Introduction
Radiodermatitis (also known as radiation dermatitis, radiation-induced skin reactions or radiation injury) is a significant side effect of ionising radiation delivered to the skin during cancer treatment. For example, on average 50–95% of cancer patients receiving radiation therapy will develop some form of radiodermatitis, including erythema, moist and/or dry desquamation. Reference Glover and Harmer1,Reference Seite, Bensadoun and Mazer2
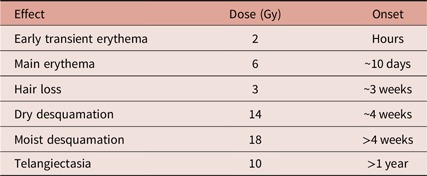
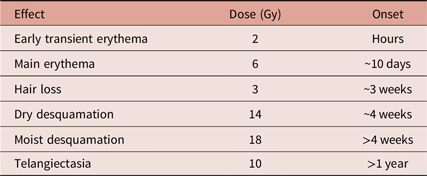
The tissue damage arising from ionising radiation occurs in the acute stage, primarily as an epidermal event. Reference Hopewell3 The causative agents are thought to be reactive oxygen species such as hydroxyl radicals which damage DNA in the basal keratinocytes. Reference Karbownik and Reiter4 The onset of radiation-induced reactions can be related to the dose received (see Table 1). Late-onset damage is a function of radiation effects on the vasculature; this produces dermal atrophy after 16–26 weeks. Dermal necrosis develops at this time after high doses. A second phase of dermal thinning is seen to develop after greater than 52 weeks, and this later phase of damage is associated with the appearance of telangiectasia. Reference Kinoshita, Ishimine and Shiraishi6 As a consequence of these findings, the use of antioxidants has been evaluated. While the scientific rationale behind the use of antioxidants in treating radiodermatitis is strong, clinical studies have been far less consistent. Even in large-scale randomised controlled trials, findings have been limited by the inconsistent use of topical pharmaceutical applications and placebos.
Table 1. Some radiation-induced skin reactions as related to dose (in Grays) and approximate time of onset. Derived from Koenig et al.Reference Koenig, Wolff, Mettler and Wagner5
These radiation-induced skin reactions result in a myriad of complications, including delays in treatment, pruritus, pain, diminished aesthetic appeal and reduced quality of life. Reference Singh, Alavi, Wong and Akita7 They can also have the negative impact of hospital admission and curtailing treatment (thereby reducing the cumulative dose given). Radiodermatitis occurs early on in the treatment period or appears months or up to several years later. Acute radiodermatitis is, in effect, an inflammatory condition which varies in severity according to both treatment (i.e., dose and duration) and inherent patient factors. There are several factors that can increase the incidence and severity of radiotherapy-induced skin reactions including higher total dose, paler skin colour, Reference Turesson, Nyman and Holmberg8 larger overall treatment volume, adjuvant chemotherapy, location of treatment (such as areas with skin folds, the head and neck and perineum) the use of bolus material Reference McQuestion9 and genetic predisposition to radiosensitivity. Reference Rattay and Talbot10 While the amalgamation of this wide variety of factors in the individual patient makes it difficult to predict if and when they will develop a skin reaction, certain patient groups are at greater risk.
For radiotherapy patients at high risk of skin reaction (those undergoing radiotherapy to the head and neck, anal canal and vulval area) radiation-induced erythema can often escalate to moist desquamation (MD) which can require specialised costly dressings. At a time when cost-saving and efficiency are important in the NHS, avoidance of the development of erythema and subsequent escalation to MD would be of great benefit. Most acute reactions resolve after several weeks but some persist and can cause complications. Late-onset radiodermatitis is characterised by telangiectasia (i.e., permanent dilation of superficial blood vessels) which forms in atrophic, fragile skin. These reactions could have a significant negative impact on future treatment and patient body image.
Recent advancements in radiotherapy delivery (such as intensity-modulated radiotherapy) and treatment regimens have only been successful in partly ameliorating these adverse effects. New, effective treatments to either treat, or better still, to avoid such reactions are required. Radiation-induced skin reaction is the most prevalent side effect of radiation therapy. Not only does it have a significant effect on patients’ quality of life, Reference Sutherland, Bennett and Herst11 but it also results in poor follow-up and, of great clinical significance, the enforced delay of radiotherapy. Reference Hymes, Strom and Fife12 This latter complication often has an impact on survival outcome. Reference Salvo, Barnes and van Draanen13 Several variations in skin care practices and topical applications have been studied including skin washing, Reference Roy, Fortin and Larochelle14 topical steroids Reference Haruna, Lipsett and Marignol15 and mechanical skin barriers. Reference Scott16 The Society and College of Radiographers produced guidance for the multiprofessional radiotherapy workforce to aid in delivering optimal skin care advice to patients undergoing radiotherapy treatment. 17 The guidance recommended discontinuing the use of creams containing sodium-lauryl sulphate (SLS) which led many radiotherapy departments to review and change their local practices. The evidence base provided in the guidance was not strong enough to support or disregard the use of any particular product for topical application. This article summarises the current knowledge on the pathogenesis and clinical manifestations of radiation-induced skin damage.
Flamigel® is a hydroactive colloid gel formulated specifically for the topical management of radiation-associated dermatitis (this same product has recently been rebranded as Flamigel RT®). When applied to the skin, Flamigel® provides a barrier that protects the skin from damage and breakdown due to radiotherapy. Arginine increases the moisturising properties of the gel and accelerates wound healing. Previous experience from the experimental use of Flamigel® in two large-scale clinical studies Reference Censabella, Claes, Orlandini, Braekers, Thijs and Bulens18,Reference Censabella, Claes and Orlandini19 on affected skin has proven sufficiently positive to warrant an evaluation of its use in the prevention and treatment of radiotherapy-induced skin reactions. The spectrum of adverse cutaneous reactions is evaluated in a UK-based multicentre cohort of cases. The aim of this investigation is to evaluate the use of this new product to study how effective it is in the prevention and/or treatment of radiation-induced skin damage.
Methods
For this study, the UK standard scoring system for skin reactions guided by the ‘Radiation Therapy Oncology Group’ (RTOG) was used, Reference Cox, Stetz and Pajak20 this being the usual system employed by all the clinicians and centres involved. The RTOG is an established assessment tool that classifies radiodermatitis by severity. Mild radiodermatitis (RTOG Grade 1) is characterised by mild, blanchable, erythema. The onset is typically within days to weeks of initiating therapy and symptoms may fade within a month. Reference McQuestion9 Dry desquamation (RTOG Grade 2a) may be associated with pruritus, epilation, scaling and possibly changes in skin pigmentation. Patients with mild radiodermatitis may report that their skin feels ‘tight’. Reference McQuestion9 Hair loss occurs in the treatment field and is often temporary, but may become permanent in some patients. Reference McQuestion9
MD (RTOG Grade 2b and above) indicates that the integrity of the dermis is impaired as the epithelial barrier is lost. The skin will look red and inflamed and the wound will secrete exudate. This area can be quite painful and if not treated accordingly patients are at increased risk for infection with Staphylococcus aureus. Reference Hymes, Strom and Fife12 Following the change in practice from recommending SLS-containing products (such as aqueous cream) for use as a leave-on moisturiser during radiotherapy, a number of radiotherapy centres were investigating the efficacy of topical applications to reduce the severity of skin reactions in high-risk patients and increase their comfort. A prospective study using an evaluative survey on the use of Flamigel® (conceived by Flen Health, Luxembourg) was conducted among radiotherapy specialist teams in dedicated UK radiotherapy centres between 01 January 2017 and 31 October 2017. A sample of centres were approached to participate and the survey was designed by Flen Health, Luxembourg. Ethics approval was not required as Flamigel® was a registered product available for the management of skin reactions. Patients at a high risk of skin reaction were selected and following their consent patients were assessed prior to radiotherapy and regularly (weekly where possible) during their treatment by specialist nurses and/or review radiographers. Each participating centre had been supplied with Flamigel® in 40 g tubes and the following information was collected via the survey:
-
Level of skin reaction present (if any) and changes weekly during treatment (RTOG)
-
Locality of the reaction (body site)
-
Duration of reaction
-
Frequency of application of Flamigel®
-
Flamigel® clinical performance: erythema, pain, MD, pruritus
-
Opportunity was provided for the documentation of any other local reactions, including adverse events, clinician and patient comments
-
Flamigel® was continued for a minimum of 2 weeks following completion of radiotherapy treatment before the final skin assessment was undertaken at follow-up in the radiotherapy centre
-
The clinicians were asked to rate Flamigel® performance according to ‘meeting expectations’ and ‘recommendation to colleagues’.
Following collection of all survey forms, the data were tabulated and subjected to summary statistical analyses. The statistical test was a standard ‘binomial test’ with 95% confidence intervals (CI) calculated using the Wilson method. Reference Brown, Cai and DasGupta21 All p values reported were one-tailed. The software used was GraphPad Prism version 7·00 for Windows.
Results
A total of 108 subjects were recruited from 16 clinical centres by 22 practitioners. The scale of radiodermatitis upon recruitment was recorded in 103 subjects and was predominantly RTOG 1 (65%). Of the remaining subjects, skin reaction was recorded as RTOG 0 (no skin reaction) in 9%, RTOG 2a in 24% and RTOG 2b in 2%. Radiodermatitis, when present, was on the breast area, neck or perineum in all cases.
In terms of erythema, 48 (n = 99; 48·48%) said it improved and 51 (n = 99; 51·52%) reported ‘no change’ or ‘worsening’ (Figure 1: although clinician comments suggested this was a normal reaction to radiotherapy). The difference was not statistically significant, p = 0·4204, 95% score CI 38·88–58·2%, indicating that the number of patients reporting an improvement in erythema was not larger than would be expected due to chance. It is, however, important to note that in most patients Flamigel® was started after radiotherapy rather than from the beginning of treatment. As a consequence, the onset of RT-mediated cell damage occurred prior to Flamigel® treatment. Further investigation may be required to identify whether appearance of erythema could be diminished if Flamigel® is applied from the onset of radiotherapy as previous studies have identified that when applied from day 1 the onset of MD for breast radiotherapy patients was delayed. Reference Censabella, Claes, Orlandini, Braekers, Thijs and Bulens18,Reference Censabella, Claes and Orlandini19

Figure 1. n = 99.
With respect to discomfort/pain assessment, of the 76 that responded, 69 (90·79%) said it soothed the area upon application and 7 (9·21%) said it did not (Figure 2). The difference was highly statistically significant, p < 0·0001, 95% score CI 82·19 to 95·47%, indicating that the number of patients reporting ‘soothing’ upon application was larger than would be expected due to chance.

Figure 2. n = 76.
For direct patient experience of pain, of the 93 that responded, 67 (72·04%) said pain was ‘reduced’ and 26 (27·96%) reported ‘no change’ or ‘worsening’ (Figure 3). The difference was highly statistically significant, p < 0·0001, 95% score CI 62·19–80·15%, indicating that the number of patients reporting a reduction in pain during treatment was larger than would be expected due to chance. However, as pain was not assessed at the start of treatment, a ‘no change’ score is not necessarily a negative finding.

Figure 3. n = 93.
A statistically significant number of patients stated that pruritus (itching) was improved by using Flamigel®. Of the 90 that responded, 58 (64·44%) said itching was improved and 32 (35·56%) reported ‘no change’ or ‘worsening’ (Figure 4). The difference was statistically significant, p = 0·0040, 95% score CI 54·15–73·56%, indicating that the number of patients reporting an improvement in itching was larger than would be expected due to chance.

Figure 4. n = 90.
The binomial test was used to test whether a significant number of patients said Flamigel® met or even exceeded their expectations. Of the 105 that responded, 102 (97·14%) said expectations were met or exceeded and 3 (2·86%) said they were not (Figure 5). The difference was highly statistically significant, p < 0·0001, 95% score CI 91·93–99·22%, indicating that the number of patients reporting that Flamigel® met or exceeded their expectations was larger than would be expected due to chance.

Figure 5. n = 105.
Note: I combined ‘met’ and ‘exceeded’ and asked the question ‘did Flamigel meet or exceed your expectations, or not?’
Asked whether they were happy to continue recommending Flamigel® and/or likely to recommend it to a colleague, 106 clinician responses indicated that the majority 99·06% (n = 105) said ‘yes’ and only one (0·94%) said ‘no’ (Figure 6). The difference was highly statistically significant, p < 0·0001, 95% score CI 94·85–99·95%, indicating that the number of responders happy to use Flamigel® and/or recommend larger than would be expected due to chance.

Figure 6. n = 106.

Other question with no statistical analyses possible:
Discussion
The current evidence for topical agents in managing (or avoiding) radiodermatitis is equivocal. Corticosteroids have been found useful but are accompanied by the usual side effects known for such agents. There is a clear clinical need for a safe and effective topical preparation which can alleviate pain and soothe the affected area.
The evidence available from this study suggests that early use of Flamigel® at the development of erythema can serve to extend the duration of radiotherapy and to ensure that the patient completes their radiotherapy course. Feedback from patients was positive particularly with regard to the ‘cooling effect’. Two large studies conducted and published by Censabella et al involved a combined total of over 700 patients undergoing breast radiotherapy. Data indicate that by plotting a Kaplan–Meier estimate of MD-free survival at 50Gy dosage, Flamigel® offers a statistically significant time free from MD when compared with dexpanthenol cream. Reference Censabella, Claes, Orlandini, Braekers, Thijs and Bulens18,Reference Censabella, Claes and Orlandini19
Limitations of the study reflect the inherent variation in comparing radiotherapy patients such as individual patient factors (age, skin type, dose and fractionation and area treated) and the challenges in terms of accurate, timely, comparable and accessible records regarding skin reactions. Patient preference in terms of creams used is important and future research involving a randomised controlled study supplemented by a qualitative element could be beneficial. Future research in this area with a large patient cohort and collaboration between radiotherapy centres is important as skin reactions are common and reducing the incidence and severity these reactions would reduce the impact on resources, reduce dressing costs and improve the patient experience.
Conclusions
This preliminary, non-comparative survey has demonstrated that regular use of Flamigel® on skin exposed to radiotherapy has the potential to reduce erythema, itch and pain, and to soothe the irradiated area. No safety issues were reported in association with the use of Flamigel®. The clinicians involved in this study stated that the product met or exceeded their expectations and that they were happy to recommend it to colleagues. Each of these measured parameters proved to be statistically significant as well as being clinically significant.
On the basis of the evidence presented here, Flamigel® merits first option status for patients receiving, or about to receive, radiotherapy.
Acknowledgements
The authors thank the radiotherapy departments and patients who took part: Lingen Davies Oncology Centre (Shrewsbury and Telford), Genesis Care (Elstree), Genesis Care (Nottingham), James Cook Hospital (Middlesbrough), Velindre Cancer Centre (Cardiff), Poole Hospital (Dorset), Hull University Teaching Hospitals, Bristol NHS Trust, Queen Elizabeth Hospital (Kings Lynn), Ipswich Hospital, Royal Derby Hospital, North Cumbria NHS Trust (Carlisle), St James University Hospital (Leeds), Torbay and South Devon Hospitals, Nuffield Health Cambridge Hospital, Colchester Hospital and Tissue Viability at Lincolnshire NHS Trust.