Introduction
Squamous cell carcinoma (SCC) is the most common type of cancer arising in the upper aerodigestive tract. SCC of the head and neck (SCCHN) is a serious public health problem, especially in developing countries, due to its high incidence and prevalence and is a leading cause of mortality.Reference Jemal, Bray, Center, Ferlay, Ward and Forman1 In 2018, there were more than 830,000 new cases (the tenth most common cancer) and more than 430,000 deaths due to head and neck cancer worldwide (the sixth most common cause of cancer mortality),Reference Bray, Ferlay, Soerjomataram, Siegel, Torre and Jemal2 with the highest incidence in South and Southeast Asia.Reference Mehanna, Paleri, West and Nutting3,Reference Gupta, Johnson and Kumar4
The well-known main risk factors are tobacco and alcohol consumption,Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5–Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7 but there are other specific risk factors such as betel nut chewing for oral cavity cancer,Reference Reichart, Philipsen, Mohr, Geerlings and Srisuwan8–Reference Liao, Wallace and Lee10 and human papillomavirus infection for oropharyngeal cancer.Reference Ang, Harris and Wheeler11–Reference D’Souza, Anantharaman and Gheit13 Although SCC is histologically similar, the specific site of the primary cancer can affect the outcome of treatment due to the dissimilar risk of metastasis to the cervical regional lymphatic channel and haematologic dissemination.Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Li, Di, Shang, Zhou, Cheng and He14–Reference Cadoni, Giraldi and Petrelli18
The 5-year overall survival (OS) rate of SCCHN at 24–65% differs among continents and even within the same country and has been linked to the prevalence of risk factors.Reference Jemal, Bray, Center, Ferlay, Ward and Forman1,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15–Reference Pulte and Brenner20 In general, the options of curative treatment at an early stage of the cancer are either surgery or radical radiotherapy, which have resulted in similar oncological outcomes.Reference Lefebvre, Coche-Dequeant, Buisset, Mirabel, Van and Prevost21,22 There are many options for the treatment of locally advanced stage cancer, such as surgery with postoperative radiotherapy, concurrent chemoradiotherapy, induction chemotherapy followed by concurrent chemoradiation or a combination of radiotherapy with targeted therapy.Reference Li, Di, Shang, Zhou, Cheng and He14,Reference Lefebvre, Coche-Dequeant, Buisset, Mirabel, Van and Prevost21–Reference Moskovitz, Moy and Ferris23 Most patients present with locoregionally advanced disease and already have diminishing health, thus making them unsuitable for intensive curative treatment.Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15–Reference Cadoni, Giraldi and Petrelli18,Reference Prakash Saxena, Unnikrishnan, Rathi, Kotian and Reshmi24–Reference Chitapanarux, Traisathit and Komolmalai29
In Thailand, SCCHN is a common type of cancer predominantly affecting males.Reference Pruegsanusak, Peeravut and Leelamanit15 According to the National Cancer Registry, primary cancer of oral cavity is the most common SCCHN followed by the larynx, oropharynx and hypopharynx.Reference Kowalski and Carvalho30 From 2010 to 2012, the most common newly diagnosed SCCHN cancer in northern Thailand was in the oral cavity with an age-standardised incidence rate per 100,000 population of 4·1 for males and 3·2 for females. Compared to the previous decade, the incidence is not declining,Reference Khuhaprema, Srivatanakul, Attasara, Sriplung, Wiangnon and Sumitsawan31,Reference Imsamran, Chaiwerawattana and Wiangnon32 and survival rates are not improving.Reference Li, Di, Shang, Zhou, Cheng and He14,Reference Moskovitz, Moy and Ferris23 A previous study of SCCHN patients treated at Songklanagarind Hospital, southern Thailand, reported 5-year OS rates for the whole cohort, oral cavity, oropharynx, hypopharynx and larynx of 24·1, 25·91, 19·2, 13·4 and 38·0%, respectively.Reference Pruegsanusak, Peeravut and Leelamanit15 Meanwhile, the previous study in northern Thailand reported that the OS rate of oral cavity SCCHN patients was 17%.Reference Chitapanarux, Traisathit and Komolmalai29 These figures are evidence that the incidence of and mortality due to SCCHN can differ significantly within the same country and have been linked to dissimilar risk factors.Reference Jemal, Bray, Center, Ferlay, Ward and Forman1,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15–Reference Pulte and Brenner20
This study aims to evaluate the survival outcomes by gender, primary cancer site and stage, and age at diagnosis of all patients with SCCHN at the Maharaj Nakorn Chiang Mai Hospital, regardless of treatment approach. The result would be useful for people to be aware of avoiding risk factors and prioritising screening programmes. Moreover, it reflects on the importance of improving management as well as prevention, screening and monitoring programme in northern region of Thailand.
Materials and Methods
Data collection
This was a retrospective observational study of new SCCHN patients who had been diagnosed at Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, Chiang Mai, Thailand between 2007 and 2014. Demographics and clinical data were collected on the date of diagnosis (baseline) from the Chiang Mai Cancer Registry, which is a tertiary care centre in northern Thailand. The end of follow-up was on 31st December 2018. The OS rate was calculated from the date of diagnosis of the index primary tumours to the date of death from any cause. Censored observations were cases that lose contact at mid-study or cases that did not experience death before the end of the study. Those observations were censored at the date of loss to follow-up or at the end of the study period.
Tumour sites were classified according to the International Classification of Diseases version 10 (ICD-10) as oral cavity (C01–C06), oropharyngeal (C10), laryngeal (C32) and hypopharyngeal (C13).33 Staging of the cancer (I–IV) was classified using the American Joint Committee on Cancer guidelines, 7th edition.Reference Edge, Byrd, Compton and Friz34 This study separated metastasis stage from locally advanced stage because treatments used were different and difference survival rates were expected. Early stage cancer was defined as stages I and II, locally advanced stage was defined as III to IVB and metastasis stage was defined as IVC.
Statistical analyses
Descriptive characteristics are presented as medians and interquartile ranges (IQRs) for continuous variables and as frequencies and percentages for categorical variables. Fisher’s exact test was used to compare the characteristics of the tumour site groups. The Kaplan–Meier method was used to estimate survival rates, and the log-rank test was used to compare the survival function. The Cox proportional hazard model was applied to assess the independent prognostic factors. Variables with p-value <0·25 in the univariate analysis were included to the multivariate analysis,Reference Mickey and Greenland35 while those with p-value <0·05 were considered statistically significant. All statistical analyses were performed using Stata version 16.
Results
Of 2,095 patients with newly diagnosed as SCCHN, oral cavity cancer was the most common among the various anatomical sites (53·3%). The baseline characteristics by tumour site are reported in Table 1. The median age of the patients was 61 years (IQR: 52–72). Approximately 50% of the patients were elderly, and those with laryngeal cancer had relative older presentations than other sites. The highest proportion of advanced stage (III–IVC) cancer was found in patients with hypopharyngeal tumours (89·1%) compared to other tumour sites.
Table 1. Baseline characteristics

a p-values from the Fisher exact test.
Median follow-up duration was 1·4 years (IQR: 0·6–7·6). There was a total of 1,445 deaths during follow-up, the majority of which (80·5%) occurred in the first 2 years after tumour diagnosis. The highest mortality rate was found in patients with hypopharyngeal cancer (84·7%), followed by oral cavity, laryngeal and oropharyngeal cancers (70·6, 65·4 and 63·2%, respectively). The 5- and 10-year OS rates for all patients were 30·1% [95% confidence interval (CI): 28·0–32·2%] and 22·8% (95% CI: 20·7–25·1%), respectively. The OS rate was not statistically different between gender (p-value = 0·254), while the survival curves declined in an orderly fashion from younger to older patients (log-rank test, p-value < 0·001).
Figure 1 shows the OS rate uniform decline from high to low cancer stage (p-value <0·001). The OS rate was significantly different by tumour site (p-value <0·001). Patients with hypopharyngeal cancer had a statistically significant lowest survival rate compared to patients who had other sites of SCCHN [5-year OS rate was 13·4% (95% CI: 8·6–19·4%) and 10-year OS rate was 9·7% (95% CI: 5·4–15·4%)]. Meanwhile, patients with oropharyngeal cancer had the highest 5- and 10-year OS rates (36·3% with 95% CI: 31·2–41·5% and 29·8% with 95% CI: 24·4–35·3%, respectively), followed by laryngeal (35·0% with 95% CI: 30·1–39·9% and 24·4% with 95% CI: 19·3–29·9%, respectively) and oral cavity (29·0% with 95% CI: 26·1–31·9% and 22·1% with 95% CI:19·2–25·2%, respectively) (Figure 2). In stratified analysis of the survival time by cancer stage, there were significant differences in the 5- and 10-year OS rates for any cancer site in both the early stage and the locally advanced stage (Table 2); the results showed the same survival pattern when the stages were combined. Moreover, patients with hypopharyngeal cancer had the (statistically significant) lowest survival rate and patients with oropharyngeal cancer had the highest. In contrast, for the early stage, the 10-year OS rate was highest in patients with oral cavity cancer, followed by oropharyngeal and laryngeal cancers (p-value =0·014). For the metastasis stage, patients with hypopharyngeal and laryngeal cancers died within the first year after diagnosis, while three patients with oral cavity and oropharyngeal cancer survived after 5 years.
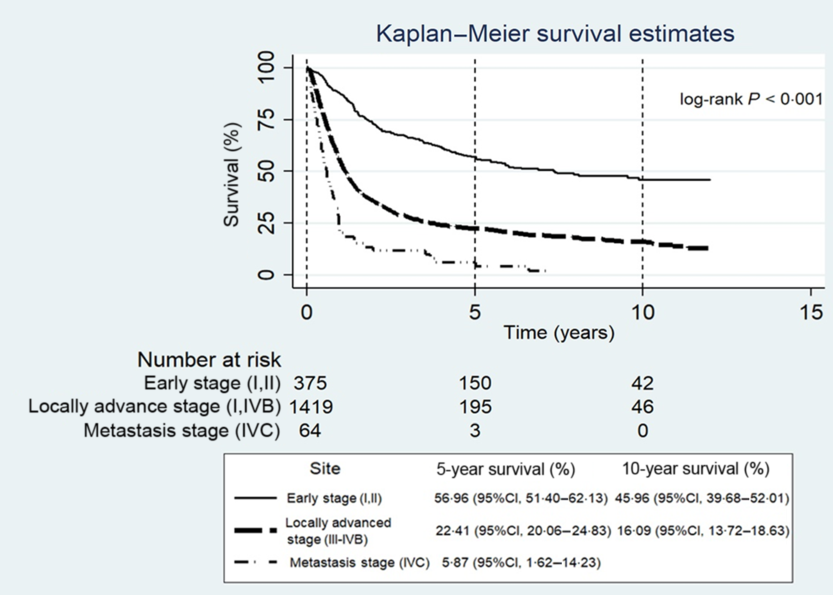
Figure 1. Kaplan–Meier estimates of overall survival rate by cancer stage.

Figure 2. Kaplan–Meier estimates of overall survival rate by tumour site.
Table 2. Kaplan–Meier estimates of stage group survival by site analysis

a There were 236 patients with an unknown cancer stage.
b Not available, because the patients could have died during the calculated year or survived at the end of the study.
Abbreviation: CI, confidence interval.
Table 3 summarises the results of the univariate and multivariate Cox proportional hazard regression analysis of four potential prognostic factors of SCCHN mortality. In the univariate analysis, age, tumour site and cancer stage were significantly associated with the risk of mortality, while in the multivariate analysis, age [adjusted hazard ratio (aHR): 1·7, 95% CI: 1·5–1·9 for age 60–69 years and aHR: 1·9, 95% CI: 1·7–2·2 for age >69 years compared to age <60 years; p-value <0·001], tumour site (aHR: 1·4, 95% CI: 1·2–1·8 for hypopharyngeal and aHR: 1·3, 95% CI: 1·1–1·5 for oral cavity cancer compared to oropharyngeal cancer; p-value <0·001) and cancer stage (aHR: 2·8, 95% CI: 2·5–3·5 for the locally advanced stage and aHR:5·1, 95% CI: 3·8–3·5 for the metastasis stage compared to early stage; p-value <0·001) were also independently associated with a higher risk of mortality.
Table 3. Univariate and multivariate cox proportional hazard regression analysis
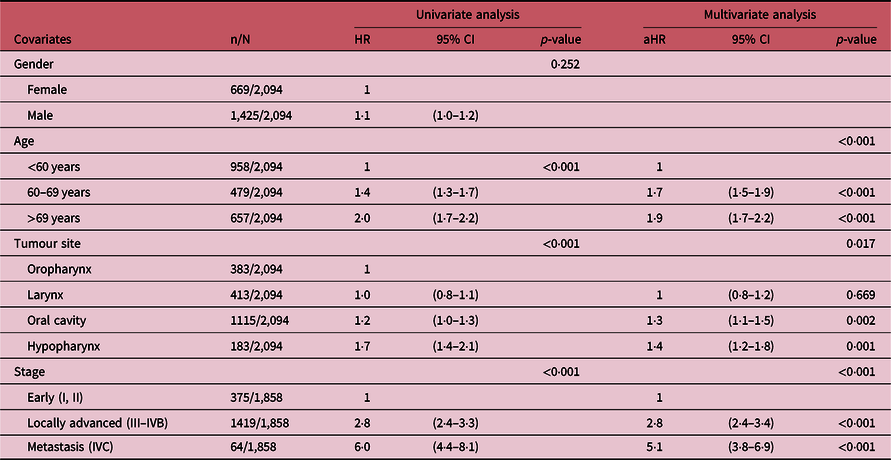
Abbreviations: HR, hazard ratio; aHR, .adjusted hazard ratio; CI.confidence interval.
Discussion
In this retrospective study of 2,095 new cases of SCCHN, oral cavity cancer was the most common among the various anatomical sites. The results show a higher proportion of locally advanced stage (67·7%) than early stage. Two-thirds of SCCHN patients had died by the end of the present study. The 5-year OS rate of SCCHN patients in this study was slightly higher than in Southern Thailand,Reference Pruegsanusak, Peeravut and Leelamanit15 but it was very much lower compared to the United States and Europe, probably because of higher prevalence of advanced stage at diagnosis of patients in this study.Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Abrahão, Anantharaman and Gaborieau16–Reference Cadoni, Giraldi and Petrelli18,Reference Pulte and Brenner20 Moreover, most deaths occurred within the first 2 years after diagnosis.
This study revealed that the OS rates were significantly different by site, with oropharyngeal cancers having the highest 5-year OS rate, followed by laryngeal, oral cavity and hypopharyngeal. It should be noted that the 5-year OS rate of oral cavity cancer was almost twice as high as reported in Malaysian patients.Reference Razak, Saddki, Naing and Abdullah26 In this study, hypopharyngeal cancer was associated with the highest risk of mortality compared to other tumour sites, which might be due to its known aggressive behaviour and no specific early symptoms.Reference Gatta, Botta and Sánchez36,Reference Milisavljevic, Stankovic, Zivic, Popovic and Radovanović37 Thus, it is not surprising that the highest proportion of patients with an advanced stage cancer was in this category in this study.
When focusing on the locally advanced stage, the 5-year OS rate in the study in India was much higher for oral cavity (46% versus 19%) and laryngeal (53% versus 29%) cancer compared to this study.Reference Nandakumar, Rath and Kataki28 This was probably due to the fact that patients with incomplete treatment and those given only palliative/supportive care were excluded, while this study incorporated all of the patients diagnosed during the study period in northern Thailand, including patients who had completed treatment and those who had refused treatment, received incomplete treatment or were receiving palliative treatment.
Consistent with other studies, it was found an association between tumour stage at diagnosis and the risk of mortality.Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15–Reference Cadoni, Giraldi and Petrelli18,Reference Pulte and Brenner20,Reference Prakash Saxena, Unnikrishnan, Rathi, Kotian and Reshmi24,Reference Yeole, Ramanakumar and Sankaranarayanan25 Patients with locally advanced stage and metastasis stage cancer had an approximately 3- and 5-fold higher risk, respectively, compared with early stage at diagnosis. These findings add to the growing body of evidence that advanced stages of SCCHN at the time of diagnosis are associated with a shorter survival time.Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15–Reference Cadoni, Giraldi and Petrelli18,Reference Pulte and Brenner20,Reference Prakash Saxena, Unnikrishnan, Rathi, Kotian and Reshmi24,Reference Yeole, Ramanakumar and Sankaranarayanan25 Unfortunately, a large proportion of patients with SCCHN are often not diagnosed until their disease has reached an advanced stage, requiring multimodal and costly treatment that often leads to severe physical and psychological disabilities.Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5,Reference Short, Vasey and Tunceli38
The results were found that patients aged ≥60 years were significantly associated with a 2-fold higher risk of mortality compared to younger patients, which was consistent with previous studies in southern Thailand, the USA, Canada and Scotland.Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15,Reference Tiwana, Wu, Hay, Wong, Cheung and Olson17,Reference Pulte and Brenner20 Some studies have suggested that the poor outcome in older patients could be related to co-morbidity, treatment-related morbidity and the higher prevalence of debilitating illnesses associated with ageing.Reference Prakash Saxena, Unnikrishnan, Rathi, Kotian and Reshmi24,Reference Chen, Matson, Roberts and Goepfert39,Reference de Cássia Braga Ribeiro, Kowalski and Latorre Mdo40
In addition, it was found that the majority of SCCHN occurs in males than females, which is consistent with previous studies,Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5,Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15–Reference Cadoni, Giraldi and Petrelli18,Reference Pulte and Brenner20,Reference Prakash Saxena, Unnikrishnan, Rathi, Kotian and Reshmi24 especially for laryngeal and hypopharyngeal cancers (6:1 and 4:1, respectively). Some studies revealed that the higher occurrence rate could be related to differences in smoking amount and alcohol consumption.Reference Reyes-Gibby, Anderson, Merriman, Todd, Shete and Hanna5–Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7 However, consistent with several previous studies, gender was not significantly associated with OS rate.Reference Ingarfield, McMahon, Douglas, Savage, Conway and MacKenzie7,Reference Pruegsanusak, Peeravut and Leelamanit15,Reference Abrahão, Anantharaman and Gaborieau16
There was limitation in this study. It did not examine the association of treatment with the risk of mortality and did not include the treatment factor in the analysis due to the fact that treatments in this study were based on the cancer stage (specific treatment for early stage and combined treatments for locally advanced stage) and some patients who had refused treatment, had received incomplete treatment or were receiving palliative care were still included.
Conclusion
These results emphasise the association of tumour site, stage of cancer and age at diagnosis on the risk of mortality in SCCHN patients and highlight the importance of prevention and pre-cancer screening for the early detection of SCCHN. Survival and quality of life in SCCHN are directly linked to stage of tumour at the first detection. Therefore, the results of this study suggest that people should be aware of avoiding risk factors and enrol in gender and age-appropriate cancer screening programmes. Moreover, post-treatment surveillance is important to detect early recurrent disease. To improve quality of regional and national healthcare services, it is beneficial to recruit primary care practitioners and oncological specialists.
Acknowledgements
This research was supported by Chiang Mai University.
Author Contributions
SC and WB contributed to the writing of the study protocol. SC, PS and IC contributed to data collection. WB and PT conducted the statistical analyses. WB wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and the final version of the manuscript.
Funding
This study did not receive any funding.
Conflict of Interest
None to declare.
Ethics Approval
This study was approved by The Research Ethics Committee, Faculty of Medicine, Chiang Mai University.








