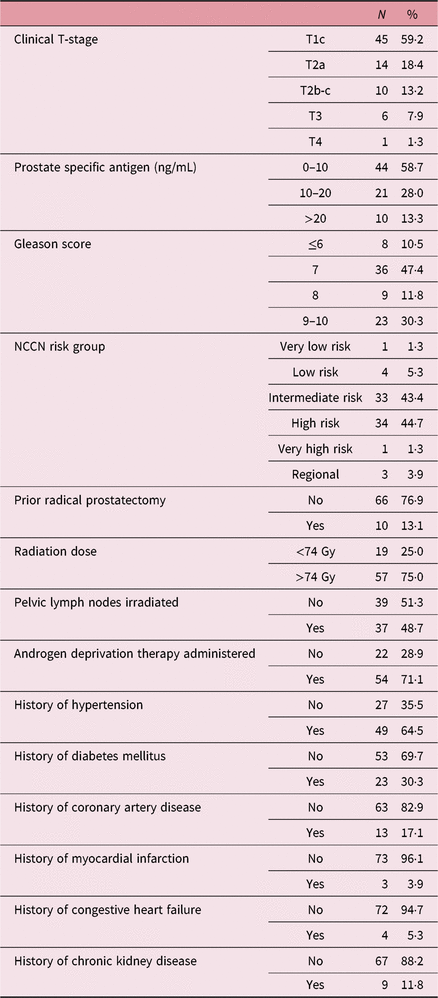
Demographic information about the patients is shown in Table 1. The median patient age was 67·5 years (interquartile range (IQR) 62–72). The majority had intermediate-risk (43·4%) or high-risk (44·7%) prostate cancer; 88% underwent definitive RT, with 12% receiving adjuvant or salvage RT after a prior radical prostatectomy. All patients received intensity-modulated RT to the prostate or prostate bed with doses ranging from 64 to 81 Gy, with 48·7% of patients also receiving pelvic nodal irradiation. A total of 71·1% patients received neoadjuvant/concurrent/adjuvant ADT, which typically consisted of leuprolide with or without concurrent bicalutamide. Pre-existing comorbidities included hypertension (HTN, 64·5%), coronary artery disease (CAD, 17·1%), diabetes mellitus (DM, 30·3%), chronic kidney disease (CKD, 11·8%), congestive heart failure (CHF, 5·3%) and myocardial infarction (MI, 3·9%).
Table 1. Patient demographics

Abbreviation: NCCN, National Comprehensive Cancer Network.
A total of 36 (47%) patients developed RAH, with the majority (75%) developing RAH on treatment, and 25% developing RAH during post-treatment visits. Within the group that developed RAH on treatment, 66% remained hypertensive post-treatment as well. Among the patients who developed RAH, 27% were prescribed additional anti-hypertensive medications. Median changes in BP among patients who did and did not develop RAH are shown in Table 2. Within the subgroups of patients who experienced RAH on treatment and post-treatment, there was a median increase in SBP, DBP and MAP compared to pre-treatment measurements of 20 mmHg (IQR 140–161 mmHg, p = 0·0002), 6 mmHg (IQR 75–88 mmHg, p = 0·02), 11 mmHg (IQR 97–112 mmHg, p = 0·0001); and 6 mmHg (IQR 142–161 mmHg, p = 0·14), 10 mmHg (IQR 84–94 mmHg, p = 0·008), 15 mmHg (IQR 106–115 mmHg, p = 0·02), respectively. The median time to development of RAH was 7 days (IQR 3–19) within the on-treatment RAH group.
Table 2. Comparison of blood pressure measurements obtained before, during and after radiation therapy among patients who did and did not develop RAH
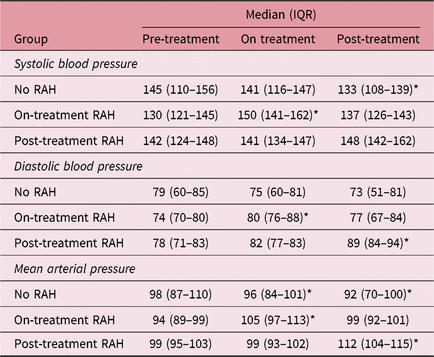
Abbreviations: RAH, radiation-associated hypertension; IQR, interquartile range.
* Statistically significant difference compared to pre-treatment blood pressure.
Statistical analysis demonstrated that only the presence of pre-existing hypertension was significantly associated with the development of RAH [p = 0·03, odds ratio = 2·9 (1·1–7·9)]. There was also a trend towards an association between RAH and pre-existing DM (p = 0·09). Other factors that were not significantly associated with RAH included patient age, history of CAD, history of CHF, history of MI, history of CKD, NCCN prostate cancer risk group, prior radical prostatectomy, ADT administration, prescription radiation dose, use of nodal irradiation or use of brachytherapy boost. Multivariate analysis was not performed as only a single variable was significant. Within the post-treatment period, two patients who experienced RAH developed symptomatic CAD, requiring percutaneous coronary intervention (PCI), two others experienced a MI, and one of these patients subsequently developed CHF. Of note, one patient who did not develop RAH died from an MI during treatment.
Discussion
Heart disease is the most common cause of death in the United States, and prostate cancer is the most common malignancy in men.15–16 For those patients who undergo treatment for prostate cancer, exacerbation of pre-existing CVD or the development of new CVD via uncontrolled hypertension may have substantial effects on the quality and duration of life. Therefore, it is critical to understand and adequately treat potential cardiovascular complications of prostate cancer therapy. The current study characterises changes in systemic BP in patients undergoing prostate RT. Our findings indicate that nearly half of patients will experience elevations in BP during or within 1 year after treatment, highlighting the importance of close monitoring of this often elderly patient population with multiple comorbidities.
The mechanism of this novel phenomenon is unclear and warrants further study; possibilities may include the release of inflammatory cytokines or other vasoactive substances from a dying tumour or normal tissue, transient prostatitis with mild acute kidney injury related to the obstruction of urine flow, stress or white coat hypertension related to a cancer diagnosis or frequent doctor visits, or direct effects of radiation on the pelvic or abdominal vasculature.Reference Milutinovic, Darcy and Thompson17 Vascular changes, in particular, are difficult to demonstrate on routine clinical evaluation, though prospectively evaluating Doppler impedance studies could lead to some physiologic insight in this area. Interestingly, as Table 2 shows, individuals who did not develop RAH experienced a decrease in SBP and MAP, similar to findings in head-and-neck carcinoma patients undergoing treatment.Reference Leibowitz, Grossman and Berkovitch7 While the significance of this observation is unknown, within head-and-neck cancer patients, similar results were associated with parameters linked to poor cardiovascular outcome.Reference Goyal, Shukla and Gupta8
ADT is associated with an increased risk of cardiovascular ischaemic heart disease, heart failure, arrhythmia, hypertension, stroke and hyperlipidemia.Reference O’Farrell, Garmo and Holmberg12,Reference Klotz, Boccon-Gibod and Shore13 In particular, previous reports found that individuals with more pre-existing comorbidities as defined by the Charlson Comorbidity Index were at a higher risk of developing cardiovascular and metabolic sequelae due to ADT.Reference D’Amico, Chen and Renshaw11 ADT was not found to be associated with RAH in our study; however, this may have been due to the fact that the incidence of ADT-induced hypertension is only approximately 4%, a difference less likely to be detected in our relatively small cohort of patients.Reference Klotz, Boccon-Gibod and Shore13 Notably, pre-existing hypertension was correlated with RAH, perhaps suggesting a proclivity towards increased systemic BP based on a patient’s underlying disease. Notably, other radiation-related factors were not associated with RAH, including radiation dose, use of brachytherapy or treatment of pelvic lymph nodes.
While many patients were appropriately treated for their increase in systemic BP, the majority were not. Within the short follow-up period, five patients who developed RAH also developed significant cardiovascular sequelae (e.g., MI, CHF, CAD requiring PCI). Although this study was not adequately powered to investigate such outcomes, the relationship between RAH and long-term cardiovascular risk is of great concern. Furthermore, the optimisation of BP before, during and after treatment may decrease such risks and improve all-cause mortality of prostate cancer patients who undergo RT. An integrative approach termed ‘cardio-oncology’, which involves the collaboration of cardiologists and oncology specialists, may be of value in identifying and treating such patients.Reference Cardinale18 A simple ABCDE approach for heart and vascular wellness following a prostate cancer diagnosis has been introduced.Reference Guan, Khambhati and Jones19 A (awareness and aspirin), B (BP), C (cholesterol and cigarettes), D (diet and diabetes), and E (exercise) should be reviewed and optimised at each patient visit. Based on our findings, a prudent strategy may include attention to hypertension optimisation prior to RT, and RAH screening in the early weeks following initiation of therapy. Because some patients will develop post-therapy RAH, heightened awareness should continue throughout follow-up. Specific recommendations for anti-hypertensive drug class of choice cannot be made at this time given a lack of mechanistic understanding of our novel findings. However, contemporary hypertension guidelines recommend a treatment goal of at least systolic BP of 130 mmHg and a diastolic BP of 80 mmHg.Reference Whelton, Carey and Aronow14
Conclusion
The current study identifies an association between RT for prostate cancer and elevations in systemic BP. The conclusions are limited by the retrospective nature of the report, relatively small sample size and the fact the data were gathered from a single institution. Future investigations will focus on the relationship between RAH and subsequent cardiovascular sequelae, as well as the role of anti-hypertensive medications in possibly mitigating this risk.
Acknowledgements
There are no stated acknowledgements.
Conflicts of interest
The authors do not have any conflicts of interest to disclose.