Introduction
The role of positron emission tomography–computed tomography (PET-CT) in radiation oncology has grown significantly over the past decade, impacting diagnosis, treatment planning, assessment of treatment response and monitoring for recurrent malignancy.Reference Heron, Andrade and Beriwal1 PET and PET-CT data has been incorporated into the radiation treatment plan of cancers of the head and neck (HNC) for over a decade at several institutions with several studies showing its impact on tumour staging and gross tumour volume (GTV) contoured on images used in the planning process.Reference Ciernik, Dizendorf and Baumert2, Reference Deantonio, Beldi and Gambaro3
Though the use of combined metabolic and anatomic information in the radiation treatment plan has become an established practice in many institutions, only a small number of studies including relatively small numbers of patients have been published evaluating their clinical outcomes.Reference Heron, Andrade and Flickinger4–Reference Rothschild, Studer and Seifert6 In the current climate of healthcare, clinical outcomes data for highly technologically advanced therapies is becoming increasingly important. The purpose of this study was to retrospectively evaluate the long-term clinical outcomes of patients with HNC who received definitive intensity-modulated radiation therapy (IMRT) with or without chemotherapy, planned with PET-CT.
Methods
The methods of this study were approved by the local institutional review board (IRB) and the same IRB monitored the ongoing progress of the study. Data reported to the California Cancer Registry was queried to identify all patients treated by the institution with HNC based on ICD-9 diagnosis between 1 January 2002 and 31 December 2010. Current procedural terminology codes required for billing of IMRT and PET imaging were cross referenced to identify eligible cases. Inclusion criteria included a new diagnosis of HNC treated with IMRT, PET-CT performed for treatment planning and completion of all planned radiation sessions. Patients with recurrent cancer or metastatic disease from a separate cancer site were excluded from evaluation. Retrospective chart review was used to develop a database of eligible subjects. The dataset included patient demographics, IMRT dose summary data, primary site of cancer, surgical treatments, clinical lab values, pathology findings related to the detection of cancer, cancer diagnosis, chemotherapy treatments, clinical findings related to cancer, presence and timing of cancer recurrence and the date and cause of death. A master’s prepared nurse with clinical experience in oncology performed all chart reviews.
PET-CTs for HNC radiation treatment planning were performed following a standardised protocol. All patients were imaged using a flat bed and were positioned using the custom-made mask to be utilised during IMRT sessions. Orthogonal lasers were utilised to position the patient and positioning coordinates were aligned between the PET-CT imaging system and the radiation treatment planning software. While remaining in position on the PET-CT system, an additional CT scan through the head and neck was performed during the intravenous administration of 100 mL iodinated contrast at 2 mL/minute. MaxSUV for the primary tumour was collected for each scan.
Data for all health encounters were captured in an electronic medical record. The electronic records for diagnostic imaging, clinical consultation, treatment planning and follow-up visits were abstracted. Each visit was reviewed to capture the following variables: imaging findings, treatment provided inclusive of IMRT treatment summary data and chemotherapy regimen, clinical or radiographic disease progression, incidence of new cancers and death. Adjunctive chemotherapy regimen data were also collected and categorised into five groups: platinum-based treatment, platinum with taxanes, epidermal growth factor receptor (EGFR) inhibitor, no adjunctive chemotherapy and no data. Recurrence was defined as any clinical or radiographic evidence of tumour confirmed by tissue biopsy or a change in treatment plan. Time to recurrence was a calculated variable reported in days by subtracting the date of initial diagnosis from the date of recurrence diagnosis. Disease was further categorised as either locoregional or distant metastases.
Data were then categorised by site to include nasopharyngeal, oropharynx, oral cavity, salivary gland and larynx/hypopharynx. The oropharynx classification was given to all cancers of the tonsils and base-of-tongue. The oral cavity classification was given to all cancers of the oral tongue or other components of the oral cavity. All data were entered into an electronic spreadsheet (Microsoft Excel 2010) with data encryption software and password access to protect subject privacy. In those cases where a date of death could not be verified (n=10), the last date of follow-up was taken to represent the last known survival date.
Survival rates were analysed by actuarial curves using the life table method. Log Rank test was used to test the difference between curves. Cox proportional hazards regression model was used to evaluate the effects of different factors on survival and recurrence. T-test was used to test the differences between groups and subgroups for continuous variables, while χ 2 test was used to test differences for categorical variables. SAS 9·2 was used in this data analysis.
Results
A total of 1,200 cases with a diagnosis of HNC were reviewed by retrospective chart review. A total of 261 cases met the inclusion criteria and were included in the study. All subjects included in the study received intensity-modulated radiotherapy using computerised inverse treatment planning algorithms to achieve a conformal dose and homogeneity of the target volumes, utilising information from PET-CT in the treatment plan (Figures 1–3). Patients had tumours of various stages as summarised in Table 1.

Figure 1 Axial PET (a) and PET-CT fusion (b) images showing the contours for the radiation treatment plan in a patient with a right tonsillar cancer and metastatic lymph nodes in the neck bilaterally. The GTV of the primary tumour (red line) is delineated based upon the hypermetabolic region on PET. Nodal GTV (yellow line) is also shown as contours of normal structures. Abbreviations: PET, positron emission tomography; CT, computed tomography; GTV, gross tumour volume.
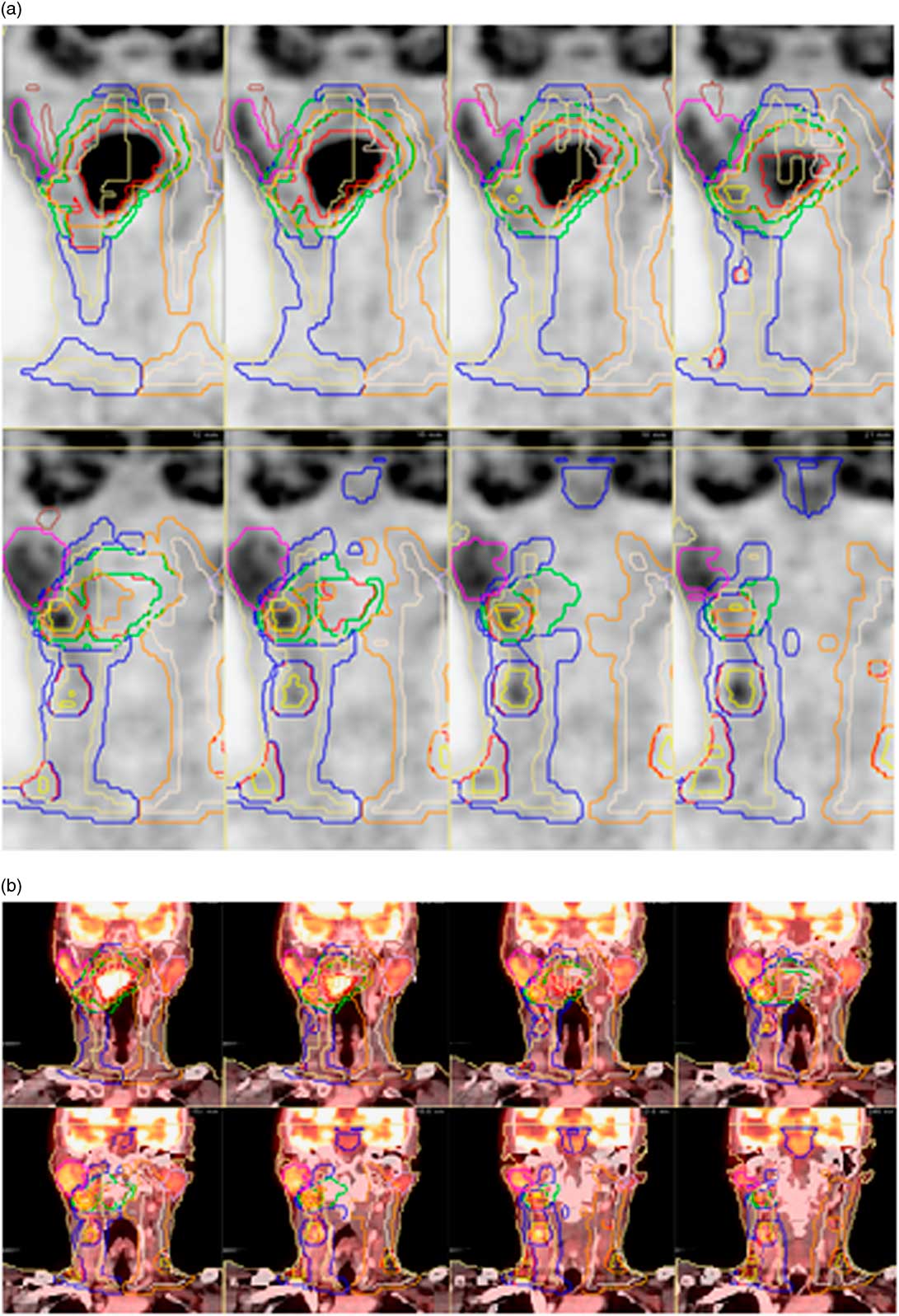
Figure 2 Coronal PET(a) and PET-CT fusion (b) images in a patient with a right-sided tonsillar cancer. PET assists in identifying GTV of the primary malignancy (red line), GTV of lymph node metastases (yellow line) and normal structures such as the parotid glands (pink line on right, white line on left). Abbreviations: PET, positron emission tomography; CT, computed tomography; GTV, gross tumour volume.

Figure 3 Treatment volumes in a radiation therapy plan utilising PET-CT. Areas of hypermetabolism on PET assist in planning radiation of volumes involved in malignancy, including the primary tumour (red line) and small and large lymph node metastases (yellow contours). An 8×5 mm node is a non-specific finding based on CT size criteria (white arrow), but it is included in the GTV due to its hypermetabolism on PET (black arrow). Abbreviations: PET, positron emission tomography; CT, computed tomography; GTV, gross tumour volume.
Table 1 Representation of primary sites and stages in patients with cancers of the head and neck, treated with intensity-modulated radiation therapy with PET-CT-based planning

Abbreviation: PET-CT, positron emission tomography–computed tomography.
The mean follow-up was 26·4 months (1·2–84·7 months). A total of 72% of participants were male with an average age of 62 years. Cancers were located frequently in the oropharynx (45%: 118 cases). There were 79 cases (30%) of cancers of the oral cavity, 26 cases (10%) of nasopharyngeal cancer, 12 (5%) cases of cancer of the larynx or hypopharynx and 26 (10%) cases of salivary gland malignancy. The 3-year cumulative locoregional recurrence rate was 22·6% and 3·07% of all patients had distant metastasis during the follow-up period. Over the total time period studied, 191 patients were confirmed alive at the end of 3 years following IMRT while 60 were confirmed to be deceased. Ten other patients were either lost to follow-up or had records unavailable.
The overal survival and disease-free survival rates for the entire group of HNC patients were 0·883 and 0·791, respectively, at 1 year; 0·793 and 0·688, respectively, at 2 years; and 0·732 and 0·619, respectively, at 3 years. Overall survival for all patients studied as well as subgroups based on site of the primary malignancy may be found in Figure 4. Disease-free survival for all patients studied as well as subgroups based on site of the primary malignancy may be found in Figure 5. The cumulative locoregional recurrence for all cancer types may be found in Figure 6. Median time to recurrence was 345 days. There was an overall low incidence of distance metastatic disease (3·07%) and additional cancers (8·05%). Survival data are summarised in Table 2.

Figure 4 Life table survival curve from original diagnosis to death for (a) all patients and (b) broken down according to site of primary malignancy.

Figure 5 Life table disease-free survival for (a) all patients and (b) broken down according to site of primary malignancy.
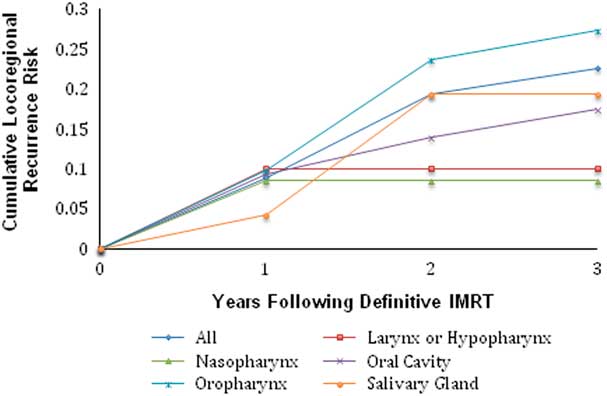
Figure 6 Cumulative locoregional recurrence in patients with cancers of the head and neck, treated definitively with IMRT planned using PET-CT. Abbreviations: IMRT, intensity-modulated radiation therapy; PET-CT, positron emission tomography–computed tomography.
Table 2 Overall and disease-free survival at 1, 2 and 3 years following treatment with intensity-modulated radiation therapy with PET-CT-based planning

Abbreviation: PET-CT, positron emission tomography–computed tomography.
Of concommitant chemotherapy regimens, platinum-based treatment was most common (27%), followed by EGFR inhibitor (22%), no adjuvant chemotherapy (21%) and platinum with taxanes (11%). No data regarding chemotherapy regimen was present in 15% of cases. Three subjects were excluded from analysis due to receipt of a study drug. When compared with patients who did not receive chemotherapy during the period of radiation, patients who received chemotherapy had a significantly prolonged survival (p=0·023) but no significant difference in their rate of recurrence (p=0·207).
Discussion
IMRT is a technique that has been applied to HNC widely over the past decade. IMRT seeks to spare damage to critical normal tissues, many of which are in close anatomic proximity to the targeted malignancy. Specifically, the use of IMRT aims to avoid complications of other radiation techniques, such as dysphagia, osteoradionecrosis, xerostomia and dental caries.
PET-CT, an imaging modality that combines functional (PET) and anatomic (CT) information, has been utilised directly in developing the IMRT plan for patients with HNC for over a decade at some centres, and the technique has been widely adopted by radiation oncologists.Reference Ciernik, Dizendorf and Baumert2–Reference Heron, Andrade and Flickinger4 Clinical PET utilises 18F-flurodeoxyglucose (FDG), a glucose analogue, to identify areas of high metabolic activity. Early prospective studies of PET in patients being considered for primary radiation therapy demonstrated new information over that included in the anatomic imaging alone in a high fraction of cases that resulted in alterations in therapy.Reference Ha, Hdeib and Goldernberg7, Reference Dietl, Marienhagen and Kuhnel8 A recent retrospective study found that PET provided greater sensitivity than CT in identifying the GTV of the primary tumour in patients with squamous cell carcinomas of the head and neck, and that determination of the GTV combining data from PET and CT was superior to GTV based upon contrast-enhanced CT alone.Reference Kajitani, Asakawa and Uto9 The use of PET data can also conceivably decrease the observed large variation in target volume delineation in radiation treatment planning by more easily distinguishing between tumour and non-malignant soft tissue.Reference Steenbakkers, Duppen and Fitton10
However, the utilisation of PET data in the radiation treatment plan brings its own challenges. Incorporation of anatomic abnormalities on CT into the radiation treatment plan is important, sometimes regardless of their FDG-avidity on PET as heterogeneous patterns of FDG uptake within primary HNC lesions are well known and necrotic lymph node metastases can show little or no FDG uptake on PET.Reference Dos Santos, Lima and Chojniak11 Image level thresholding can have significant effects on tumour delineation for radiation treatment planning on PET-CT.Reference Yaremko, Riauka and Robinson12 Registration between PET and CT images is critical and may require advanced image registration algorithms, particularly when PET-CT is not acquired in the treatment position with a flat table and the head/neck stabilisation system and face mask that will be utilised in the radiation therapy suite.Reference Hwang, Bachrach and Yom13 In the current study, all patients were imaged in the treatment position and underwent positioning by radiation therapists.
The purpose of this study was to retrospectively evaluate the long-term clinical outcomes of patients with HNC who received IMRT with or without chemotherapy, planned with PET-CT. Because of all of the potential variabilities introduced by the data provided by PET-CT, as well as the labour- and cost-intensive nature of radiation treatment planning with PET-CT, it is important to understand long-term outcomes of patient who have undergone radiation treatment planning with PET-CT. Only a small number of studies including relatively small numbers of patients have been published evaluating the clinical outcome of patients with HNC who have undergone definitive radiotherapy with integrated PET-CT treatment planning.Reference Vernon, Maheshwari and Schultz5, Reference Rothschild, Studer and Seifert6, Reference Soto, Kessler and Piert14 A retrospective study of 45 patients with stage IVA oro- or hypopharyngeal carcinoma staged with PET-CT and treated with definitive chemoradiation with IMRT over a 3-year period with case-control matching with median follow-up time of 18 months showed that the use of PET-CT and treatment with IMRT improved cure rates compared with matched cases who did not receive either PET/CT nor IMRT. The study reported an overall survival of patients with PET-CT and IMRT of 97 and 91% at 1 and 2 years, respectively. Notably, staging data from the PET-CT was considered in the overall treatment, however, the study did not specifically evaluate patients treated with IMRT planned with integrated PET-CT.Reference Rothschild, Studer and Seifert6 A separate study of 42 patients with HNC who received definitive radiotherapy with integration of PET-CT in the treatment plan over a 4-year period with median follow-up of 32 months reported survival and disease-free survival of 82·8 and 71%, respectively, at 2 years and 74·1 and 66·9%, respectively, at 3 years. The cumulative risk of recurrence was 18·7%.Reference Vernon, Maheshwari and Schultz5 The overall survival rates at 2 and 3 years in the present study of 261 patients are comparable, as is the disease-free survival rate at 2 years. Overall survival was potentially underestimated in the current study as the conservative assumption was made that, in those cases where a date of death could not be verified (n=10), the last date of follow-up represented the last known survival date. In addition, overall survival in the current study was not cause specific, though the vast majority of deaths in patients with HNC are typically secondary to their malignancy.
The survival and disease-free survival data from the present study suggest potential improvements in survival rates over the past decade for patients with cancers of the oropharynx and nasopharynx treated with primary radiation therapy.Reference Robertson, Gleich and Barrett15 A recently published analysis of data in the Surveillance, Epidemiology, and End Results-Medicare database showed evidence of improved cause-specific survival in patients with HNC treated with IMRT when compared with those treated with non-IMRT radiation techniques.Reference Beadle, Liao and Elting16 In a large trial evaluating various methods of delivering radiation fractions for conventional combined chemoradiation therapy in patients with head and neck carcinomas, overall 5-year survival ranged 56–59% with locoregional failure rates ranging 28–31%.Reference Ang, Pajak and Wheeler17 Though the present study analysed 3-year survival and recurrence rates, the majority of locoregional recurrence occurred within the 1st year, suggesting that the 73·2% overall 3-year survival and 22·6% cummulative recurrence rate compare favourably to previously published data. Notably, PET-CT was not necessarily included in the treatment plan in these previously published series and, therefore, the use of these series for comparison is intended only to determine whether the present IMRT outcomes are within an expected range.
The finding that the addition of chemotherapy provided a statistically significant survival benefit in the current analysis is in keeping with prior published data. The recurrence rate was not significantly different between those patients who did receive chemotherapy and those who did not in the current analysis, though disease-free survival has previously been reported to be affected by the addition of chemotherapy.Reference Huncharek and Kupelnick18
Notably, only 261 of 1,200 (21·75%) patients treated for HNC over the study period met criteria for inclusion in the present study. All subjects included in the study were required to have received intensity-modulated radiotherapy using computerised inverse treatment planning algorithms to achieve a conformal dose and homogeneity of the target volumes, utilising information from PET-CT in the treatment plan itself. There were many contributing reasons for patients to fail inclusion criteria. The geographic region of the practice was large and some patients chose not to travel to sites that could deliver IMRT, choosing conformal radiotherapy at a nearby facility instead. In addition, patterns of implementation of IMRT were heterogeneous between providers early in the study period as the technique had only recently been introduced into the community healthcare setting. Given that the first PET-CT system became available to the studied population 1 year before the beginning of the study period, radiation oncologists varied in their level of integration of the imaging method in the IMRT treatment plan. Some providers reserved integrated PET-CT planning for more advanced disease while other providers used integrated PET-CT planning in every case where IMRT was employed. Some patients could not tolerate PET-CT planning using a customised mask, and therefore their data could not be used directly in the treatment plan due to uncertainty of image registration. The larger number of patients included in the later portion of the study period attests to the gradual shift in application and acceptance of the methodology into the practice environment.
Radiation treatment plans for the patients included in this study were generated by qualitative evaluation of the combined PET and CT data by the radiation oncologist, with incorporation of areas more metabolically active than surrounding soft tissue on PET and the primary mass and enlarged locoregional lymph nodes on CT into the GTV contour. This dependence upon judgment of the radiation oncologist could be viewed as a shortcoming to the present analysis, but it represents one methodology utilised widely for clinical tumour delineation in radiation oncology. There are numerous other methodologies, including automatic segmentation of PET-CT images based upon specific thresholds of metabolic activity (e.g., % maxSUV or absolute maxSUV thresholds), however, the superiority of any one of these methodologies has not been demonstrated. Certainly, the choice of any one of these contouring methods may impact the final result.Reference Wang, Heron and Clump19 In one study evaluating the relationship of pretreatment FDG-PET biological target volume and the anatomical location of failure in patients with HNC following radiation therapy, all locoregional treatment failures were inside the GTV and only one of these had a recurrence volume that mapped outside the pretreatment biologic tumour volume as determined on PET, suggesting recurrences cannot necessarily be avoided even if those areas are in the original radiation volume.Reference Soto, Kessler and Piert14
The integration of other PET-derived molecular data into the radiation treatment plan, such as areas of hypoxia, may also become more prevalent in the future.Reference Thorwarth, Eschmann and Scheiderbauer20, Reference Postema, McEwan and Riauka21 The feasibility of dose escalation to areas with high cell proliferation based on PET-CT with 18F-fluorothymidine and sophisticated methods to determine tumour volumes based on regions containing uptake of these radiopharmaceutical biomarkers are also been explored.Reference Troost, Bussink and Hoffman22, Reference Arens, Troost and Hoeben23 Prospective multicenter studies will need to be performed to validate these approaches, but it appears that PET-guided, molecular-based planning for radiation therapy, and IMRT in particular, is integral to the future of treatment of HNC.Reference Hoeben, Bussink and Troost24
Conclusion
Overall and disease-free survival outcomes of a large cohort of HNC patients treated with definitive radiotherapy following treatment planning with PET-CT shows a similar high level of disease control and mortality rate as previously published outcome studies over a shorter term or including a smaller numbers of patients. Given the widespread use of IMRT in HNC and the increasing number of centres that provide integrated PET-CT radiation treatment planning, the field of radiation oncology would benefit from a greater number of analyses evaluating outcomes of large sets of patients in whom PET-CT is directly integrated into the IMRT plan. Future studies could focus on the outcomes of patients using varying PET-CT planning techniques in IMRT for HNC or other highly conformal techniques for other cancer types.
Acknowledgement
The authors are grateful for the statistical assistance provided by Guibo Xing of the University of California, Davis.











