Introduction
The developments in accelerator physics have made possible the efficient use of charged particles for tumour treatments with a main focus on proton beams and carbon ion beams. Ions and proton beams have an advantage over γ or X-rays making them more effective for the treatment of certain cancerous tumours. Indeed, their energy is strongly deposited in a localised region of a few millimetres called the Bragg peak, where the tumour is located.Reference Fix, Frei, Volken, Born, Aebersold and Manse 1 – Reference Garonna, Amaldi, Bonomi, Campo, Rizzogglio and Verdu Andrs 3 In Europe, epidemiological studies have evaluated that ion beam therapy could benefit patients in about 13·5–16% of all radiotherapy treatments.Reference Baron, Pommier, Favrel, Truc, Balosso and Rochat 4 An ideal radiation therapy treatment delivers sufficiently high dose to cancerous cells without adverse effects to normal tissues.
There has been for the last two decades a considerable interest in studying dosimetry properties in the presence of high atomic number nanomaterials (NMs). In fact, introducing nanoparticles (NPs) to tumour cells can potentially lead to improve techniques for cancer treatment.Reference Herold, Das, Stobbe, Iyer and Chapman 5 One of the most bio-compatible materials and high atomic number is gold.Reference Berrezoug, Dib and Belbachir 6 , Reference Tsiamas, Liu and Cifter 7 Researchers are also studying radio-sensitisers and radio-protectors, NMs that enhance a cell’s response to radiation. Several agents are under study as radio-sensitisers and many of them are currently being studied as potential radio-protectors.Reference Connell and Hellman 8 With advances in the syntheses of a variety of NMs, NPs become an interesting surge in biomedical application.Reference Dongkyu and Sangyong 9
In particular, gold NPs (GNPs) can be used as a good material for diagnosis and treatment of cancer cells.Reference Heath and Davis 10 , Reference Jiao, Zhou, Chen and Yan 11 Because of their physical, chemical properties and bio-compatibility,Reference Giljohann, Seferos, Daniel, Massich, Patel and Mirkin 12 numerous experimental and theoretical researchers are interested in GNPs, particularly for the use in biomedical applications such as in cancer treatment. GNPs can be delivered intravenously or orally for the most part by intravenous way or drugs. Drug must be soluble in water in order to travel through the bloodstream. For the simple reason, tumour cells are bigger and more numerous than normal cells, they have the capacity to consume more substances. Therefore, each tumour cell has the ability to contain more than one GNPs. Several works have investigated the effects of adding GNPs on tumour cells.Reference Chow, Leung, Fahey, Chithrani and Jaffray 13 , Reference Ricketts, Castoldi and Guazzoni 14 Hadron Therapy with NPs is an advanced radiotherapy technique for cancer treatment. It offers a better irradiation ballistic than conventional techniques and therefore requires appropriate quality assurance procedures.Reference Morris 15 Until now, most techniques in proton therapy based on the control of the protons beam energy. In other words, proton beam energy should be controlled to locate exactly the Bragg peak energy in the tumour area. For such therapy, the proton passes through the human body and deposited most of its energy in a very narrow area of a few millimetres. However, the non-homogeneity of the human body is not always the same and changes from one person to another.
In this article, we are especially interested in the role of NMs injected into a tumour. These bio-NMs trapped in the tumour will increase the absorption of the energy of the proton beam. We selected an appropriate simulation environment (physics models and parameters) in order to produce simulations using measurements Monte Carlo codes for deposited energy in brain with including several types of NMs in a tumour. Our main goal is to investigate the effect of adding some NMs into a tumour during a proton therapy. Therefore, we have focussed our simulation on materials such as platinum, silver and gold the most popular material in nanomedicine researches.
Methods and Geometry
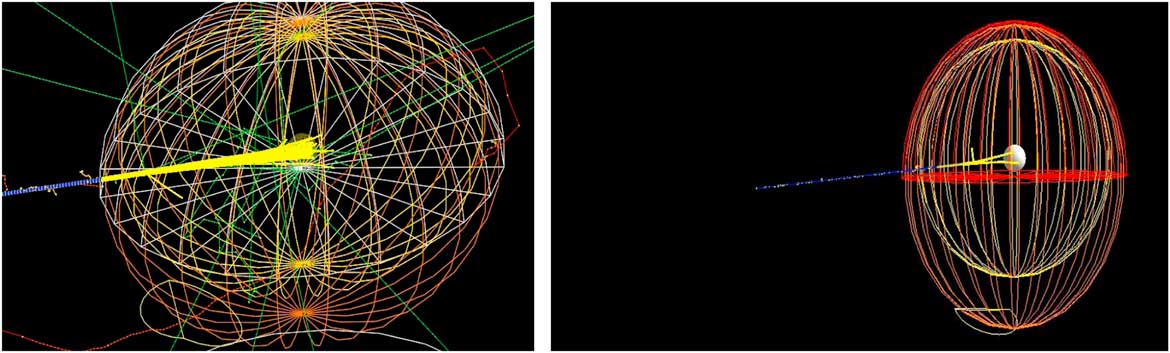
Our main study is to investigate the effect of bio-NMs injected into a tumour during a proton therapy. For this, we have simulated a spherical tumour localised in the centre of a human head. Then, the human head is exposed to a monoenergetic proton beam placed at 1 m from a patient (see Figure 1).
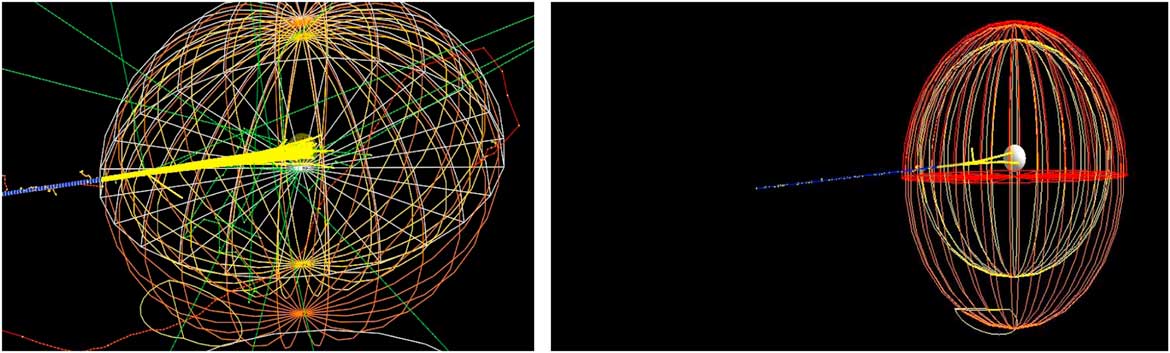
Figure 1 Monte Carlo simulation of 106 beam interaction with a human head.
Monte Carlo simulations
Geant4Reference Agostinelli, Allison and Amako 16 , Reference Allison, Amako and Apostolakis 17 is a platform for the simulation of the passage of particles through matter using Monte Carlo methods. It is used in various application domains,Reference Chauvie, Francis and Guatelli 18 , Reference Pandola 19 including high energy physics, astrophysics and space science, and medical physics. This work is based on the G4HadronInelasticQBBC and G4HadronHElastic Physics packageReference De Napoli, Agodi and Battistoni 20 ; these packages contain hadronic and electromagnetic processes.
Materials and geometry
Simulation of a tumour inside a human head
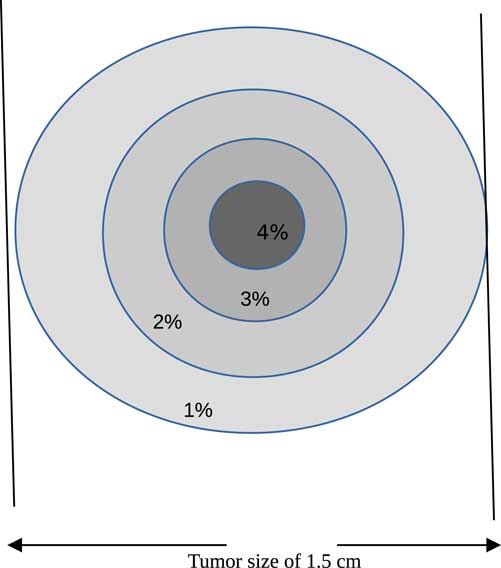
Proton therapy was simulated with the Monte Carlo code of a tumour within a human head. The tumour is assumed to have a spherical shape with a diameter of 1·5 cm and localised at the centre of the head (see Figure 1). The best way to inject NMs in a tumour localised in a sensitive organ like a human head is by the bloodstream. Carmeliet et al.Reference Carmeliet and Jain 21 and Avnesh et al. (2013)Reference Thakor and Gambhir 22 studied the development of blood vessels in the tumour cells; they noticed that the blood vessels are more concentrated in the centre of a tumour. Consequently, NMs will be distributed with a non-homogeneous way and concentrated more towards the centre of the tumour. In this simulation, the concentration of NMs in the tumour is assumed ranging between 0 and 4%. Figure 2 shows the concentrations of bio-NMs localised in a tumour taken in our simulation.

Figure 2 Distribution of bio-nanomaterials in tumour taken in our simulation.
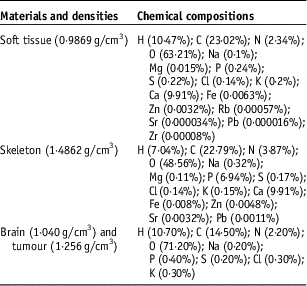
The geometry of the human head is composed essentially of a skeleton of thickness of 0·8 cm and brain. Then, a soft tissue with thickness of 0·2 cm covered the skeleton. The tumour is placed in the centre of the brain. The chemical compositions and densities of skeleton, brain, soft tissue and tumour are taken from Geant4 database and represented in Table 1.
Table 1 Chemical compositions and densities of materials used in our geometry
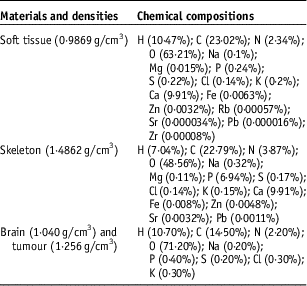
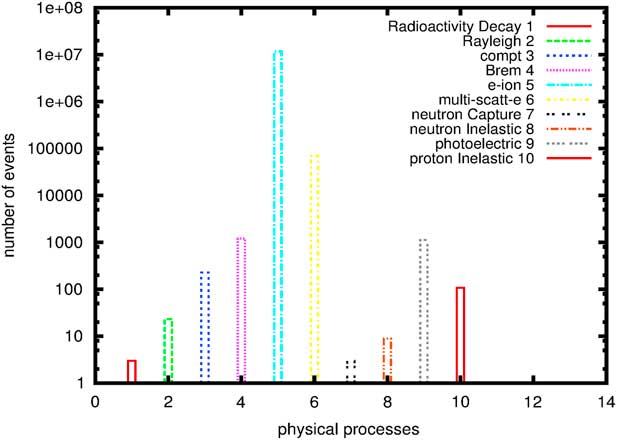
Figure 3 shows processes that occur during a simulation of 106 proton through a head containing GNPs within tumour. In order to have the Bragg peak energy exactly in the tumour location, the proton beam energy should be in the energy range between 116 and 132 MeV. We notice here that processes such as electron-ion and multi-scattering of electrons are the most dominating in our simulation. These are due to secondary electrons created by NMs when irradiated by proton beams. Chow et al.Reference Chow, Leung, Fahey, Chithrani and Jaffray 13 have calculated secondary electrons outside and inside GNPs irradiated by low-energy electrons at cellular level, similarly Walzlein et al.Reference Walzlein, Scifoni, Kramer and Durante 23 have calculated secondary electrons created from heavy atom NPs irradiated by proton. They both concluded that the additional of secondary electrons from NMs may enhance the dose absorption.

Figure 3 Physical processes generated during a proton therapy. Abbreviations: e-ion, electron-ion; multi-scatt-e, multi-scattering of electrons.
Results and Discussion
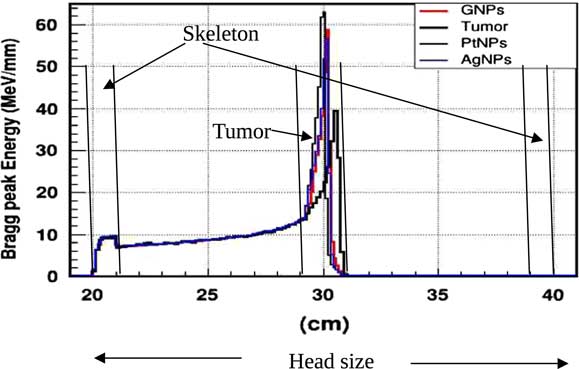
In practice, proton therapy can be used in two ways known as passive scattering and pencil beam. The first technique is based on a single scattering foil (made out of lead) used to broaden the beam. In the second technique, the proton beam is deflected with a magnetic field to generate a narrow monoenergetic pencil beam and scan it magnetically across the tumour. Both of these techniques should be used carefully. Our principal goal is to investigate on how NMs affect on proton therapy. The deposited energy along the head is plotted in the Figure 4. This plot is obtained from a Monte Carlo simulation of 106 protons beam energy of 125 MeV. This figure shows that NMs enhance in a significant way the deposited energy in the tumour. Furthermore, the platinum NPs (PtNPs) present the more efficiency. In order to investigate deeply the enhancing proton therapy by NMs in tumour, we have plotted the deposited energy only along the tumour ranging between 29·2 and 30·8 cm.
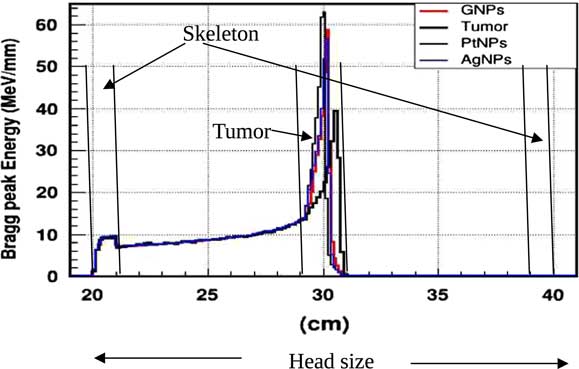
Figure 4 The deposited energy of a monoenergetic proton beam along a head. Notes: The Bragg peak energy issued from 106 protons placed at 1 m from the head. The proton beam energy is 125 MeV. Abbreviations: GNPs, gold nanoparticles; PtNPs, platinum nanoparticles; AgNPs, silver nanoparticles.
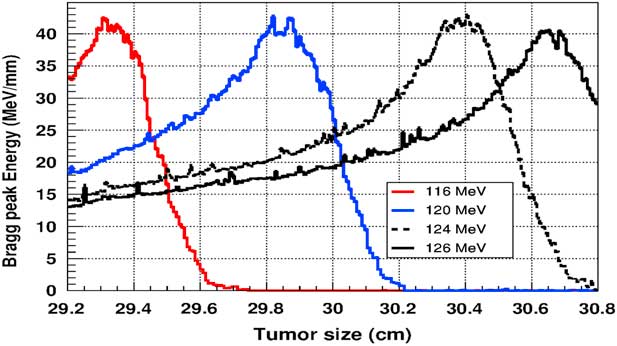
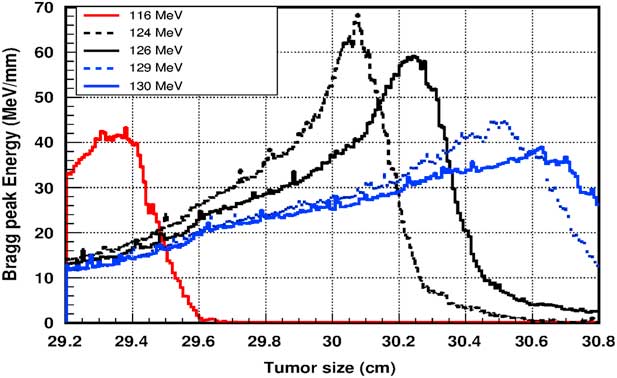
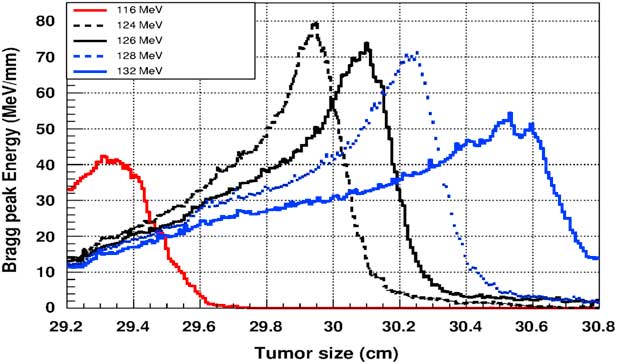
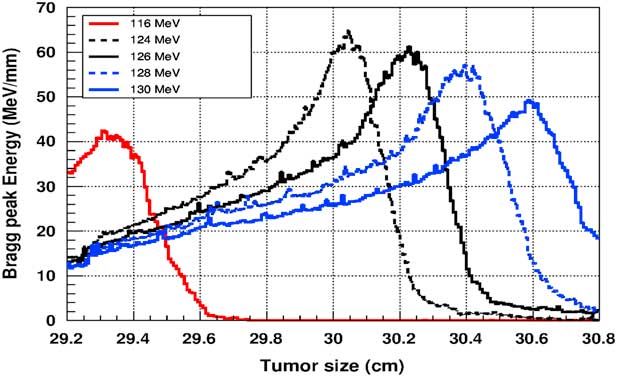
Figure 5 shows the plot of the deposited energy of a monoenergetic proton beam along the tumour size. As this figure shows, to surround completely a such tumour we should use a proton beam energy, ranging between 116 and 126 MeV. For this range energy, the Bragg peak energy is around 40 MeV and the half-value width for each Bragg peak is about 50% of the tumour size. The effect of adding gold in the tumour on the Bragg peak energy is shown in Figure 6. In this figure, we notice that the Bragg peak is localised in the tumour for the proton beam energy ranging between 116 and 130 MeV. The Bragg peak energy at the centre of the tumour is greater than without GNPs, in this case, with the presence of GNPs, the proton therapy is enhanced up to 75%, this is due to the concentration of GNPs at the centre of the tumour. Moreover, for high proton beam energy like 130 MeV, the width at half height of the Bragg peak is around of 75% of the tumour size. Comparing with previous results, the presence of GNPs in the tumour makes the width at half height of Bragg peak larger. This result shows that adding GNPs in tumours makes the proton therapy easier in clinical medicine and presents more benefit. Similarly in Figure 7, we plotted the deposited energy in the tumour with the presence of nanoplatinum materials. In this figure, the Bragg peak is localised in the tumour for the proton beam energy ranging between 116 and 132 MeV. Comparing with previous results, the width at half height of the Bragg peak is spread over 85% of the tumour. Moreover, the deposited energy at the centre in the tumour is almost double comparing with the same results in Figure 6. In the case of the use of silver NPs during this therapy, the plot of the deposited energy has the same shape as in the case of the use of GNPs (see Figure 8).
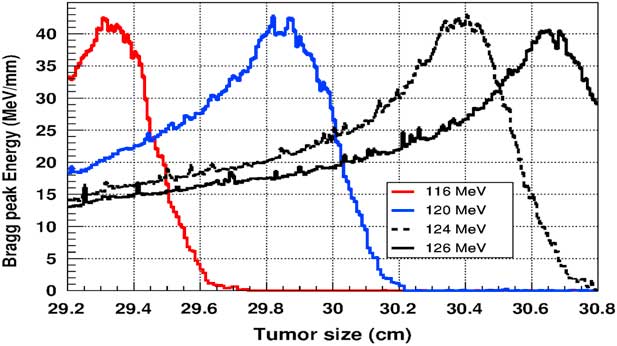
Figure 5 The deposited energy of a monoenergetic proton beam into a spherical tumour with a diameter of 1·5 cm. Notes: The proton beam energy is ranging between 116 and 126 MeV.
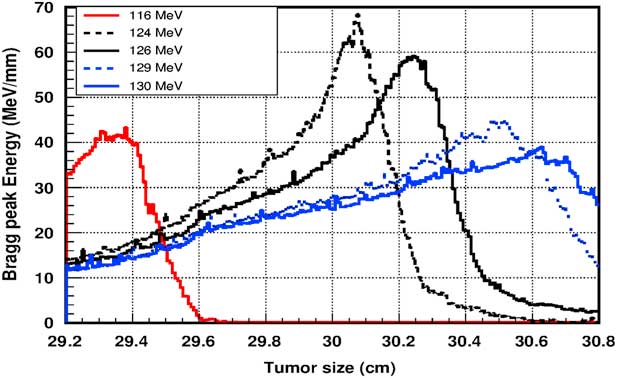
Figure 6 The deposited energy of a monoenergetic proton beam into the tumour with adding gold nanoparticles. Notes: The proton beam energy is ranging between 116 and 130 MeV.
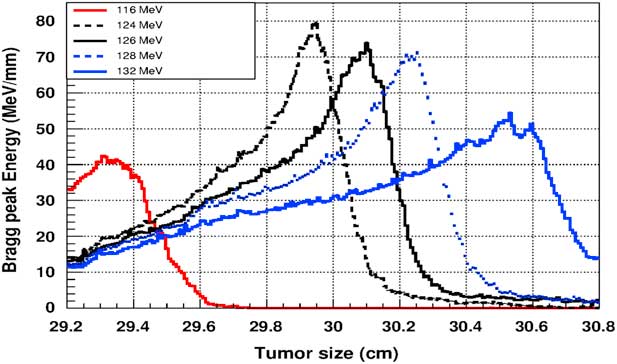
Figure 7 The deposited energy of a monoenergetic proton beam into the tumour with adding platinum nanoparticles. Notes: The proton beam energy is ranging between 116 and 132 MeV.
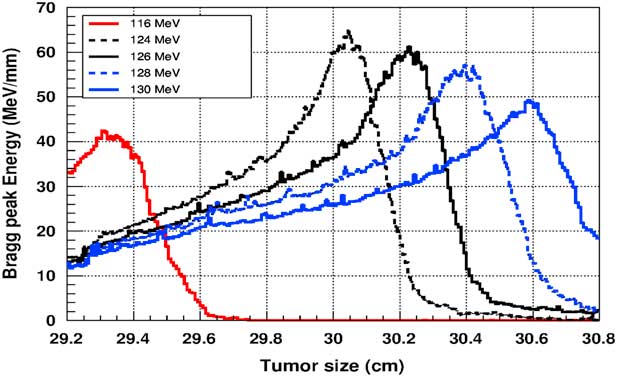
Figure 8 The deposited energy of a monoenergetic proton beam into the tumour with adding silver nanoparticles. Notes: The proton beam energy is ranging between 116 and 130 MeV.
From these figures, we notice that the platinum metal is the most effective in proton therapy. Our results are in good agreement with several researchers.Reference Walzlein, Scifoni, Kramer and Durante 23 , Reference Porcel, Liehn and Remita 24 This can be a hope of a new treatment options in the near future.
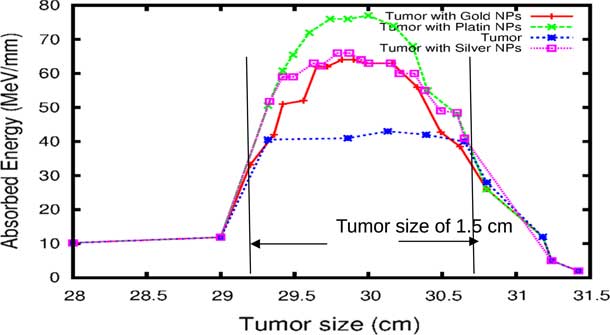
The comparison of our results concerning the energy deposited into a tumour in the presence of NMs is represented in Figure 9. As the figure shows, the gold and silver enhance the proton therapy and increase the absorbed energy in the tumour up to 55%, on the other hand, platinum increases the absorbed energy up to 80%. Lin et al.Reference Lin, McMahon, Scarpelli, Paganet and Schuemann 25 have compared radiotherapy with protons and radiotherapy with photons and concluded that the proton therapy can enhanced significantly the absorbed dose only if the GNPs are in close proximity to the biological target.
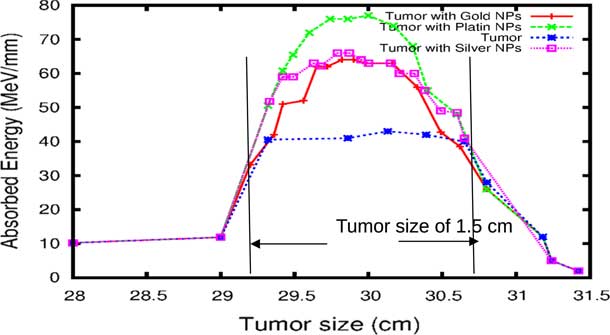
Figure 9 The deposited energy of a polyenergetic proton beam into the tumour with adding nanomaterials (NMs). Notes: The proton beam energy is ranging between 116 and 126 MeV in the tumour without any NMs, and between 116 and 130 MeV in the tumour with gold NPs (GNPs) and silver NPs (AgNPs). In the case of platinum NPs (PtNPs), the proton beam energy is ranging between 116 and 132 MeV.
Although GNPs have been very popular with nanomedicine researchers,Reference Lin, McMahon, Scarpelli, Paganet and Schuemann 25 our findings show that platinum metal is more effective in proton therapy. A French–Japanese research group has demonstrated that PtNPs strongly enhance the biological efficiency of radiations.Reference Porcel, Liehn and Remita 24
Conclusion
Over the last decades, a large number of NMs delivery systems have been developed for radiation therapy, including organic and inorganic materials. Many of nanoparticles are currently in the preclinical stages of development. The use of GNPs in radiation therapy show an exponentially increasing, especially in imaging and diagnosis of tumour cells and because of their bio-compatibility and ability to convert energy radiation in heat. In this paper, we have realised a Monte Carlo simulation based on Geant4 code of a proton therapy of a tumour inside human head and we have investigated the effect of adding metal nanoparticles in this tumour in order to enhance the proton therapy. Our results show that platinum is more effective than gold and silver to enhance proton therapy.
Acknowledgements
The authors thank Dr K. Laihem from the Aikhen University in Germany for the fruitful discussions and the help he provided the authors during his visit.