Introduction
Gynaecological cancers vary across the globe in terms of prevalence, incidence and mortality rates. According to Globocan 2018 data, cervical cancer is the second most common cancer and cause of cancer-related mortality in Indian women. Reference Bray, Ferlay and Soerjomataram1 Recent data has shown a declining trend of advanced cervical cancer incidence due to screening and prevention strategies but conversely, it has also predicted a rise in the incidence of endometrial cancer due to increasing exposure to risk factors. 2,Reference Balasubramaniam, Sushama and Rasika3 In early endometrial and cervical cancers, certain histological risk features warrant multimodality management involving surgery followed by vaginal vault brachytherapy (VBT) alone or with external beam radiation therapy (EBRT). Reference Small, Beriwal and Demanes4,Reference Nout, Smit and Putter5 Most of the available data on VBT stem from two-dimensional (2D) plain film-derived conventional plans. The most important drawback of this approach is that the dose to adjacent organs, mainly rectum and bladder may be inadequately assessed. With the advent of computed tomography (CT)-based three-dimensional (3D) planning systems, prescribed dose to the target volume and concomitant dose to normal organs can be computed and optimised. However, the International Commission on Radiation Units and Measurements (ICRU) report 38 defines only bladder and rectal reference points in 2D plans, which may not represent actual 3D volumetric dose distribution. 6 Even more worrying is the fact that doses to the small bowel and sigmoid colon are ignored in conventional VBT plans. In post-hysterectomy status, small bowel loops and sigmoid colon tend to migrate to the pelvis and lie in close proximity to the vaginal vault, which results in a significant proportion of the prescribed dose to these organs. This is especially important when combined with EBRT. Although the Groupe Européen de Curiethérapie – European Society for Radiotherapy and Oncology (GEC-ESTRO) group and the American Brachytherapy Society (ABS) provide practice guidelines for gynaecological brachytherapy, there is a lack of consensus on the optimal bladder volume during treatment and threshold doses for bowel resulting in inadequacy in the post-operative brachytherapy guidelines. Reference Small, Beriwal and Demanes4,Reference Dimopoulos, Petrow and Tanderup7 In a dosimetric study by Pathy et al., significant small bowel doses were reported in post-hysterectomy VBT using ovoid applicators and about one-fifth of their observations had doses more than 9·9 Gy EQD2. Reference Pathy, Kumar, Sharma, Biswas, Sharma and Binjola8
In view of paucity of robust data, this study was undertaken primarily to compare a conventional plan to a customised volumetric plan and dosimetrically analyse if there was an advantage in terms of dose to the tumour and organs at risk (OARs). The secondary objective was to analyse the effect of bladder distension on target volume and normal tissue dosimetry.
Materials and Methods
Between April 2017 and February 2018, 46 patients who were planned for post-operative VBT were recruited in this prospective study after approval from the institutional review board. All patients who had undergone modified radical hysterectomy +/− pelvic and para-aortic nodal dissection for cervical or endometrial carcinoma with risk factors necessitating adjuvant radiation therapy were enrolled in this study after obtaining written informed consent. The decision on adjuvant therapy was made in the gynaecological oncology multidisciplinary tumour board. All patients underwent a gynaecological examination to assess the vaginal vault, vaginal length and to determine the appropriate applicator diameter prior to radiation therapy planning.
Planning CT scan protocol
All patients underwent 2 planning CTs (92 observations) with a vaginal applicator in the treatment position. A single-channel vaginal cylinder applicator with stackable cylinders was used for all patients. Each cylinder was 2·5 cm in length and a maximum of four cylinders could be used. Cylinder diameters ranging from 2 to 3·5 cm were available for use and the widest cylinder that the patient could comfortably accommodate was inserted to obtain optimal coverage. A bladder protocol was followed for each planning CT. Distended bladder protocol was followed for the first CT, that is, the patient was asked to void completely 1 hour prior to the CT, consume 1L of water and not void thereafter until planning CT was completed. Empty bladder protocol was followed for the second CT. Initially, this was achieved by inserting a Foley’s catheter under aseptic precautions to drain the urine completely. However, with patients reporting catheterisation-related discomfort, this was subsequently discontinued, and patients were asked to void completely prior to the planning CT to attain an empty bladder for the same. Planning CT protocol was standardised and images with a slice thickness of 2 mm were taken to cover the pelvis from the sacral promontory to 5 cm below ischium.
Contouring and planning
OARs such as bladder, rectum, sigmoid colon and bowel bag were contoured on the CT images according to Radiation Therapy Oncology Group (RTOG) pelvic normal structure delineation guidelines. Reference Gay, Barthold and O’Meara9 All planning CTs were contoured by the principal investigator and was checked by a senior radiation oncologist. The treatment length was defined as proximal two-thirds of the total vaginal length. The mould cylinders were contoured apex downwards covering the treatment length, and the clinical target volume (CTV) was defined by an isometric expansion of 5 mm. Brachytherapy planning was done on Oncentra® Brachytherapy Treatment Planning System (Version: 4.5.3.30, Elekta-Nucletron BV, Stockholm, Sweden).
The applicator was digitally reconstructed, and two plans were created for each CT dataset, one library-based 2D plan and an optimised 3D plan. The dose was prescribed to 5 mm depth from the cylinder’s surface. The 2D plan was generated on the CT using dwell positions and weightings from standard loaded library plans without any dose optimisation. This was considered as a surrogate for a conventional point-based plan. Dose optimisation was done only in the 3D plan to minimise normal organ doses and to achieve the best possible coverage (at least D95>90%). Dose to 5 mm depth was reported in terms of percentage of the prescribed dose received by 90% (D90) and 95% (D95) of the target volume, respectively. Target volume that received 100% dose (V100) and 150% dose (V150) were also recorded. The OAR volume dose was defined as the percentage dose received by 0·1cc, 1cc and 2cc of the respective OAR (D0·1cc, D1cc and D2cc). Dose parameters were compared between 2D and 3D plans, and between the full bladder and empty bladder plans. All the patients were treated using Iridium-192 with a Microselectron® high-dose rate (HDR) after loading system (Elekta-Nucletron BV, Stockholm, Sweden). The equivalent of 2 Gy dose (EQD2) was calculated for the normal tissues (α/β = 3) for a better understanding of the biological dose received.
Statistical methods
The analysis was done using the Statistical Package for Social Services (SPSS)® software (Version 21.0, Armonk, NY, USA: IBM Corp.). Based on the normality of data, the parametric single mean paired t-test, or the non-parametric Wilcoxon signed-rank test was applied to the data. All p-values were two-sided, with p < 0·05 considered to be statistically significant.
Results
A total of 46 patients were enrolled in the study and 92 observations (planning CTs) were included for analysis. Their median age was 49 years (range: 24–69 years). Patient characteristics are listed in Table 1. Median EBRT dose was 50·4 Gy (range: 45–50·4 Gy). Patients who received brachytherapy as a boost after EBRT received 6 Gy × 3 fractions [RTOG 0921] and 7 Gy × 3 fractions [PORTEC 2] was prescribed for brachytherapy alone. Reference Nout, Smit and Putter5,Reference Viswanathan, Moughan and Miller10 Median cumulative tumour EQD2 for tumour (α/β = 10) from EBRT and VBT was 68·3 (range: 68·3–73·6) and brachytherapy alone was 29·8. Although cylinder diameters of 2–3·5 cm were available, 70% (n = 32) of the patients required a 3 cm diameter cylinder and 30% (n = 14) required a 2·5 cm diameter vaginal cylinder. Median treatment length was 4·5 cm (range: 4–5 cm).
Table 1. Patient characteristics

Abbreviations: EBRT, external beam radiation therapy; IMRT, intensity-modulated radiotherapy; 3DCRT, 3D conformal radiotherapy; Co60, Cobalt-60.
Dosimetry of target volume—2D versus 3D
Mean CTV volume was 53 cc (range: 36·3–68). The mean percentage of CTV that was encompassed by the prescribed dose (V100) in 2D plan was 89·54% (range: 77·9 – 99·7) and 87·17% (range: 75·17 – 93·78) for 3D plan. Mean percentage of prescription dose to 95% of CTV (D95%) was 92·8% (range: 82·7–117·36) for 2D plan and 89·75% (range: 82·96–98·41) for 3D plan. Mean percentage of prescription dose to 90% of CTV (D90%) was 100·2% (range: 89–124·57) for 2D plan and 96·9% (range: 90–105·27) for 3D plan. The difference between CTV coverage in terms of 2D and 3D plans were marginal and not significant (p = 0·11) [Table 2].
Table 2. Dose parameters for CTV and organs at risk (OARs) in 2D (library) and 3D plans

Abbreviations: SD, standard deviation; CTV, clinical target volume.
Dosimetry of OARs—2D versus 3D
All dosimetric parameters (D0·1cc, D1cc and D2cc) of OARs of 2D and 3D plan were compared and tabulated in Table 2. A statistically significant dose reduction was observed in all the dosimetric parameters of bladder and rectum with the 3D plan (p < 0·001). Sigmoid colon and bowel dosimetric parameters also had a statistically significant dose reduction, albeit, to a lesser extent [Table 2, Figure 1a–d].

Figure 1. 2D versus 3D dose–volume comparison in (a) bladder, (b) rectum, (c) sigmoid and (d) bowel.
Effect of bladder filling
The mean volume of full bladder was 368cc (range: 144–669·5cc) and mean volume of empty bladder was 70cc (range: 23–148cc). The full bladder and empty bladder status had no significant effect on CTV dosimetry [Table 3].
Table 3. Dose parameters for CTV and OARs in full bladder and empty bladder plans

Abbreviations: SD, standard deviation; CTV, clinical target volume.
All the OARs parameters were compared in full and empty bladder states in 2D and 3D plans. All parameters reported in Table 3 were in the 3D volumetric plan. D2cc of bladder showed a statistically significant reduction in bladder dose in an empty state (volume <150 cc) as compared to full state. In full bladder state, 6·12% (p = 0·047) increase in D2cc of bladder was noted. But the mean dose (D50%) to the bladder was observed to be reduced by 13·7% in full bladder state (p < 0·001). Interestingly, a steep increase in mean bladder dose was noted in patients with bladder volume more than 300cc. Changes in rectal and sigmoid dose parameters were marginal and insignificant. Bladder distension was observed to reduce the D0·1cc, D1cc and D2cc of the bowel by 27%, 22% and 20% which was statistically significant (p < 0·001) [Figure 2].

Figure 2. Dose (D2cc) variation in organs at risk (OARs) in full bladder state.
For a better understanding of the biological representation of the dose and clinical relevance, the percentage dose was converted to physical dose and cumulative EQD2 was calculated. The mean cumulative EQD2 (α/β = 3) for 50·4 Gy (1·8 Gy per fraction) dose of EBRT and 18 Gy (6 Gy per fraction) dose of brachytherapy for D2cc of bladder, rectum, sigmoid and bowel was in empty and full bladder state, and is tabulated in Table 4. Full bladder treatment increased the mean bladder D2cc by nearly 3 Gy (63·4→66·1), but reduced the bowel D2cc by 4 Gy (54·3→50·1). It was also noted that bowel may receive as high as 69 Gy EQD2 with empty bladder treatment as compared to 54·7 Gy EQD2 in full bladder treatment. Mean EQD2 for CTV in the full bladder with brachytherapy alone (7Gy × 3) is 29·8 Gy, bladder was 22·6 Gy and bowel was 6·3 Gy.
Table 4. D2cc physical dose parameters for OARs and cumulative EQD2 for EBRT and brachytherapy

Abbreviations: SD, standard deviation; EQD2, equivalent dose at 2 Gy per fraction; EBRT, external beam radiotherapy.
Discussion
VBT has been commonly used in the treatment of carcinoma of the endometrium, cervix and vagina. Despite significant technological advances in medical imaging, EBRT planning and delivery techniques, few developments have occurred with VBT over the years. The 2016 ABS survey showed that although many centres have CT simulators, cross-sectional imaging is often used only to confirm applicator position and library plans are used for treatment. Reference Harkenrider, Grover and Erickson11 One of the reasons why 3D volume-based planning has not been used in VBT is that the published outcome data with conventional planning is excellent.
In our study, the variation in target volume (CTV) dosimetry between 2D and 3D-based plans were observed to be marginal and not statistically significant. This was consistent with a study by Kim et al., where the CTV parameters of 2D and 3D-based planning did not have a significant difference. Reference Kim, Kim and Beriwal12 Holloway et al. evaluated 3D dosimetry for VBT and concluded that there was no advantage in reporting dose to OARs beyond the initial fraction. In our study as well, CT was performed before the first brachytherapy fraction. Reference Holloway, Macklin, Cormack and Viswanathan13
Although the presence of uterus in itself provides additional protection to small bowel during pelvic radiation therapy, studies investigating intracavitary brachytherapy have shown a significant reduction in bowel dose with distended bladder. Reference Kim, Shen, Lin, Spencer and De Los Santos14,Reference Mahantshetty, Shetty and Majumder15 Although similar studies have also been done in the past on the effect of bladder filling in VBT, a wide variation exists in the technique of bladder filling leading to disparity in a standard protocol. A stringent bladder protocol was followed in studies by Hoskin et al., Guler et al. and Hung et al., who evaluated the effect of bladder volume by saline injection of various volumes such as 35, 70 or 100 mL (Hoskin) and 180 mL (Gular and Hung), using a Foley’s catheter. Reference Hoskin and Vidler16–Reference Guler, Onal and Acibuci18 Whereas, in studies by Stewart et al. and Kobzda et al., patients were asked to drink 32 fluid ounce (948 mL) of water 1 hour prior and 400 mL of water 40 minutes prior to CT, respectively. Reference Stewart, Cormack and Lee19,Reference Kobzda, Cikowska-Wozniak and Michalska20 In our study, patients were asked to drink 1L of water 1 hour prior to CT, which was similar to the protocol by Stewart et al. We found this protocol pragmatic and reproducible in the clinic without causing catheter-related discomfort to the patient.
In the study by Hoskin et al., they found a 57·5% reduction in small bowel volume exposed to the high-dose region. These values were based on 2D measurements and volumetric information of other OARs was not taken into consideration. Stewart et al. reported that bladder distention increased the D2cc of bladder and a significant increase in cylinder-to-bowel distance (0·57–1·16 cm). They also correlated 2D-based maximal bladder and rectal points with the volumetric information and concluded them to acceptable surrogates for D2cc volumetric assessment. Reference Hoskin and Vidler16 Hung et al. demonstrated a 269 cGy decrease (−39·7%) in the small bowel dose (D2cc) with distended bladder protocol. But this was accompanied by a non-significant increase in D2cc of bladder (+5·7%), but a significant decrease in mean dose to the bladder (−36·7%). Reference Hung, Shen and De Los Santos17 Gular et al. reported a significant increase in bladder dose (D2cc) in full bladder and a decrease in the sigmoid and bowel doses, which fell short of statistical significance. They also reported increasing mean cylinder-to-bowel distance (1·69–1·20 cm) similar to Stewart et al. Reference Guler, Onal and Acibuci18,Reference Stewart, Cormack and Lee19
In line with the aforementioned studies, our study with the largest dataset also showed significant benefit with full bladder treatment in reducing the dose (D2cc) to bowel by 20%, but at the cost of higher D2cc to the bladder yet a significant decrease in the mean dose (D50%) to the bladder. In a distended state, the posterior wall of bladder is in proximity to the brachytherapy applicator resulting in the high-dose overlap. In addition to this, the posterolateral wall of the distended bladder bulges into either side of the vaginal vault to form ‘bladder horns’ around the high-dose region which may have contributed to the steep increase in mean dose to the bladder observed in our study in bladder volume more than 300 mL [Figure 3a–d].
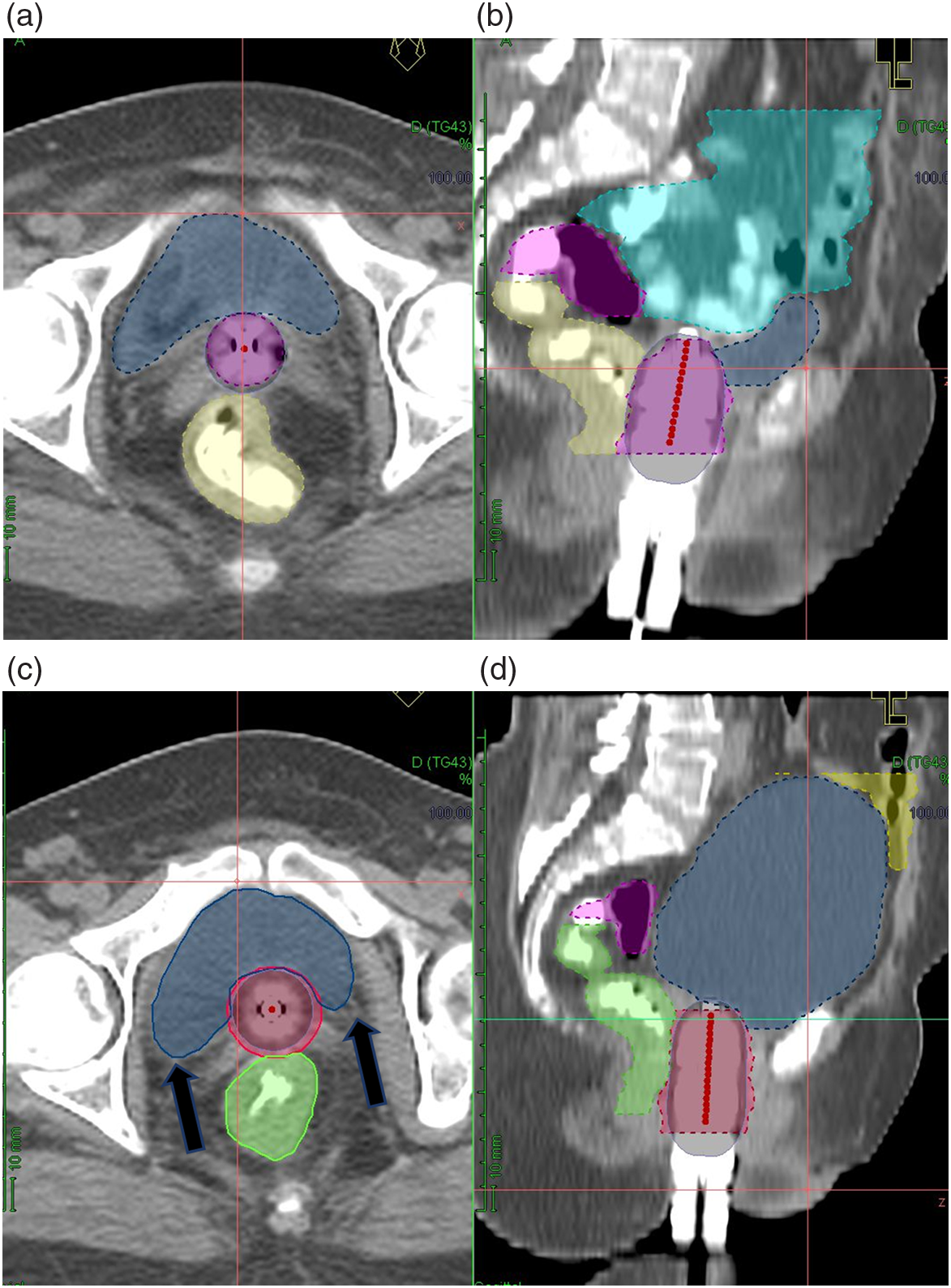
Figure 3. Differences between empty bladder (a—axial, b—sagittal, dark blue—bladder, cyan—bowel, pink—sigmoid) and full bladder (c—axial, d—sagittal, dark blue—bladder, yellow—bowel, pink—sigmoid). Black arrows—Bladder horns.
In our study, the dose to sigmoid did not have a significant reduction (−3·43%, p = 0·085) as compared to a study published by Hollway et al., which showed a 20·3% reduction. This is likely because of interfraction variation in the position of the mobile sigmoid, and its location posterior to the bladder, which makes it less likely to be pushed away from the applicator by a distended bladder.
For better clinical relevance, EQD2 was also reported in our study. Mean cumulative EQD2 (EBRT+VBT) in the full bladder plan for bladder and rectum was found to be within recommended dosimetric limits and a 4 Gy mean EQD2 reduction in D2cc of bowel was observed. Reference Dimopoulos, Petrow and Tanderup7 Treatment with full bladder will be more beneficial in decreasing dose to the small bowel loops, which have a lesser radiation tolerance threshold than bladder. This bowel sparing effect facilitated by a distended bladder must be considered essential especially in patients who receive brachytherapy after EBRT. Despite various reports on dosimetric advantages of bladder filling, a 2019 survey among ABS membership on practice patterns of radiotherapy in post-operative endometrial cancer, bladder filling as a technique to displace the bowel was practised only among 28% of the responders. Reference Martell, Doll and Barnes21 This inertia to change practice may be attributed to the unavailability of robust clinical and toxicity outcome data correlating with the dosimetric benefits.
There were certain limitations in this study. First, as this study was undertaken as a dosimetric study, the clinical implications of our findings could not be demonstrated. The sample population will remain under our clinical follow-up and the clinical correlation will be reported after a considerable follow-up period. Furthermore, normal organ contouring on planning CT was based on RTOG normal organ contouring guidelines, which are not specific for brachytherapy contouring and contouring of OARs were subject to interobserver variation. To minimise variation and for standard volumes, all contouring was done by the principal investigator. A less stringent bladder protocol was adopted for the reproducibility in daily practice and convenience of the patients, resulting in a wide variation in bladder filling among the groups.
Conclusion
Our study demonstrates the dosimetric advantages of 3D CT-based planning for VBT over 2D-based conventional planning. 3D planning helps to decrease dose to critical organs without compromising target volume coverage by individualising the dosimetry according to each patient’s anatomy. This study also illustrated the dosimetric benefit of bladder distension in significantly lowering small bowel dose, and highlights the need for further clinical studies evaluating the acute and late toxicity outcomes.
Acknowledgement
None.
Financial Support
Institutional fluid research grant from Christian Medical College, Vellore, India (IRB Min No. 10488 dated 05.01.2017).
Conflict of Interest
There are no conflicts of interest disclosed by the authors.










