Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death in the male sex and the second in the female sex in developed world. During the course of the disease, more than half of patients with local advanced disease will receive thoracic radiotherapy as part of the treatment.Reference Tyldesley, Boyd, Schulze, Walker and Mackillop1 The natural history of the disease shows that about a quarter of patients with locally advanced disease (stage III) will develop an isolated thoracic recurrence of disease, although it is known that the majority of them will have a systemic metastasis. The risk of developing metachronous tumour in patients with NSCLC is around 5–10%.Reference Curran, Paulus and Langer2,Reference Martini, Bains and Burt3
The lengthening of life expectancy of patients with NSCLC following therapeutic innovations, both in the pharmacological field, with the new target and immunological therapies, and in the radiotherapy field, with the new methods of irradiation, place the need to submit again at chest irradiation an increasing number of patients, both for recurrent and metachronous disease. Therapeutic options of patients with recurrent or metachronous tumour are rather limited, since they have already been previously excluded from surgical treatment and subjected to radical cancer treatments.
Thoracic re-irradiation is a clinical challenge, due to the high risk of toxicity, mainly pulmonary. In these cases, even if there are no predictors of toxicity, the previously irradiated doses and volumes are certainly important. Stereotactic body radiation therapy (SBRT) is a potential treatment option for patients who develop a relapse within or marginal to a previously irradiated volume. Re-irradiation is relatively rarely used as salvage after primary radiotherapy, because of reduced knowledge on efficacy and morbidity related to the treatment. SBRT gives high-precision irradiation with tight margins, sparing exposure of normal tissues, and it may therefore potentially have an advantage to conventional radiotherapy in re-irradiation of relapsing tumours.
In this paper, we want to evaluate the pros and cons of re-irradiation with SBRT as a salvage therapy in patients with metachronous NSCLC not eligible for surgery or who refused it, reporting on our initial experience in this field with regard to local control and toxicity.
Patients and Methods
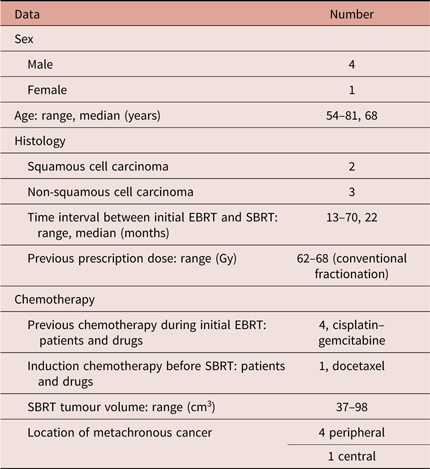
From January 2015 to April 2018, five patients (male/female: 4/1; age 54–81 years, median 68) with previously irradiated NSCLC presented with a second primary lung tumour. All patients had received a previous external-beam irradiation (EBRT) with three-dimensional conformal (3D-CRT) or intensity-modulated technique (IMRT) and the new lesions had occurred outside the fields of the previous radiotherapy. All five patients were judged not eligible for surgery, as was the case during the course of the previous disease, due to medical reasons or patient’s refusal. A pneumological evaluation was done in every case before the start of treatment, to know the residual respiratory function following the combined treatment with EBRT.
Previous treatment information, regarding target volumes, prescription dose, dose distribution, dose–volume histograms (DVHs) to the volumes of interest, field arrangements were available from our archives or reconstructed at treatment planning systems using imaging fusion for patients treated at other institutions (see Table 1). Previous EBRT was given with radical intent to the primary and involved nodes, without elective nodal irradiation; prescription dose of first irradiation ranged between 60 and 68 Gy. Time interval from initial EBRT to SBRT ranged between 13 and 70 months (median 22 months). Chemotherapy was previously received by four patients, with cisplatin-based schedule, and one patient received an induction chemotherapy with docetaxel before SBRT. None of the five patients showed a metastatic disease at 18-FDG-PET-CT scan and metachronous NSCLC was histologically confirmed in all of them, with a squamous cell carcinoma detected in two patients and non-squamous cell lesions in the other three. The tumour volume for SBRT treatment ranged between 37 and 98 cm3, with a median of 51 cm3, the lesions were peripherlly located in four cases, and centrally in the last one.
Table 1. Patient characteristics and previous treatment data
SBRT was carried out by a 6 MV Siemens ONCOR Impression Linear Accelerator (Siemens AG, Erlangen, Germany) using a dynamic micro-multileaf collimator (DMLC 3Dline, Reggio Emilia, Italy), with multiple consecutive arcs (5–6 arcs, producing an final arc ranged from 180° to 225°, in order to reduce the dose to the contralateral hemithorax). Patients were treated in supine position on a SBRT body frame positioning system [C. Charol Solutions, Montanera (CN), Italy] composed by head and superior arms fixation system, a carbon fibre base with a vacuum cushion, an indexed arc provided with a diaphragmatic compressor to reduce the respiratory movements and a knee/feet fixation device (see Figure 1). Target volume was defined based on a combination of CT and PET scan, giving 8 mm isotropic margin to the gross tumour volume (GTV): lesions were peripherally located in four cases, and centrally in one patient. The tumour volumes ranged from 37 to 98 cm3. Dose prescription was 48 Gy, given in four fractions of 12 Gy every other day. We calculate the biological equivalent dose (BED), to evaluate the efficiency of the treatment dose in terms of local control and toxicity, as follows:

Figure 1. SBRT positioning system.
(Legend: TD = Total dose; DFx = dose per fraction; α/β = 10 for tumour responseReference Patel, Lanciano and Sura4).
Before each fraction, the image-guidance through a cone beam computed tomography scan (CBCT) was used to facilitate the target volume repositioning, by comparing planning CT scan with current CBCT scan to correct the patient’s position throughout irradiation. Treatment planning was performed using a 6 MV pencil beam and dose distribution calculation was made by Elekta Ergo Software (CMS Software® of Elekta, Stockholm, Sweden), used at our institution for intensity-modulated thoracic treatments.Reference Spatola, Tocco and Milazzotto5,Reference Spatola, Militello and Tocco6 During planning session, the previous EBRT dose distributions and DVH of the organs at risk (OAR) such as lungs, spinal cord, heart and oesophagus were taken into account. Doses to the OARs were limited to the lowest amount possible, adapting the Timmerman indicationsReference Timmerman7 and using the following constraints:
Whole lung: V12 Gy < 20% volume
Heart: V15 Gy < 10% volume
Cord: Dmax < 14 Gy
Oesophagus: <5 Gy/fraction.
Results
For all patients, a follow-up of at least 12 months is available, and it varies from 12 to 45 months, median 28 months. Response was evaluated with PET/CT at 3, 6, 12, 18 and 28 months; a pneumological evaluation was programmed at the same intervals. At the time of analysis, all five patients are alive, only one of them had a disease progression with the development of brain and bone metastases. A partial response (PR) was seen in four patients (see in Figure 2 a case of a very good PR), one complete responses in the fifth. Three patients did not undergo chemotherapy during the management of metachronous NSCLC, except one who received single-agent docetaxel as induction to SBRT and another who had, as specified above, progressive disease after 9 months.

Figure 2. CT images of 72-year-old female at the time of diagnosis of metachronous right NSCLC (a) and 6 months after the SBRT treatment, showing a very good partial response (b).
The overall toxicity was determined according to Common Terminology Criteria for Adverse Events (CTCAE 4.03) criteria of European Organization for Research and Treatment of Cancer. It was acceptably low: two patients experienced a grade II asymptomatic radiation pneumonitis after 6 and 12 months from the end of SBRT, resolved with cortisone therapy. No acute or late oesophageal or cardiac toxicity was found.
Discussion
In patients with isolated pulmonary mass, both recurrence and metachronous second primary NSCLC, elective treatment is represented by surgery, according to National Comprehensive Cancer Network guidelines 2018.
Surgical salvage and/or systemic therapy are dependent on patients’ pulmonary function and performance status, and often not recommended. Salvage chemotherapy alone, for those who are eligible, offers a low probability of disease control. The response rates to second-line chemotherapy are low without the prevision of durable control.Reference Noble, Ellis, Mackay and Evans8 Nevertheless, patients who had previously been excluded from surgery for locally advanced disease, medical reasons or for their refusal are rarely eligible for this treatment in case of recurrent or metachronous NSCLC.
Following a thoracic combined radio-chemotherapy with curative intent, therapeutic options in case of recurrence or metachronous NSCLC are rather reduced due to the high risk of acute and late toxicity. There is no consensus regarding the use of systemic therapies, either chemotherapy or immunotherapy or target therapy, since they are indicated in locally advanced or metastatic disease.
In the cases treated in this paper, we have opted, after acquiring informed consent from the patients, for a thoracic re-irradiation with a SBRT technique. Its clinical use has been expanding in recent years, thanks to the increase of survival of cancer patients. Traditionally, re-irradiation was indicated for symptomatic or emergency, as severe dyspnoea, airway obstruction or bleeding. Recently, indications to re-irradiation were extended also to non-symptomatic local progression with a single-institution case-by-case evaluation.Reference Drodge, Ghosh and Fairchild9 We taken into account the time interval from the previous EBRT (that was at least 13 months, median 22 months), to allow for normal tissue radiation recovery, the dimension of target volume (less than 100 cm3 in all patients), the localisation of the metachronous tumour (outside the fields of the previous radiotherapy in all patients) and the patient’s performance and respiratory status.
SBRT is conventionally accepted to be the best radiation option when a small volume is to be treated and tumour ablation or curative intent is the goal of treatment. The definition of a standardised dose in thoracic SBRT is far from being settled, but some authors agreed that a local control and survival improvement in early-stage NSCLC is achievable when a dose of >100 Gy BED is delivered.Reference Willner, Baier and Caragiani10 Controversies already exist on radiation dose in recurrent tumour or previously irradiated patients, due to the limited tolerance level of surrounding normal tissues. We used a dose of 48 Gy in four fractions, which correspond to BED10 (biological equivalent dose for α/β = 10) = 105 Gy, that was safely deliverable considering the OARs DVHs and dose distribution of previous EBRT.
Jeremic et al. reviewed 11 studies of conventionally fractionated EBRT re-irradiation for recurrent NSCLC. The studies showed an improved overall survival after retreatment with higher doses compared to low-dose retreatments accompanied by increased rates of grade 2–3 oesophagitis and pneumonitis. They have summarised that conventional external-beam radiotherapy (EBRT) for re-irradiation of recurrent lung cancer yields suboptimal local control rates of 50–60% and a 3–5% risk of grade 3 or higher toxicity.Reference Jeremic and Videtic11
Kilburn et al.Reference Kilburn, Kuremsky and Blackstock12 presented their results with 33 patients previously treated with either EBRT or SBRT who were re-irradiated using only the SBRT approach. The local control rate (2 years) was at 67%, relatively smaller than that of other series, where a rate of about 90% is reported, because of heterogeneous tumour location and a higher BED value (50 Gy in 4 fractions versus 50 Gy in 10 fractions).
Onishi et al. proved a local control rate of 92% for those receiving SBRT with a BED > 100 Gy compared to 74% with a BED < 100 Gy.Reference Onishi, Araki and Shirato13 Regarding OARs tolerance, acute toxicity from SBRT can be 40% common and includes cough, fatigue, haematological suppression and erythema; late toxicities are less common and included scarring, pneumonia, worsening lung function, rib fractures, chest wall pain, oesophageal lesion and brachial plexopathy. Pneumonia seems to be the most common side effect from reprocessing, up to 40% in some cases.Reference Kelly, Balter and Rebueno14
The grade 4 of toxicity may be rare but possible. Trovò et al.Reference Trovo, Minatel and Durofil15 had two treatment-related deaths in their search for re-irradiation of centrally located recurrences. One patient died of fatal haemoptysis 2 months after the completion of SBRT, the other patient developed fatal pneumonia. From this study, it could be hypothesised that there is a correlation between toxicity and central versus peripheral re-irradiation.
In our study, we reported a local disease control in all cases with an acceptable toxicity, since treatment was tolerable for all patients. Due to the limited number of cases in this study and the short follow-up compared with other studies, no general conclusions can be done about our preliminary experience.
Starting from our initial experience, we have been able to highlight how the re-irradiation with SBRT can be carried out safely and with good results. A clinical consideration on re-irradiation should always include the patients’ age and co-morbidities. Elderly patients and patients with severe co-morbidity may not tolerate re-treatment and specific co-morbidities such as chronic obstructive pulmonary disorders and interstitial lung disease or hepatitis/cirrhosis may reduce the tolerability of re-irradiation considerably. The evolving literature in the field made us aware of the potential benefits and possibilities in SBRT re-irradiation.
Conclusions
Although we have not made a direct comparison with other methods of SBRT (VMAT, Tomotherapy, Cyberknife, etc.), which would probably allow a reduction of the margins around the GTV, we believe it is crucial to respect the dose limits to the surrounding healthy organs and to pay maximum attention to patient selection. It must be based on the evaluation of the performance status, the cumulative doses of the healthy tissues and time interval between EBRT and SBRT.
We can conclude that lung re-irradiation in patients with metachronous NSCLC is feasible with SBRT as a definitive treatment, as it allows good local control to be achieved, without relevant toxicity. Future studies should be directed to the comparative evaluation of the different SBRT modalities in order to improve the safety profile and the clinical results.





