Introduction
Cancer is a major disease that accounts for significant mortality. Radiotherapy as a treatment modality has been used for cancer management for more than 100 years.Reference Jonathan, Christopher, David and Glen 1 External beam radiotherapy has become an integral part of the standard treatment for most malignant conditions. In the treatment of tumours arising in the thoracic and abdominal regions, these are susceptible to physiological motion. This motion can cause inter-fractional and intra-fractional variation in the target location and geometry. Any tumour motion has the potential to negatively impact on the goal of radiation therapy, and depending upon the tumour location, size, shape, dose per fraction and proximity to sensitive structures, the impact of motion can result from trivial to clinically significant consequences.
Tumours in the lung, breast, liver and pancreas are commonly affected by respiratory motion. Tumour and organ motions are triggered by respiratory motion, cardiac motion and gastrointestinal motion. The International Commission on Radiation Units (ICRU) report no 62 recommends an internal margin (IM) around the clinical target volume (CTV) to compensate for tumour movement. 2 In July 2006, the American Association of Physicists in Medicine (AAPM) report no 91 suggests motion mitigation methods such as motion encompassing methods, respiratory gating, breath-hold techniques, forced shallow breathing with abdominal compression and real-time tumour tracking methods.Reference Keall, Mageras and Balter 3 Adopting a suitable motion mitigation technique can reduce the IM applied to the CTV, which can reduce the overall planning target volume (PTV).
The use of a breath-hold technique has the unique advantage of creating an increased lung volume and geometrically separating critical structures from the PTV. In this study, we use a breath-hold technique implemented using the active breath coordinator (ABC) (Elekta Medical Systems, Stockholm, Sweden). In a study by Rosenzweig et al, they reported a relative increase in lung volume by a factor of up to 1·9, with deep inspiration breath-hold technique.Reference Rosenzweig, Hanley and Mah 4 The ABC system has been reported to reduce the dose to heart, lung and improve the setup accuracy.Reference Harriet, Virginia and Albert 5 – Reference Welgemoed, Rogers, McNaught, Cleator, Riddle and Gujral 9
The idea of patient-specific quality assurance (PSQA) is to evaluate the delivery accuracy of the treatment plan by considering the delivery pattern and geometry. In a breath-hold treatment plan, treatment is delivered using multiple treatment beam hold and their pre-treatment quality assurance (PSQA) is often performed in uninterrupted mode. This may not address the impact of beam hold during the actual treatment delivery. The aim of this study is to evaluate the day-to-day variation in the treatment beam hold pattern and quantify its dosimetric impact in breath-hold radiotherapy using fraction-specific post-treatment quality assurance. The variation in the retrospective treatment delivery pattern in terms of both number of interruptions and monitor units (MUs) were accounted in the fraction-specific post-treatment PSQA.
Materials and Methods
A 54-year-old patient diagnosed with chondrosarcoma of right humerus (post-operative), received radiotherapy for lung metastasis. This patient was planned for intensity-modulated radiation therapy (IMRT) combined with moderate deep inspiration breath hold (mDIBH) delivery. The lung function of the patient was fair, and he was conscious and comfortable. The patient was immobilised using a vacuum prepared cushion in supine position. The ABC system is used to train and treat the patient using mDIBH. The patient was given a full explanation of the mDIBH process and the components of the ABC system. The patient underwent breath-hold training for 3 days until he could hold a consistent lung volume. We determined the threshold lung volume, above which he should inhale and the duration for which he was required to hold the breath, in training sessions. The patient was comfortable in holding a 0·9 L threshold volume. A monitor was kept near the patient, where he could observe his own breathing pattern and adjust along with that. Computed tomography (CT) simulation was carried out in mDIBH as well as free breathing condition. Target volumes and organs at risk (OAR) were delineated as per the ICRU 62 recommendations. 2
ABC system
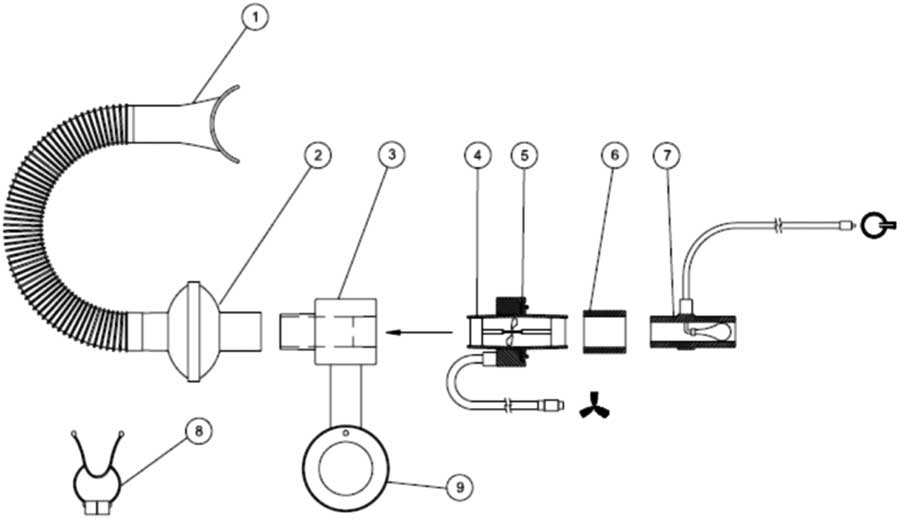
The position, volume and shape of a tumour in the thorax and abdominal region can change with instantaneous lung volume. Reproducing the predefined lung volume helps in reproducing the tumour position with sufficient accuracy. The ABC system is one of the tumour motion mitigating tools discussed in the AAPM report no. 91.Reference Keall, Mageras and Balter 3 This system was developed at the William Beaumont Hospital and commercially marketed by Elekta Inc. The major components of the ABC system are: (i) the patient respiratory system, (ii) cart system and (iii) control computer. The whole system can assist the patient to hold breath at a predefined lung volume. This system can be used for both mDIBH and DIBH. The patient respiratory system consists of (1) mouthpiece, (2) filter kit, (3) mirror support system connection port, (4) transducer turbine, (5) transducer pick-up assembly, (6) transducer/balloon valve coupler, (7) balloon valve, (8) patient nose clip and (9) rotational adjustment pole of mirror support system. These parts are indicated in Figures 1 and 2.

Figure 1 Mouthpiece used in ABC system
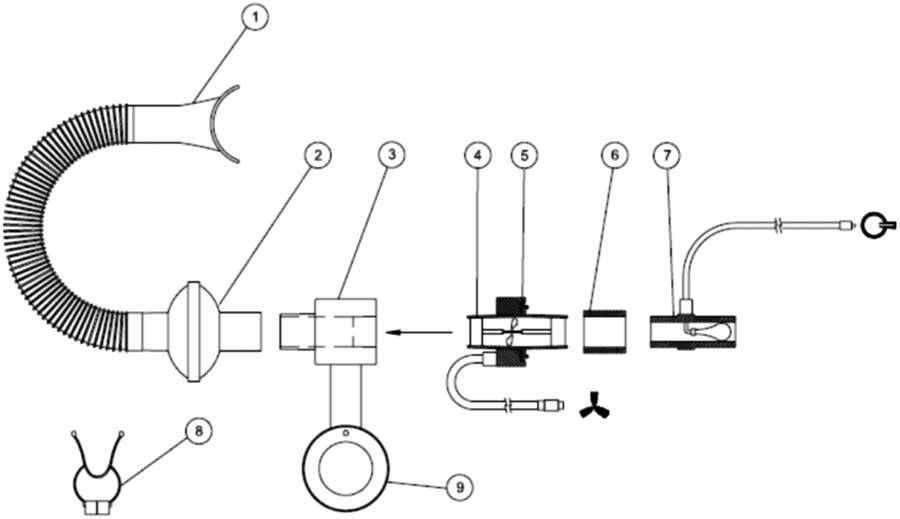
Figure 2 Patient respiratory system and its components
This system has a mouthpiece connected at the patient’s side, and is connected to a flexible breathing tube and a single-use filter kit which is then connected to a transducer. The transducer assembly transmits the number and direction of rotations of the turbine to the control module. This signal is then converted to a volume measurement. A balloon valve is connected to prevent the air flow going beyond the threshold volume set in the system. For safety reasons, the patient is given a patient control switch. The balloon valve is activated only when the patient reaches the threshold volume and can be controlled by both the patient and the operator/therapist. Breathing through the nose is prevented by using a patient nose clip, this allows patient to breath only through the mouthpiece. A mirror support system helps the patient to view the patient feedback monitor. Using this system, the patient is able to see when the operator has activated the system and is ready for the breath hold and also the duration of the breath hold once the breathing curve has entered the active threshold region.
Synergy S linear accelerator
The study carried out on a Synergy S linear accelerator (Elekta Medical Systems, Crawley, UK) capable of triple energy (4, 6 and 15 MV) photon beams. It is equipped with Beam Modulator® MLC (Elekta Medical Systems) having 40 pairs of multi-leaf collimators (MLCs) with 4 mm leaf width projected at the isocentre. The maximum field size achieved by the Beam Modulator® MLC is 16×21 cm. It can deliver conventional, conformal and intensity-modulated beams. It is also equipped with Hexapod 6D couch, which can improve the patient positioning accuracy, especially for stereotactic radiation delivery.
Treatment planning
Treatment planning was carried out in the Monaco Treatment Planning System (Elekta Medical Systems) version 5·0. A total dose of 6250 cGy over 25 treatment fractions was prescribed (250 cGy/fraction). A 6 MV photon beam with nominal dose rate was chosen for treatment planning. Nine coplanar beams were used for step and shoot IMRT planning. The isocentre was placed at the geometric centre of the PTV. Appropriate biological dose objectives/constraints were defined for the target and nearby critical structures. The X-ray voxel Monte-Carlo dose calculation algorithm was used for optimisation and final dose calculation. The dose grid spacing was 3 mm and statistical uncertainty was 1% per calculation. The final treatment plan resulted in 122 segments and 941·59 MUs for a single treatment fraction.
Patient-specific quality assurance
Most of the IMRT delivery systems are based on the use of MLCs. The leaf positioning errors are an inherent property of the IMRT delivery system.Reference Chui, Spirou and LoSasso 10 – Reference LoSasso, Chui and Ling 12 There is a higher complexity of planning, calculation and delivery as compared to three-dimensional conformal radiotherapy, because each IMRT field is composed of multiple small beamlets, and their calculations are based on corrections applied to broad-beam data, and this may not be sufficiently accurate. Additional errors can originate from the dicom export/import process and record and verify system.
Variation in treatment beam hold
Our ABC-based treatment delivery is manually driven. The number of breath holds for a given treatment field depends on the (i) breath hold capacity of the patient; (ii) active communication between the therapist and the patient; (iii) understanding of the ABC system by the patient; (iv) general condition of the patient; (v) skill of the therapist; (vi) total MU of the treatment plan and (vii) dose rate at which the treatment is being delivered. Besides these factors, the patient’s cough and unexpected hiccups in the treatment delivery unit can also modify the number of times the treatment beam is interrupted. Although most of the treatment fractions are delivered in a similar interruption pattern, there is a change in the number of beam holds and their intervals. In this study, we have documented the beam holds and their onset.
Reproducibility and linearity
Reproducibility of the uninterrupted beam was measured using delivery with open beam. This parameter indicates the combined reproducibility of the monitor chamber of the linear accelerator, electrometer and ionisation chamber. The dosimetric setup used to study the effect of the treatment beam hold was used to measure the reproducibility. With this dosimeter setup 100 MU exposed for 10 times and the meter reading is used to calculate coefficient of variation (COV).
The linearity of dose delivery was measured for MUs from 1 to 500 MU for 6 MV photon beam. An ionisation chamber kept in a slab phantom (SP34) was used to collect the charge for different MU settings. The measurement geometry used was 10·4×10·4 cm field size and 100 cm source-to-surface distance. The average of three measurements was used for each MU setting. Since the absolute output is tuned at 100 MU, the meter reading observed was normalised to the reading observed for 100 MU.
Reproducibility of interrupted PSQA dose results
Basic delivery of intensity-modulated treatment beam (step and shoot) is different from the delivery of regular un-modulated open field. Generally, the step and shoot intensity-modulated beam consists of multiple small field apertures, low MU segments and beam turn off between every successive segment. These properties can lead to dose error.Reference Ezzell and Chungbin 13 Each individual treatment beam was delivered repeatedly five times on a slab phantom (SP34) with CC13 ionisation chamber. These meter readings were noted down and their mean, standard deviation and COV were calculated. Reproducibility was then calculated in terms of COV.
Fraction-specific post-treatment quality assurance
The conventional method of PSQA verifies the error in the treatment planning process. Mostly the treatment delivery method remains the same as how it was delivered in the PSQA procedure, except (per beam verification at gantry 0°, VMAT delivered at Gantry 0°, non-coplanar beam delivered in coplanar geometry) few patterns of PSQA delivery. In breath-hold treatment delivery method, beams are not continuously delivered and the level of beam hold may not be the same for all the treatment fractions. This variable can trigger another uncertainty that adds to the systematic and random errors. In this study we attempt to evaluate and quantify the dosimetric error induced by the daily variation in ABC treatment delivery. PSQA was performed with and without beam hold setting for each treatment day following the treatment delivery. The point of beam hold and number of beam holds were adapted from the respective treatment session. Measurement results of with and without beam hold were compared to quantify the impact of beam hold.
Results
Variation in treatment beam hold
(i) Variation in number of beam hold
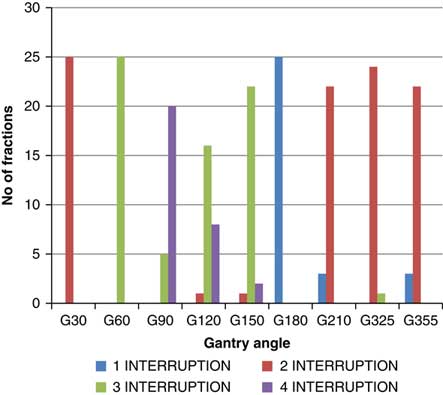
Though the total number of planned MU for a given treatment beam remains the same, the number of beam holds changes with the daily fraction delivered. The variation is different for each beam (Figure 3). Out of nine treatment beams, three beams [Gantry (G) angle G30, G60 and G180] had no change in the number of hold, four beams (G90, G210, G325 and G355) had two different numbers of hold and two beams (G120, G150) had three different numbers of hold. The beam G120 was delivered with two, three and four times beam hold for 4, 64 and 32% of the treatment fractions, respectively. The beam G150 was delivered with two, three and four times beam hold for 4, 88 and 8% of the treatment fractions, respectively. For beam G90, 20% of the treatment fractions were delivered with three times beam hold and 80% of the treatment fractions delivered were with four times beam hold. The beams G210 and G355 were delivered with one and two times beam hold for 12 and 88% of the treatment fractions, respectively. The beam G325 was delivered with two and three times beam hold for 96 and 4% of the treatment fractions, respectively. The beams G30, G60 and G180 were delivered with two, three and one times beam hold, respectively, for the entire treatment course.
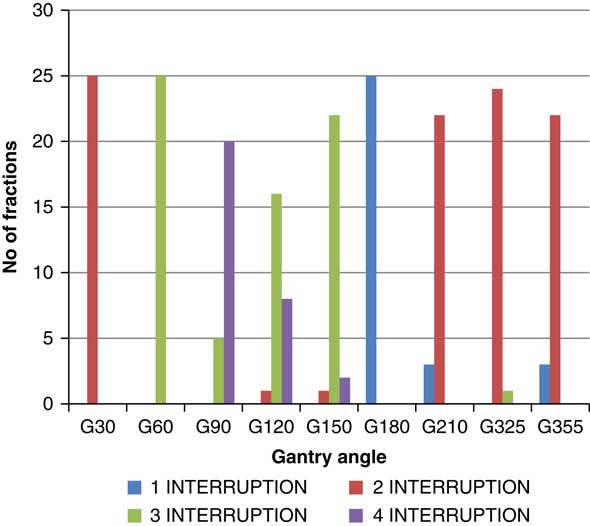
Figure 3 Variation in number of beam hold over entire treatment course
(ii) Variation in MU delivered at the point of beam hold
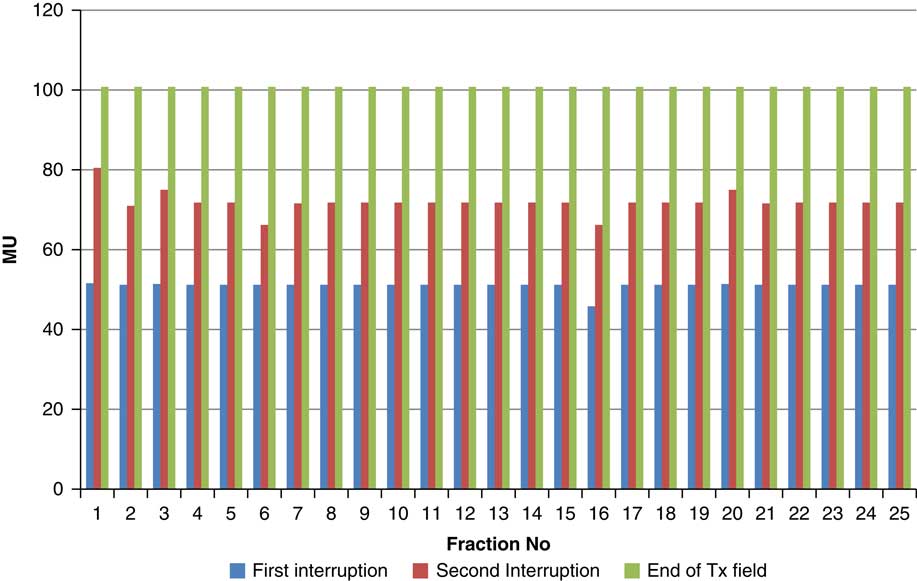
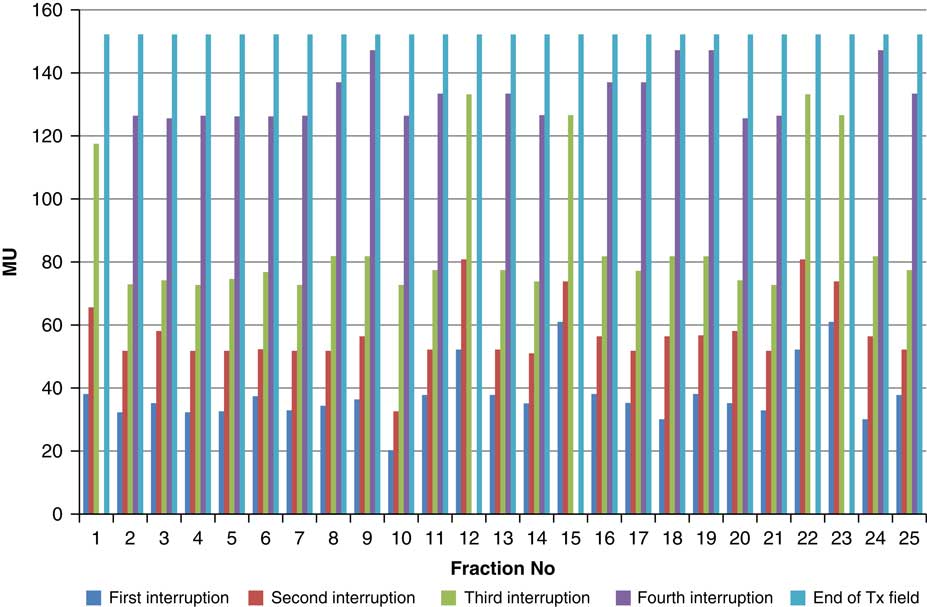
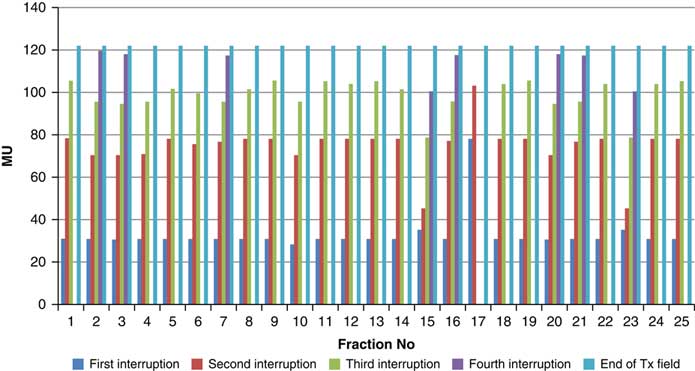
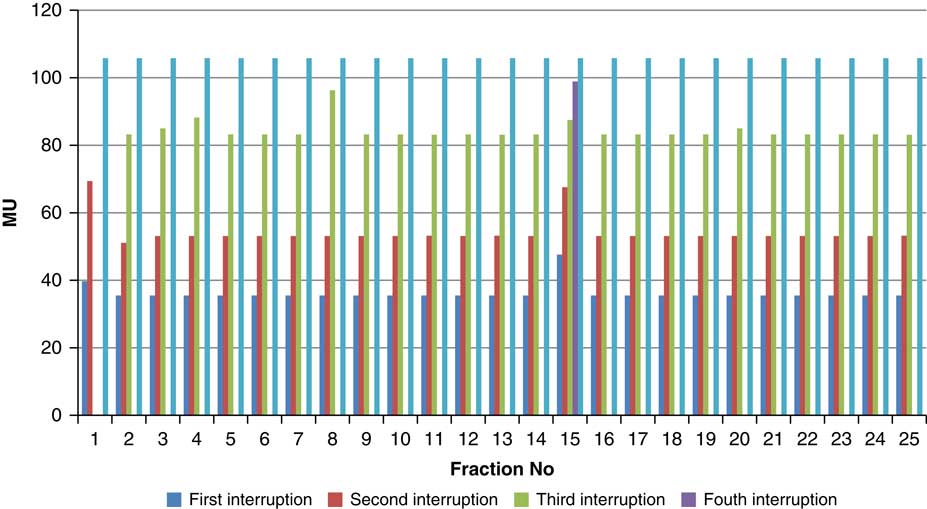
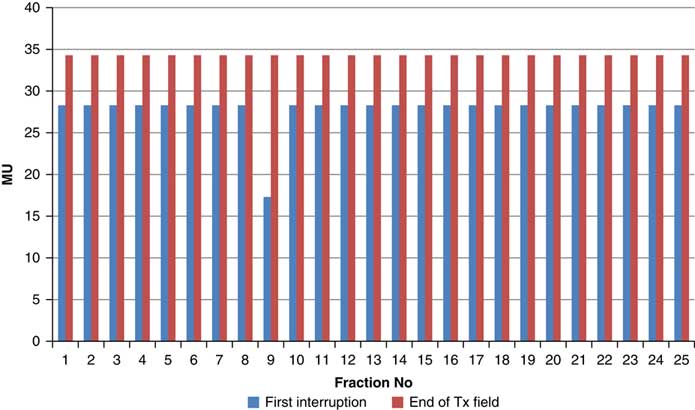
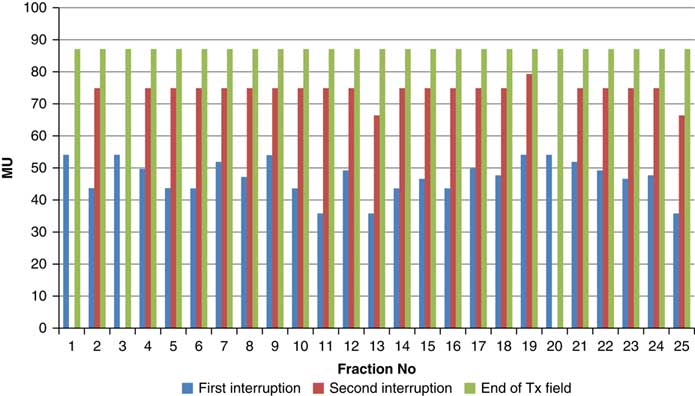
Throughout treatment, the total number of planned MU for a given treatment beam remained the same and the number of MU delivered at the point of beam hold changes with daily fraction (Figures 4–12). For the beam G30, the first beam hold occurred at 51·2 MU except for one fraction and the second beam hold occurred between 66·2 and 80·5 MU.
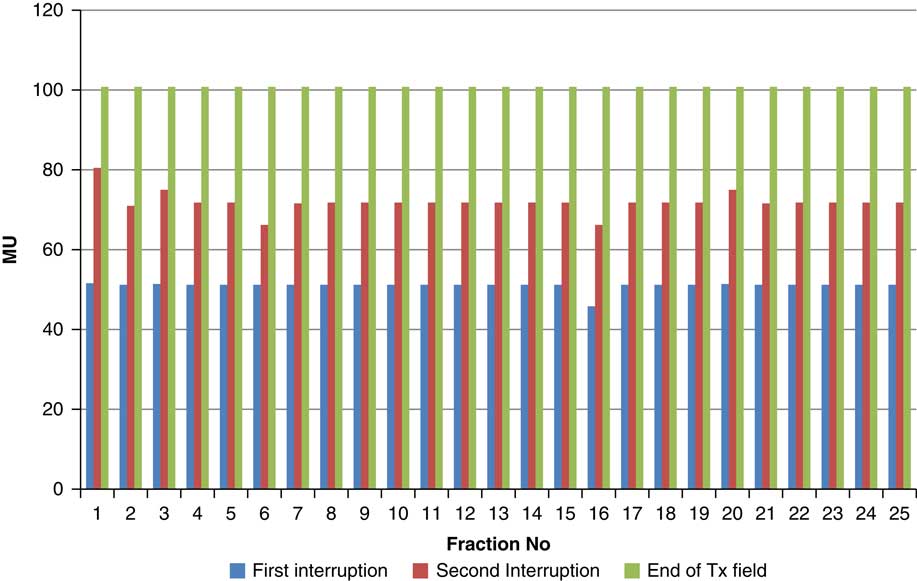
Figure 4 Variation in MU delivered at the point of beam hold for beam G30
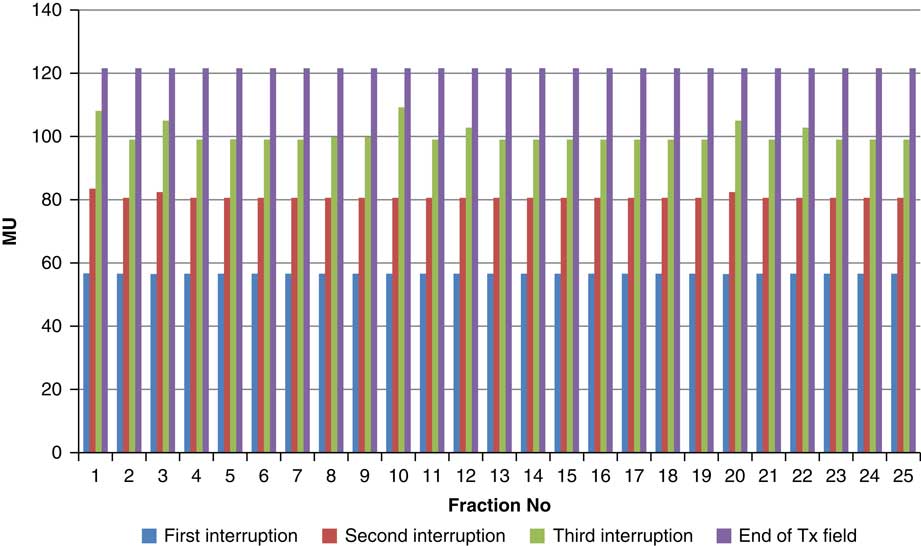
Figure 5 Variation in MU delivered at the point of beam hold for beam G60
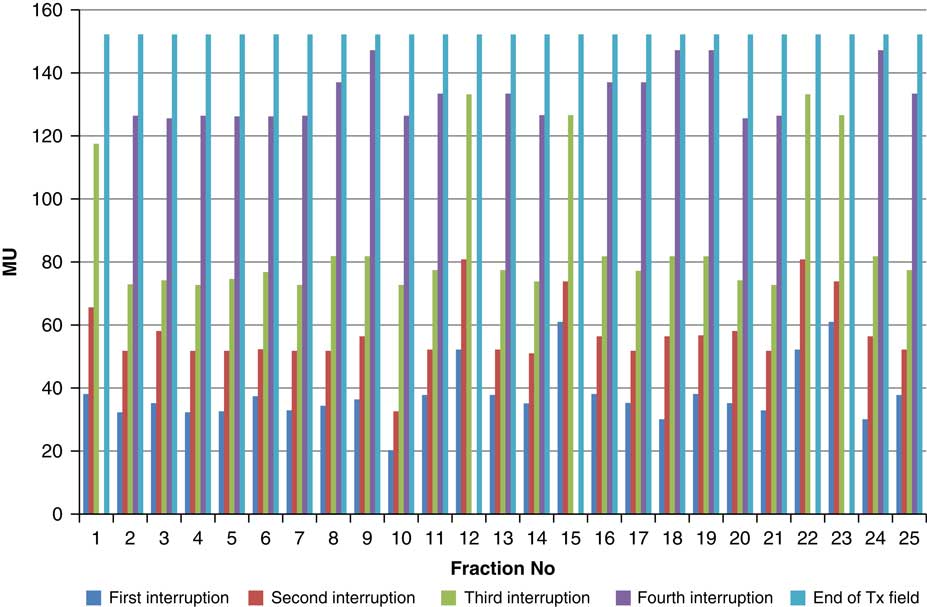
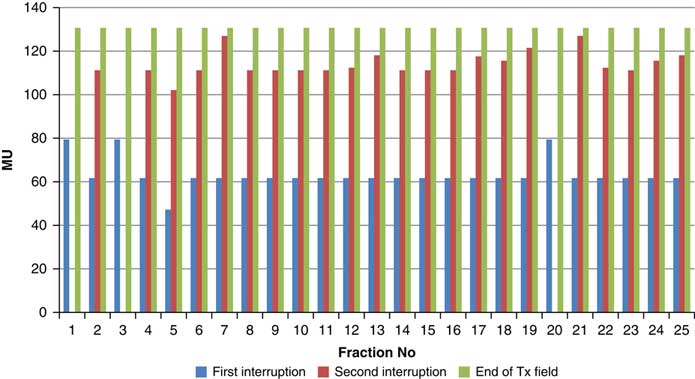
Figure 6 Variation in MU delivered at the point of beam hold for beam G90
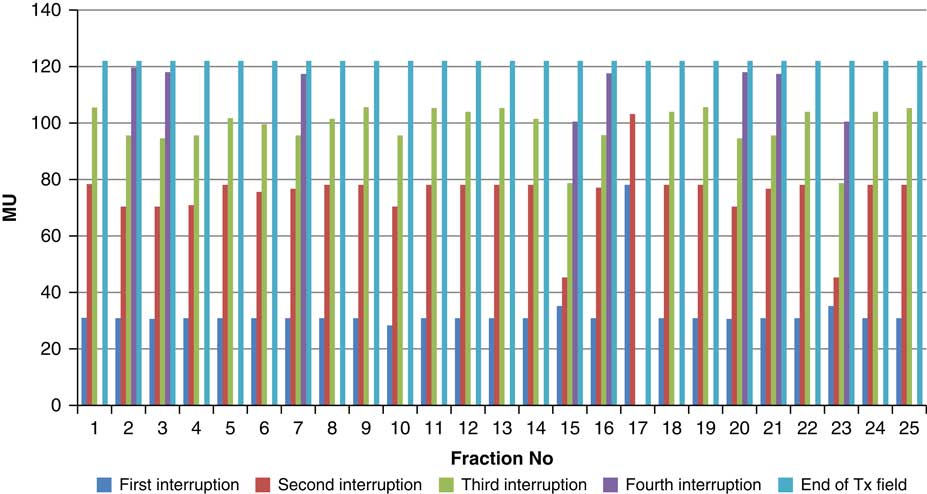
Figure 7 Variation in MU delivered at the point of beam hold for beam G120
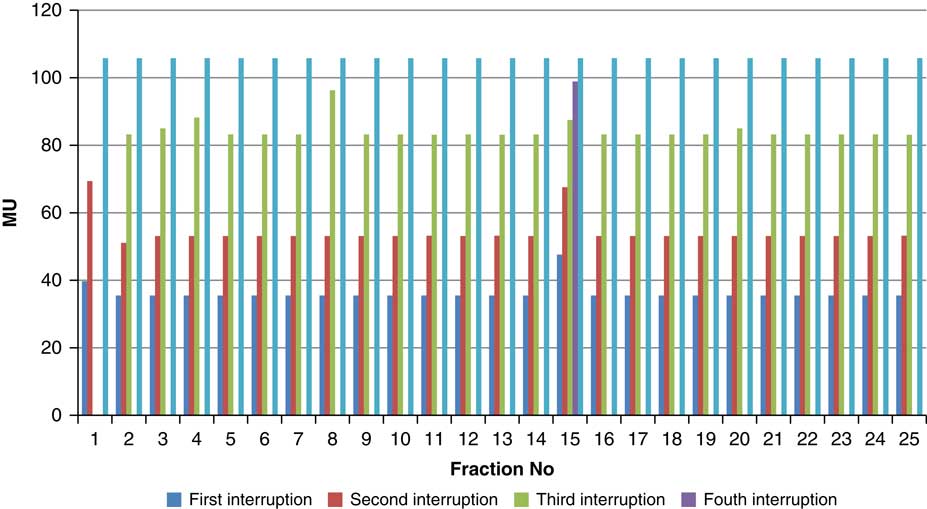
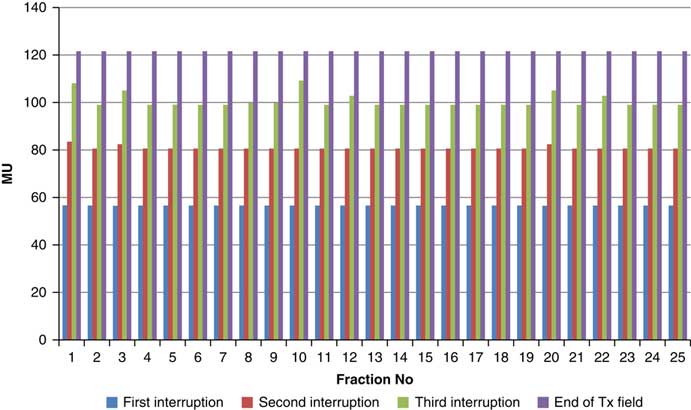
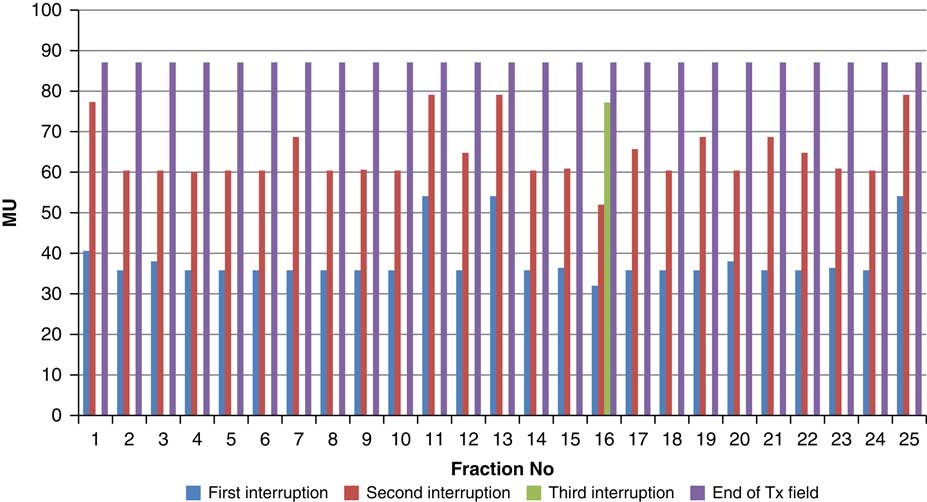
Figure 8 Variation in MU delivered at the point of beam hold for beam G150
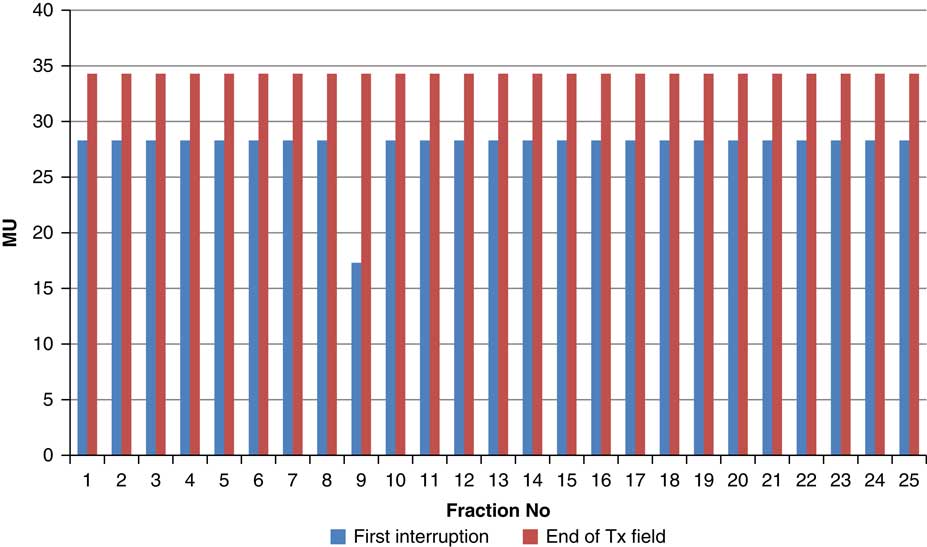
Figure 9 Variation in MU delivered at the point of beam hold for beam G180
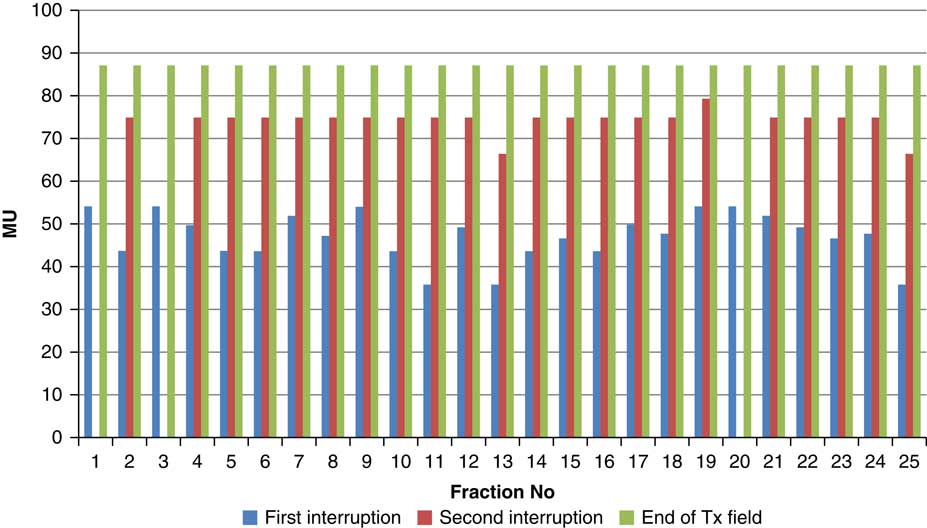
Figure 10 Variation in MU delivered at the point of beam hold for beam G210
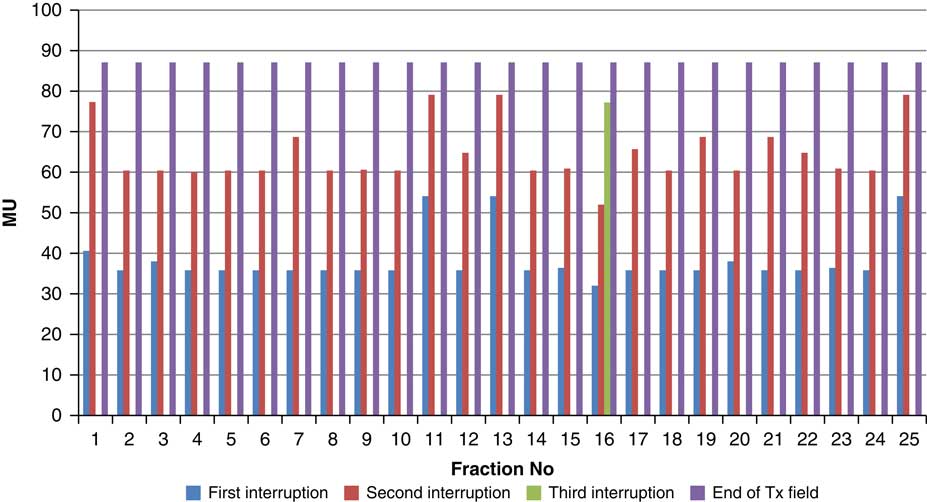
Figure 11 Variation in MU delivered at the point of beam hold for beam G325
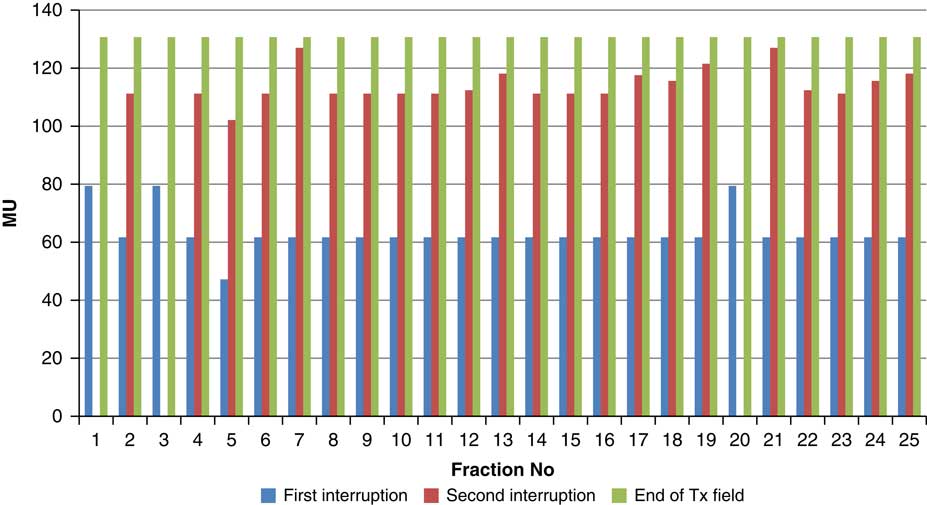
Figure 12 Variation in MU delivered at the point of beam hold for beam G355
For the beam G60, the first beam hold occurred at 56·6 MU for all fractions and for second and third beam holds occurred between 80·6–83·5 and 99–109·2 MU, respectively. For the beam G90 first, second, third and fourth beam holds occurred between 20·3–61, 32·6–80·8, 72·7–133·2 and 125·6–147·2 MU, respectively. The beam G120 had first, second, third and fourth beam holds between 28·3–78·1, 45·3–103·2, 78·7–105·6 and 100·5–119·6 MU, respectively. The beam G150 had first, second and third beam holds between 35·5–47·6, 51·1–69·4 and 83·1–96·3 MU. The beam G180 had only one beam hold that occurred between 17·3 and 28·3 MU. For the beam G210 first and second beam holds occurred between 35·8–54·1 and 66·4–79·3 MU, respectively. The beam G325 had first and second beam holds between 32–54·1 and 52–79·1 MU, respectively. The beam G355 had two beam holds that occurred between 47·2–79·4 and 102·1–127 MU, respectively.
Reproducibility
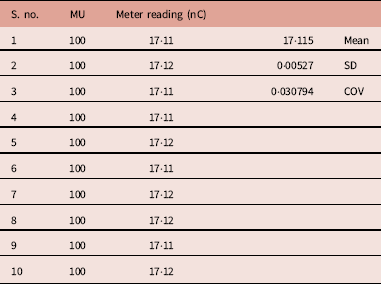
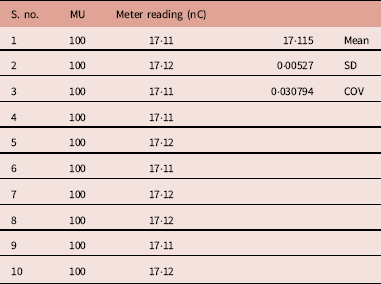
A set of repeated measurement of unmodulated photon beam showed minor variations in the meter reading (Table 1). The mean, standard deviation and COV were 17·115(nC), 0·00527 and 0·0307, respectively. In terms of COV the reproducibility is 0·0307, which is acceptable in clinical dosimetry.
Table 1 Meter reading observed for 10 repeated measurements
Linearity
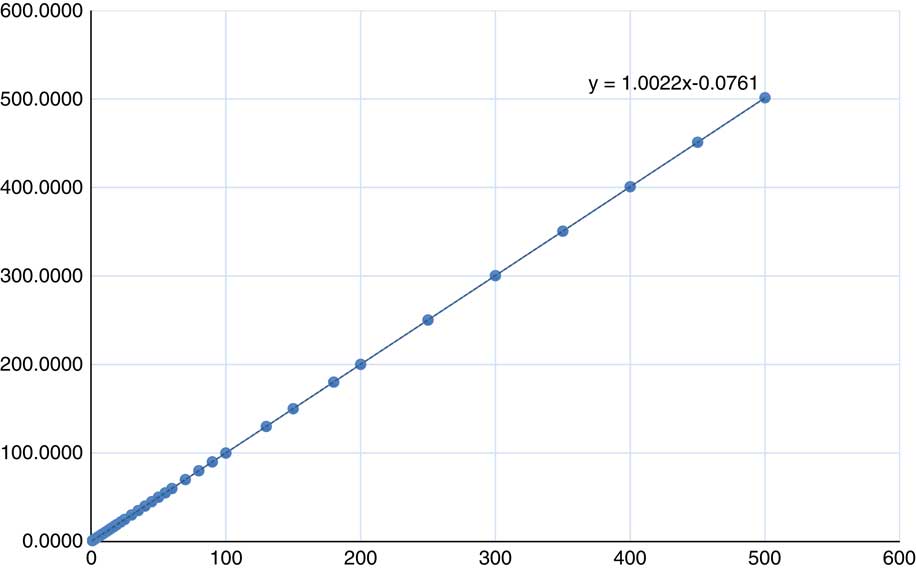
MU linearity is measured as the response of the dosimeter corresponding to the dose delivered. This response is normalised to 100 MU. For exposure <5 MU the variation in the expected meter reading (dose) is from 0·75 to 3·59%. As the MU increases from 5 to 10 MU the variation in the expected meter reading reduces to 0·1% for 10 MU. Furthermore, the variation in the expected meter reading was <0·1% for the entire range from 13 to 500 MU except in a few data, which varied up to 0·28%. The overall slope of the delivered MU versus calculated MU is 1·0022 and the end error of the trendline is 0·0761 (Figure 13).
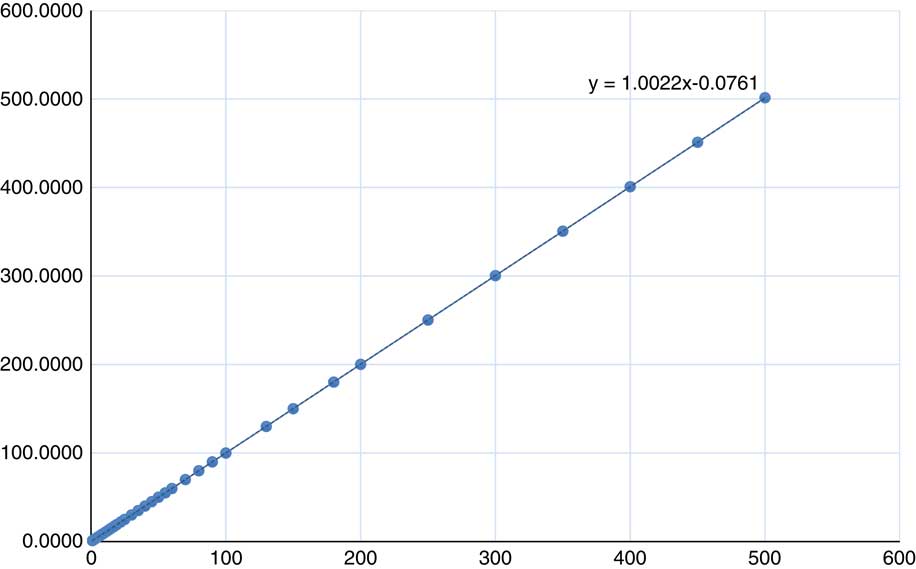
Figure 13 MU set in the treatment console plotted against the MU calculated (from measurement)
Reproducibility of interrupted PSQA/intensity-modulated treatment beam
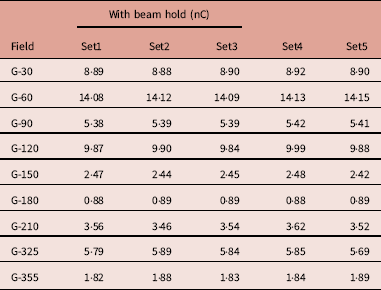
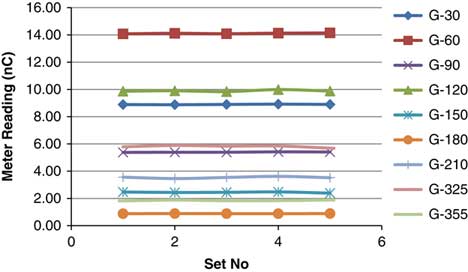
Table 2 shows five different sets of measurements taken for each beam with holds taken from day 1. Repeated measurement shows variation in the meter reading. The standard deviation was observed from 0·0036 to 0·0769 and the average COV was 0·8594 (0·1666–1·6816) (Figure 14).
Table 2 Meter reading observed for 5 repeated measurements for treatment field
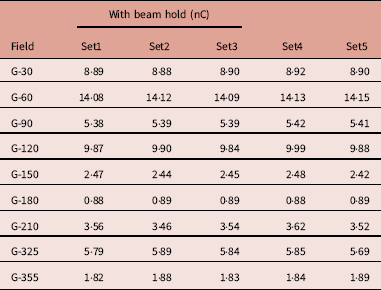
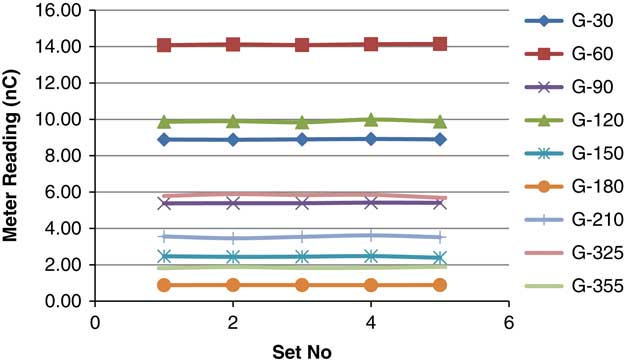
Figure 14 Reproducibility of PSQA for individual beam.
Fraction-specific post-treatment quality assurance
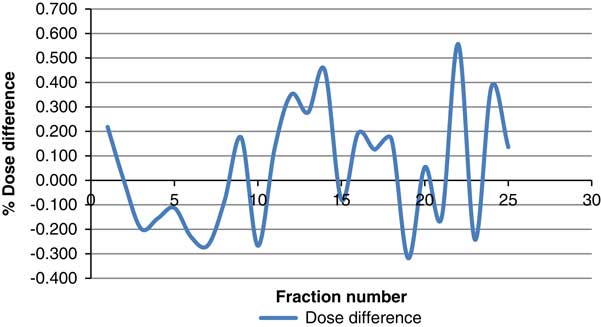
The difference between PSQA results of those measured with and without beam hold was calculated. The cumulative dose contributed by all nine beams was used to calculate the difference. The difference spanned from 0·557 to −0·318%. The average difference over the 25 measurements was 0·044% with standard deviation of 0·248. Figure 15 shows the dose difference between measurements with and without beam hold.
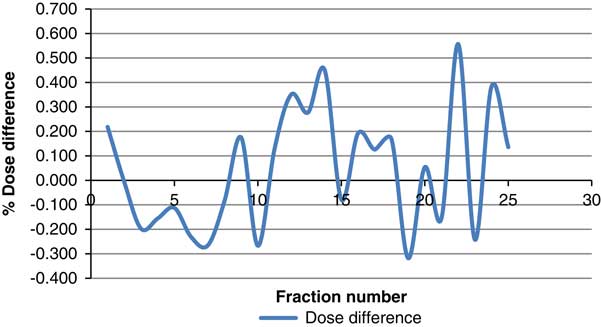
Figure 15 Dose difference between measurements with and without beam hold
Discussion
The use of mDIBH-based IMRT treatment delivery has the unique advantage of increased lung volume and geometrically separated critical structures. It increases the geometrical distance between target volume and the OAR, which gives more freedom in using beam orientation and number of beams. This advantage makes the mDIBH technique score over other motion management techniques. In addition to reducing dose to the lung, many authors reported the reduction in cardiac toxicity.Reference Bruzzaniti, Abate and Pinnarò 14 , Reference Bergom, Currey, Desai, Tai and Jonathan 15
Seco et alReference Seco, Sharp, Turcotte, Gierga, Bortfeld and Paganetti 16 studied the impact of organ motion on IMRT treatments with fewer MU segments. They reported MUs below 10–15 can induce daily dose variation up to 35%. In our study the minimum MU per segment is set to 15 MU in MLC sequencing criteria. This limit is set to have a reasonable number of segments, total MUs and to reduce the dose error induced by low MU segments. The minimum MU set falls under a safe zone found by Seco et al.Reference Seco, Sharp, Turcotte, Gierga, Bortfeld and Paganetti 16
Step and shoot IMRT delivery inherently has beam hold between every MLC segment. In addition, using ABC contributes to additional beam hold in the treatment delivery. The actual number of beam toggling between ON and OFF condition is more than that using manual beam holds. It is also noted that none of the treatment times were <15 MU due to beam hold. The change in dose due to beam hold observed in simple open field is not similar to the pattern observed in the intensity-modulated treatment field. This can be attributed to the complex shape of the beam segment, possible partial irradiation of the detector and the reproducibility of dosimetry system to the intensity-modulated treatment field.
As in the ICRU 50, the prescription dose within the target should be anywhere between 95 and 107%. 17 IROC (formerly RPC) set the acceptance criteria for lung phantom irradiation as the delivered dose be ±5% of the planned dose and the distance to agreement be ±5 mm.Reference Foster, Speiser and Solberg 18 The pre-treatment PSQA results shows 1·79% deviation from the prescribed dose. The additional dosimetric error induced in the beam hold process is found to be less; however, this difference is over and above the pre-treatment PSQA, random error.
Conclusion
In this study, we have studied the day-to-day variation in beam hold pattern of breath-hold radiotherapy delivery and its impact on the dose delivery accuracy using fraction-specific post-treatment quality assurance. Patient comfort with the ABC system and responsiveness to the therapist communication helps to maintain consistent breathing pattern, and in turn, a consistent treatment delivery pattern. The magnitude of dosimetric error found in PSQA is much less than the acceptable limits recommended by IROC. The dosimetric error induced by the beam hold is over and above the dose difference observed in conventional PSQA. The clinical user should study the treatment beam characteristics over the clinical range of MU and in addition, evaluate the change in dose output due to beam hold if the expected number of beam hold is more.
Acknowledgement
None.
Financial support
Nil.