Introduction
Total body irradiation (TBI), in combination with cytotoxic chemotherapy, is one of the most commonly used myeloablative preconditioning regimens before blood or bone marrow transplantation (BMT) for leukaemia.Reference Gupta, Lazarus and Keating1 Efforts to deliver the highest possible TBI doses with minimal side effects have led to many trials exploring different techniques, fractionation, total doses and dose rates.Reference Hill-Kayser, Plastaras, Tochner and Glatstein2–Reference Kirby, Held, Morin, Fogh and Pouliot4 Doses ranging from 500 cGy in 1 fraction (F) to 1,200 cGy in 6 F are commonly used. Doses >1,200 cGy have not shown to increase overall survival in a randomised trial.Reference Clift, Buckner and Appelbaum5 However, in single institution studies, higher TBI doses have been shown to be feasible.Reference McAfee, Powell, Colby and Spitzer6, Reference Sobecks, Daugherty, Hallahan, Laport, Wagner and Larson7 In the recent years, organ preserving approach with total marrow irradiation and total lymphoid irradiation has been evaluated.Reference Hui, Kapatoes and Fowler8, Reference Wong, Liu and Schultheiss9
The techniques used to deliver TBI can be challenging and a variety of approaches are described in the literature.Reference Quast10, Reference Quast11 In The Ottawa Hospital Bone Marrow Transplant Program (TOH-BMT), a translating couch system was developed for the delivery of TBI, and has been in use since 1991. Similar approaches have been described by other groups in the published literatureReference Quast11–Reference Hussain, Villarreal-Barajas, Dunscombe and Brown13 and have been shown to improve dose homogeneityReference Hussain, Villarreal-Barajas, Dunscombe and Brown13 and to provide better dose uniformity within the patient compared with fixed beam techniques.Reference Sarfaraz, Yu, Chen and Der14 In this technique, patients are treated on a mobile couch at ~185 cm skin surface distance (SSD) with a field size of 66·5 cm wide by 57 cm long. A computer controlled stepping motor drives the patient couch at a user-selectable speed. The total dose delivered to the patient is a function of couch velocity, field size and patient separation (Figure 1). This technique, once fully established, is relatively easy to set up and use from the physics perspective. In addition, it is well-accepted by radiation therapy staff, and takes much less overall time than our previous technique (standard standing extended beam technique).Reference Gerig, Szanto, Bichay and Genest15 It also results in fairly homogenous dose distributions, although there still tend to be increased dose at very thin regions such as legs and ankles.Reference Gerig, Szanto, Bichay and Genest15

Figure 1 Translated bed technique.
A number of potentially serious side effects have been attributed to TBI in the literature. These include pulmonary, renal, ocular, thyroid and gonadal complications. However, most often, the critical limiting organs for total TBI dose are the lungs.Reference Barrett16, Reference Ettinger and Trulock17 Radiation pneumonitis rates vary with factors including dose rate, fraction size, baseline pulmonary function status and patient age. In addition, an objective and agreed-upon definition as to what constitutes a diagnosis of radiation pneumonitis is lacking, and it is often a diagnosis of exclusion. A standard method to deliver TBI has not been established. Many factors can influence which method an institution should use and these include, but are not limited to, ease of set-up, patient comfort, good dosimetry profile and acceptable toxicity profile. Although a direct comparison of different techniques is difficult, we believe it is important to observe and document toxicity rates of TBI techniques and compare them with the available literature to ensure high quality results, including acceptable levels of toxicity.
Materials and Methods
We have retrospectively reviewed our BMT database for all patients who received TBI as part of their conditioning regimen. The goal was to determine if there was any evidence of excessive radiation-induced pulmonary toxicity. To reduce the number of variables, we designed the present study to only include acute lymphoblastic leukaemia (ALL) patients. We have included ALL patients receiving TBI in TOH-BMT from 1991 to 2011 inclusive. Conditioning regimens and doses as well as type of transplant were recorded. As per our institutional protocol, all patients were treated supine and prone with anterior/posterior fields using 18 MV photon beams at 175–185 cm SSD using a translating-bed technique. The dose rate at the patient surface was ~80 cGy/min and lung attenuators were used to limit the lung dose to that of the prescribed TBI dose. Patients were followed intensively for the first 100 days post BMT, and less frequently thereafter, by the transplant team. We have analysed all available clinical notes, radiology reports, laboratory data (including histopathological data) and pulmonary function tests in order to determine the incidence of radiation-induced pulmonary toxicities following BMT.
To avoid under reporting of radiation-induced toxicity, any pulmonary event (symptoms, signs and laboratory or radiological findings related to the lower respiratory tract) in the 1st year after BMT, regardless of the cause, has been documented as well as cases of clear radiation pneumonitis as judged by the documenting physician. When a pulmonary event was reported, we looked into its cause and divided those events into four groups; clearly infectious (culture/serology positive), non-infectious (culture/serology negative), mixed infectious and non-infectious, and a group where the data was missing or the exact cause of the event is not clear.
We then focused our attention on the later three groups to determine if the event was fatal and if the event, fatal or non-fatal, could be related to radiation. The cause of death has been documented to a great deal of accuracy by the transplant team. In the event where the cause of death was reported to be pulmonary related, we have thoroughly evaluated the patients’ records to assess the exact cause in order to determine if radiation toxicity could have contributed or was possibly the main cause of death. Baseline information including smoking history and past medical history has been documented as well. All numbers are reported as crude data.
Results
A total of 622 patients received TBI in the period from 1991 to 2011 at TOHCC. There were 88 ALL patients, 35 female and 53 male, including nine children under 18 years. Median age at BMT was 30. Patient’s characteristics as well as the conditioning regimens used are summarised in Table 1.
Table 1 Patient’s characteristics
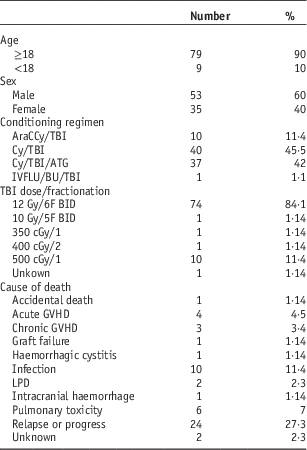
Abbreviations: ATG, anti-thymocyte globulin; AraC, cytarabine; BU, busulfan; BID, twice per day; Cy, cychlophosphamaide; cGy, centigray; F, fraction; GVHD, graft versus host disease; Gy, gray; IVFLU, intravenous fludarabine; LPD, lymphoproliferative disorder; TBI, total body irradiation.
Baseline pulmonary function tests as well as consistent follow-up pulmonary function testing was, unfortunately, not available for all patients and hence changes in these parameters, as an indication of a pulmonary event, has not been taken into account. In the 1st year following BMT, pulmonary events were reported for 24 (27%) of the patients, and the follow-up notes were unavailable for seven (8%) of the total ALL patients. Of the reported pulmonary events, eight were of clear, proven, infectious aetiology. Of the remaining 16 patients, 11 had events that were clearly non-infectious (including one patient with acute graft versus host disease (GVHD) and bronchiolitis obliterans organizing pneumonia, six patients with interstitial lung disease including pneumonitis and/or interstitial fibrosis, one patient with pulmonary infarction associated with bacterial colonisation on autopsy and three with cough and/or dyspnoea of unclear cause or suspicious viral pneumonia but negative cultures), four had events that were either of unclear nature or their data was missing and one patient had bronchopneumonia as well as pulmonary haemorrhage and diffuse alveolar damage (Figure 2) (Table 2).

Figure 2 Pulmonary events at 1 year.
Table 2 Pulmonary events in the 1st year

Notes:
a As reported by the transplant team.
b This was decided based on difficulty excluding radiation as a cause of death and not as a result of specific clinical, radiological or pathological findings.
Abbreviations: Allo, allogenieic; BMT, bone marrow transplantation; ATG, anti-thymocyte globulin; AraC, cytarabine; Cy, cychlophosphamaide; cGy, centigray; F, female; cGVHD, chronic graft versus host disease; Gy, gray; HLA, human leucocyte antigen; M, male; TBI, total body irradiation; Url, unrelated.
A total of 55 patients (63%) have died at the time of this study, 32 (36%) within the 1st year following their BMT. The most common reported cause of death was leukaemia relapse or progression, with 24 patients (27% of total death) dying with relapse/progression. Pulmonary toxicities were reported as the cause of death for five patients (6%) within the 1st year (two with fatal interstitial lung disease, the patient who had pulmonary infarction and associated bacterial colonisation of the lung on autopsy, the one with bilateral haemorrhage and diffuse alveolar damage as well as bronchopneumonia, and one of the patients in whom the documentation of the nature of the lethal pulmonary event was missing). It is believed that radiation might have contributed to the first three pulmonary deaths above due to the fact that radiation can cause those changes and not because there is a clear indication that radiation is the culprit. In addition, one patient who died of GVHD, also had severe interstitial fibrosis that could possibly be related to radiation. Therefore, we have estimated that the total number of deaths within the 1st year where the possibility of radiation-induced lung injury as a main or contributing factor to death could not be excluded was four (4·5%), and all of these patients received 12 Gy/6 F. None of these patients had baseline asthma or chronic obstructive pulmonary disease (COPD), but two were smokers. Among the 16 patients who did not have clearly infectious pulmonary events, eight (9%) patients had symptoms suggestive of grade 2–3 radiation-induced pneumonitis. Three were still alive >1 year after BMT and five have died of causes other than pulmonary toxicity. This brings the percentage of radiation related lung injury (fatal and non-fatal) in the 1st year to about 15%.
Discussion
The main reason of the paucity of clear data about radiation-induced lung toxicity in the setting of TBI is the difficulty of obtaining a valid diagnosis. With GVHD of the lung, as well as viral and bacterial infection among other contributing events, it is almost impossible, with the currently available diagnostic tests, to be certain of the true incidence of radiation-induced lung injury. In view of this, the really important issue is the overall incidence of lung related complications and adverse events and their mortality, especially when radiation injury may synergistically increase the incidence and severity of lung complications.Reference Ettinger and Trulock17 Our incidence of any significant pulmonary events and specifically those of the non-infections types compares very favourably to the published literature even though our lung TBI doses (12 Gy) is considered relatively high. We estimate that our total rate of clinically significant acute radiation related pulmonary toxicity (fatal and non-fatal) was at most 15% which, in the transplant setting, would be considered acceptable.
We have looked into potential risk factors for developing pulmonary toxicity in the sitting of TBI including smoking and baseline lung disease. No clear association between smoking and development of pulmonary toxicity could be made; however, we did not have complete data in regard to smoking status. There was also no obvious indication that patients with baseline asthma or COPD (deemed fit to undergo BMT) tolerate radiation worse.
There are few limitations to our study. First, like most other studies looking into this, this is a retrospective review and carries all biases inherent to such analyses and our numbers are in crude figures. Second, we have limited our review to acute and subacute toxicity to a great extent and one needs to be cautious not to underestimate chronic lung injury related to radiation. Finally, we have used clinical judgment to a great extent to estimate the mortality and morbidity from radiation, and hence there is a room for observer bias in our estimates.
Conclusion
TBI continues to be an important and acceptable component of the conditioning regimen for ALL patients undergoing BMT, and the incidence of radiation-induced pulmonary toxicity, using our technique and lung doses, is similar to that reported in the literature. Further work to look into the chronic pulmonary toxicity as well as other toxicities needs to be undertaken. Besides, prospective assessment of potential risk factors may, in the future, help identify a group of patients for whom TBI could be inferior to other conditioning regimens.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.






