Introduction
Since the late 1990s, whole-brain radiation therapy (WBRT) had been the preferred approach for the patient who had undergone resection of a single or solitary brain metastasis.Reference Andrews, Scott and Sperduto1–Reference Sun, Bae and Gore8 Adjuvant WBRT has been proven to reduce local recurrence from 46 to 10% and distant intracranial failure from 37 to 14%.Reference Patchell, Tibbs and Regine2, Reference Kocher, Soffietti and Abacioglu5 At the expense of modest improvement in overall survival, one important risk of WBRT is neurocognitive decline.Reference Chow, Davis, Holden, Tsao and Danjoux3, Reference Chang, Wefel and Hess6 With improved imaging surveillance and the advent of targeted therapies, the frequency and incidence of brain metastases will likely grow with time, increasing the need for more effective treatment methods.Reference Nayak, Lee and Wen9, Reference Barnholtz-Sloan, Sloan, Davis, Vigneau, Lai and Sawaya10
Adjuvant stereotactic radiosurgery (SRS) is proven to offer significant local control to the surgical cavity while also limiting the long-term neurocognitive relapses and toxicities associated with WBRT.Reference Soltys, Adler and Lipani11–Reference Smith, Lall and Lall18 Local control of SRS following resection of brain metastases was further reported in the prospective randomized trial by Mahajan et al. in 2017.Reference Mahajan, Ahmed and McAleer19
This study is a retrospective evaluation of the first 23 patients who underwent post-operative SRS/stereotactic radiotherapy (SRT) under the Novalis Tx platform at the Anne Arundel Medical Center, DeCesaris Cancer Institute. Our limited sample size showed that single-fraction SRS had comparable levels of local control to that of WBRT, with five-fraction SRT producing substandard results. These data will contribute to the established hypothesis that SRS is a viable substitution in place of WBRT and report relevant findings as post-operative SRS/SRT utilization transitions into community practice.
Methods and Materials
Patients
Since 2013, 143 patients received frameless SRS/SRT at Anne Arundel Medical Center. Twenty three (16%) patients received SRS/SRT to the surgical cavity. Cavity size did not play a role in excluding patients from treatment but did influence treatment techniques or fractionation scheme. Three out of 23 patients had received prior brain radiation.
Technique
All treatments were performed using a linear accelerator (LINAC) Novalis Tx® system (BrainLAB AG, Feldkirchen, Germany; Varian Medical Systems, Palo Alto, CA, USA) and featured a frameless technique. All patients received post-operative vector-vision magnetic resonance imaging (MRI) and computed tomography (CT) scans for total resection verification, simulation and setup. The planning CT and MRI were then transferred to BrainLab iPlan and fused for target delineation and contouring conducted by the radiation oncologist. SRS was generally executed 3–7 weeks after surgical resection. Total dose and fractionation was determined based on size of the surgical cavity. The neurosurgeon and radiation oncologist worked together to accurately define the target volumes. The clinical target volume (CTV) was determined and a 1–2 mm margin was added defining the planning target volume (PTV). One subject received a 4 mm margin to account for anatomical uncertainty. Quality assurance measures outlined by the Radiation Therapy Oncology Group were satisfied throughout the process.Reference Shaw, Kline and Gillin20
Statistical Analysis
Local failure was defined by recurrent proliferation within or adjacent to the surgical cavity. Distant failure was defined as the presence of new enhancement indicating new brain metastases or leptomeningeal disease outside of the PTV. A neuroradiologist was consulted to confirm lesions with dural/pial involvement. Time of failure was calculated to be the time difference between the radiosurgery end date and the date of the first MRI indicating relapse. Survival time was calculated from the date of SRS treatment to death or time since treatment for those still alive. p values were calculated to determine the statistical significance of the results using the Fisher’s exact test method. Analysis was performed to examine the relationship between treatment variations, failure trends, survival outcomes and prognostic factors. Tumor depth was categorized as either superficial with dural/pial involvement or deeply located. A Kaplan–Meier curve was derived for survival, accounting for follow-up time.
Results
Patient demographics
At the time of our review, the median follow-up was 12·3 months (range 1·2–38·6 months). Of the 23 patients treated with stereotactic boost to the surgical cavity, the median age was 62 years (range, 52–78 years). The median Karnofsky Performance Status was 80 (range, 60–100). Six patients (26%) were classified as RPA class I, 16 patients (70%) were RPA class II and 1 patient (4%) was RPA class III.
Two patients (9%) had a Graded Prognostic Assessment score between 0·0 and 1·0, six patients (26%) had between 1·5 and 2·0, 14 patients (61%) earned between 2·5 and 3·0 and one patient (4%) had between 3·5 and 4·0. All patients had only a single lesion grossly resected with one patient having had a prior craniotomy. The pre-operative median max axial size was 2·2 cm (range, 1·1–4·9 cm). Table 1 describes our study population and patient characteristics.
Table 1. Details patient demographics and characteristics of study sample

Abbreviations: KPS, Karnofsky Performance Status; GPA, Graded Prognostic Assessment.
SRS/SRT treatment
The median time between resection and adjuvant SRS was 5·9 weeks (range, 3·9–13·9 weeks). The median conformality index was 1·35 (range, 1·21–1·73). For single-fraction cases, the median V12 was 4·6 cc (2·3–7·3). Margins were employed in all cases, either a 1 or 2 mm expansion of the CTV, with one subject receiving a 4 mm margin due to anatomical uncertainty. 16/23 (70%) of patients received single fractions with a median dose of 16 Gy (range, 16–18 Gy). The remaining 7 received five-fraction courses with a median total dose of 25 Gy (range, 25–35 Gy). The decision to fractionate the treatment was based on cavity size and physician discretion. Table 2 further describes treatment characteristics.
Table 2. Describes post-operative SRS treatment characteristics

Local control
MRI was typically ordered every 3 months for the first year, and then every 4–6 months thereafter. Twenty patients received follow-up imaging at the time of analysis. Changes in the number of patients imaged at 3, 6, and 12 months are due to patient death before imaging, or lack of follow-up. Of those who received follow-up imaging at 6 and 12 months, local control was achieved in 13/19 (68%) patients at 6 months, and 8/16 (50%) of patients at 12 months. Of those who recurred locally, the median time to failure was 7·2 months (range, 1·8–38·5 months). Single-fraction doses demonstrated local control rates of 92% and 78% at 6 and 12 months, while fractionated cases provided local control rates 29% and 14% respectively (6mo p=0·0095; 12mo p = 0·0406). Of the six local relapses at 6 months, five occurred in surgical cavities with initial max axial lesion sizes greater than 3cm (median, 3·9 cm) (p=0·0095).
Distant failure
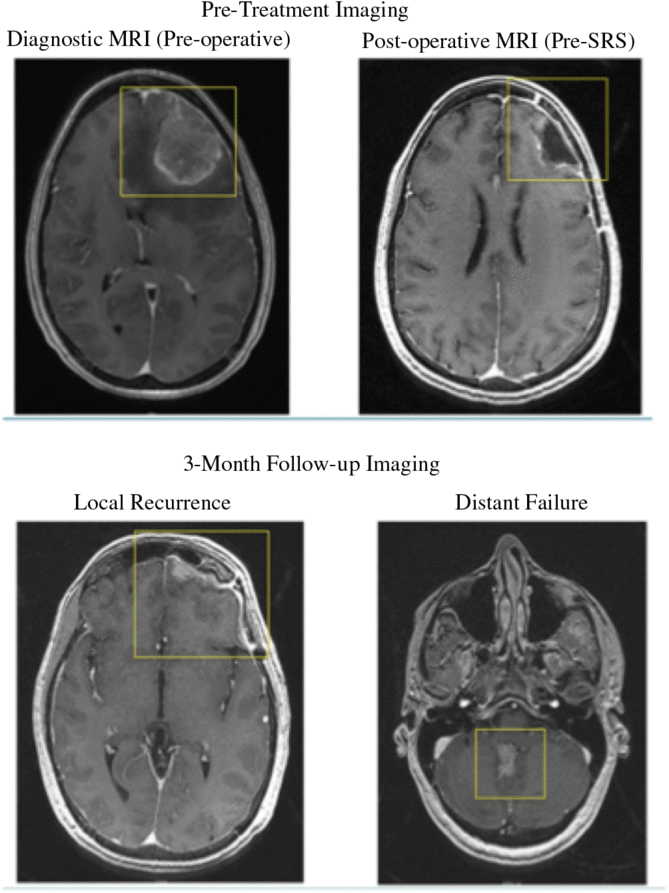
Of those who received follow-up imaging, 6/19 (32%) demonstrated distant failure at 6 months and 8/16 (50%) at 12 months. Changes in the number of patients imaged at 3, 6, and 12 months are due to patient death before imaging, or lack of follow-up. Distant failure occurred at a median time of 3·3 months (range, 1·7–20·0). 7/8 (88%) of patients who failed distantly also experienced local failure at the time of imaging. Prior to surgery, five patients demonstrated dural/pial involvement in the diagnostic MRI. Of the four dural/pial patients with 12-month follow-up imaging, all of them experienced distant failure within the first 8 months post-treatment and were associated with half of the distant failures observed (6 mo p = 0·2621; 12 mo p = ·0385). Case demonstration is shown in Figure 1.

Figure 1. Demonstrates pre-operative, post-operative, and post-SRS follow-up imaging of a superficial surgical cavity with dural/pial involvement. Local recurrence and distant failure was observed at the time of 3-month follow-up imaging.
Survival and toxicity
Overall survival at 6 and 12 months was 95 and 67%, respectively. 8/23 (35%) of patients had died at the most recent follow-up, with follow-up being lost in four other cases. The median overall survival time post-treatment was 11·1 months (range, 1·2–32·5). Kaplan–Meier curve is demonstrated in Figure 2. Dexamethasone was administered in 18/23 (78%) cases to combat edema. The remaining patients did not receive corticosteroids to reduce the risk of side effects. Radiation necrosis was observed in one patient after SRS.

Figure 2. Is a generated Kaplan-Meier survival curve estimating survival based off of available follow-up. Number failed in this graph represents number of deaths. Large confidence intervals of the result of lost follow-up.
Discussion
Surgical resection has proven to increase overall survival in patients with single brain metastases.Reference Patchell, Tibbs and Walsh21 Post-operative WBRT has been the standard of care based on evidence demonstrating an increase in local and distant control.Reference Patchell, Tibbs and Regine2 Although WBRT significantly decreases recurrence, it tends to be associated with decreased quality of life and neurocognitive decline.Reference Chow, Davis, Holden, Tsao and Danjoux3, Reference Chang, Wefel and Hess6, Reference Brown, Jaeckle and Ballman7, Reference Deangelis, Delattre and Posner22–Reference Meyers, Smith and Bezjak24 As a result, SRS has become an increasingly popular form of adjuvant therapy for treating post-operative surgical cavities. Individual institutional studies have demonstrated that SRS has the potential to offer local control comparable to that of WBRT, while having few side effects and quality of life issues.Reference Soltys, Adler and Lipani11–Reference Mahajan, Ahmed and McAleer19 Our study is one of the first community-based reports contributing to this discussion. Our work gives insight into how the use of post-operative SRS is transitioning into community practice, while calling attention to some noteworthy findings, obviously limited by small sample size and the retrospective nature of this analysis.
As review and analysis of our data, consensus guidelines on contouring the surgical cavity have been published.Reference Soliman, Ruschin and Angelov25 Our data support special attention that must be taken when considering fractionated treatment schemes, margin expansion, and qualitative factors of the surgical cavity that may influence local control and distant intracranial failure.
It was found that the majority (16/23) of patients received single-fraction SRS treatments at a median dose of 16 Gy (range, 16–18). The remaining seven patients received hypofractionated SRT treatments with a median total dose of 25 Gy (range, 25–35), 5 Gy × 5. Of the eight patients who recurred locally within the first year, six had received a hypofractionated SRT treatment, with a median margin of 2 mm (range, 1–4), suggesting a relationship between fractionated treatment and local recurrence within our limited sample size (p = 0·0406). Cavity size was another factor that was associated with local failure; of the eight patients who recurred locally, five of them had lesions with max axial sizes greater than 3 cm (median, 3·9 cm).
In our study, single-fraction treatment demonstrated effective 6- and 12-month local control at 92% (n = 12) and 78% (n = 9), respectively. In comparison, 6 and 12-month local control in the hypofractionated cohort was poor, at 29% (n = 7) and 14% (n = 7). These results agree with past studies demonstrating increased control in lesions <3 cm treated with a single fraction.Reference Soltys, Adler and Lipani11–Reference Mahajan, Ahmed and McAleer19
In recent years, different fractionation schemes have proved more effective when compared with the 5–7 Gy × 5 scheme historically utilized by our institution. A 2012 study conducted by Wang et al.Reference Wang, Floyd and Chang26 demonstrated a 6-month local control rate of 80% in patients who received 8 Gy × 3 hypofractionated SRT to the surgical site (n = 37). 2–3 mm margins were added in these cases and concluded that this hypofractionated SRT scheme offers favorable local control with limited toxicity. Another 2012 study showed by Steinmann et al.Reference Steinmann, Maertens and Janssen27 recorded similar findings with a 12-month local control rate of 71% (n = 33). This study employed a 4 Gy × 10 fractionation scheme (median) utilizing a median margin of 4 mm. More recently, a 2016 study conducted by Minniti et al.Reference Minniti, Scaringi and Paolini28 achieved 12-month local control in 91% (n = 122) implementing a 9 Gy × 3 approach with 2 mm margins, further demonstrating hypofractionated SRT efficacy in a post-operative setting. More recently, a single-institutional study by Zong et al.Reference Zhong, Ferris and Switchenko29 implemented single-fraction doses to over half (52%) of their patients with lesions >4 cm. They found no statistically convincing changes in local control, survival or toxicities.
The results of recent single-institutional reviews vary greatly from our initial finding suggesting limitations with our fractionation approach based off of our small study population. These findings may encourage the exploration of alternative approaches and fractionation schemes when treating large surgical cavities.
In 2014, Brennan et al.Reference Brennan, Yang and Hilden15 postulated that dural/pial involvement and larger tumor size are linked to high failure rates when compared with deep parenchymal tumors, and our findings strongly agreed with their claim. It was hypothesized that these high failure rates are attributed to both procedural uncertainties and anatomical factors. They theorized that superficial tumors could make determining appropriate target volumes, and disease boundaries more difficult leading to technical error and also attributed the contaminated dural/pial to increase the risk of recurrence and spread. More recently, Soliman et al.Reference Soliman, Ruschin and Angelov25 established consensus guidelines recommending increasing the margin to 5–10 mm in these cases.
A neuroradiologist was consulted to confirm that all five patients had preoperative lesions with dural/pial involvement. Four of these five patients had follow-up imaging available for review, and all four (100%) of these patients with dural/pial involvement had experienced distant intracranial failure within the first year post-treatment. This suggests a potential relationship between dural/pial involvement and 12-month intracranial failure (p = 0·0385). We are limited by the retrospective nature and limited study sample size of these data. We suggest that further studies be performed to help assess the relationship between superficial tumors with possible dural/pial involvement and intracranial failure. We also suggest exploring different contouring methods and PTV expansion for these cases, as have been supported by recent contouring guidelines.Reference Soliman, Ruschin and Angelov25
Conclusion
In our sample, SRS proved to offer substantial local control, sparing patients some of the long-term health issues linked with WBRT. These results offer additional suggestion on the potential value of SRS in the community setting. The correlation between 5 Gy × 5 hypofractionated SRT and local failure in larger lesions may encourage further investigation of fractionated dosing schemes and their impact on treatment outcomes in a post-surgical setting. Large superficial cavities with dural/pial involvement may foreshadow a high risk of local and distant intracranial failure, and therefore it may be beneficial to consider alternative treatment techniques in these cases.
Author ORCIDs
Luqman K. Dad 0000-0002-5562-7295
Acknowledgements
The authors wish to acknowledge Ms. Jacqueline P. Shanahan, RN, BSN, Oncology Nurse Navigator for her tireless effort and coordination of care for our cancer patients at Anne Arundel Medical Center.
Concept and design: Luqman K. Dad M.D.
Data collection: Zachary T. Smith B.S.
Radiology Consulting: Kerry J. Thompson M.D.; Syed U. Ashruf M.D.
Statistical methods: Charles Mylander Ph.D.
Data analysis and interpretation: Luqman K. Dad, M.D and Zachary T. Smith B.S.
Manuscript writing: Zachary T. Smith B.S. and Luqman K. Dad M.D.
Final approval of manuscript: Luqman K. Dad M.D
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Institution Ethics Review: Anne Arundel Medical Center