Introduction
Allogeneic haematopoietic stem cell transplantation (HCT) is an integral part of the management protocol of acute myeloid leukaemia (AML) or acute lymphoid leukaemia (ALL). Allogeneic HCT requires pre-conditioning of the host with immune-suppression and cytoreduction to facilitate engraftment. Pre-HCT conditioning regimens can be either myeloablative or non-myeloablative and often incorporate total body irradiation (TBI). Reference Wong, Filippi, Dabaja, Yahalom and Specht1 In myeloablative TBI, the patient receives 12 Gy in six fractions delivered twice in a day for 3 days to the whole body. Reference Wong, Filippi, Dabaja, Yahalom and Specht1 Non-myeloablative TBI or reduced intensity regimen is preferred in the elderly, patients with comorbidities, patients with poor performance status and heavily pretreated cases, and a single fraction of 2–4 Gy is delivered. Reference Wong, Filippi, Dabaja, Yahalom and Specht1 Myeloablative TBI is believed to reduce extramedullary relapse after HCT by eradicating disease from the potential sanctuary sites such as the brain and testis, where the concentration of chemotherapeutic agents may be suboptimal. Reference Yuda, Fuji and Onishi2 Interstitial pneumonitis is the most common toxicity after myeloablative conditioning with TBI Reference Gao, Weisdorf, DeFor, Ehler and Dusenbery3 ; other associated known toxicities described include parotitis, nausea, Reference Buchali, Feyer, Groll, Massenkeil, Arnold and Budach4,Reference Valls, Grañena, Carreras, Ferrer and Algara5 diarrhoea, erythema, alopecia, cataracts, Reference Benyunes, Sullivan and Deeg6 renal dysfunction and gonadal failure. Reference Tauchmanovà, Selleri and Rosa7–Reference Abboud, Porcher and Robin9 Interstitial pneumonitis is potentially fatal in TBI, and the lungs should be partially shielded to receive a mean dose of not more than 8–10 Gy. Reference Girinsky, Benhamou and Bourhis10–Reference Pinnix, Smith and Milgrom12 TBI is conventionally delivered with the patient in either standing position or lying down on the floor. The patient is placed at an extended source-to-skin-distance (SSD) (~4 m) to treat them with a single large anterior and opposing posterior field. Extended SSD reduces the dose rate at the patient’s body surface and potentially reduces the incidence of severe interstitial pneumonitis. Reduction in dose rate leads to an increased treatment time, and this prolonged treatment time is uncomfortable for the patients in standing position. These patients are often pretreated with chemotherapy resulting in reduced stamina, making prolonged immobilised standing non-viable in such cases, with a high risk of possible fall and injury. When patients are lying down on the floor, a single large field is not sufficient to irradiate the whole body due to relatively smaller SSD and radiation fields are adjoined with enormous dose heterogeneity at the junctions. Different field arrangements for TBI have been reported. Reference Wong, Filippi, Dabaja, Yahalom and Specht1 The present study reports a novel method utilising abutting radiation fields and shielding lungs for myeloablative TBI. Our technique is delivered on an isocentrically mounted linac by laying the patient on the floor. The overall aim of the study was to evaluate the efficacy of this technique in terms of homogenous dose distribution to whole body and sparing the lungs.
Materials and Methods
Patient simulation
In our institute, a total of 46 patients underwent TBI during January 2015 to June 2020. Out of these, 15 had undergone myeloablative and the remaining 31 had undergone non-myeloablative conditioning for bone marrow transplant. The prescribed dose to TBI was 12 Gy in six fractions delivered over a period of 3 days for myeloablative conditioning and 2 Gy in a single fraction for non-myeloablative conditioning. Patients were positioned on a flat couch in the supine position with hands by the side of the body, and whole-body non-contrast CT images with a slice thickness of 0·5 cm were acquired on a helical CT machine (Biograph; M/s. Siemens Healthcare, Erlangen, Germany). CT imaging data were imported to ARIA (Version 13.0.20.2; Varian Medical Systems Inc., Palo Alto, CA, USA). An expert radiation oncologist contoured the body, lungs, brain and kidneys. Planning target volume (PTV) was derived by excluding 5 mm from the surface of the body and also excluding the lungs. Reference Fog, Hansen and Kjær-Kristoffersen13
Abutting radiation fields
In this dosimetric study, we explain a novel abutting radiation field technique using jaws and multi-leaf collimator (MLC) by laying the patient on the floor beneath the treatment head (over the drum of the treatment couch) (Figure 1). An indigenously developed flat couch-top was used to shift the patient for abutment of radiation fields. The arithmetic formula behind the abutting radiation fields for myeloablative TBI is explained using Figure 2.

Figure 1. Description of abutting radiation fields for myeloablative TBI by laying the patient on the floor.

Figure 2. Formulation of abutting radiation fields for myeloablative TBI.
Let us assume a triangle ABC has been divided into three triangles such as ABD, ADE and AEC having central axes AN, AO and AP, respectively (Figure 2). The triangle ABC resembles a radiation field (ADE) is abutting with radiation fields (ABD and AEC) on either side by tilting the gantry angle to θ, given by equation 1, and their isocentres are separated to a distance, given by equation 2. θ1 and θ2 can be derived from their respective field size (FS) at source-to-axis distance (SAD).
where
For example: Let us assume FS1 and FS2 be 20 cm and 33 cm at SAD of 100 cm. FS2 has to be tilted to abut FS1 to an angle of 15° (θ1 = 5·7° and θ2 = 9·3°) and the central axis has to be separated at the isocentre plane to a distance of 26·5 cm ((20 + 33 cm)/2). Dose distribution due to the resultant abutting radiation fields on a coffin box phantom and profile at 5 cm depth is shown in Figure 3.

Figure 3. Dose distribution calculated by TPS for abutting radiation fields and the dose profile at 5 cm depth on a coffin-box phantom.
Dose validation
Point dose measurements were done using Farmer type 0·6 cc Chamber (M/s. PTW, Freiberg, Germany) in water-equivalent plastic slab phantoms (30 × 30 × 20 cm). SSD was extended to 209 cm and measurement depth was 10 cm. The phantom was positioned at the floor, and 10 cm additional backscatter was provided using the plastic slab phantom. Measured doses were verified against doses calculated by anisotropic analytical algorithm (AAA-13.0.26) on Eclipse (Version 13.0.33) (Varian Medical Systems Inc.) treatment planning system (TPS). Auto-radiographs were taken on GafchromicTM films (M/s. Ashland Specialty Ingredients, Wayne, NJ, USA) sandwiched between plastic phantom at different depths (5, 10 and 15 cm) (Figure 4) and the intensity profiles of abutting radiation fields were verified with TPS dose profiles.

Figure 4. Dosimetrical set-up to acquire auto-radiograph at the junction of abutting radiation fields: (a) gantry tilt—0° and (b) gantry tilt—15°.
Treatment planning
Dosimetrical parameters from TBI plans on whole-body CT scans of 46 patients were analysed in this retrospective study. TrueBeam STx platform Linac (Varian Medical Systems Inc.) equipped with HD120™MLC was used to plan the TBI. Opposing fields were planned on TPS to deliver the prescribed dose of 12 Gy in six fractions for the whole body. In order to facilitate abutting radiation fields, couch was set at 90° for treatment planning. The central axis of FS1 was placed on the craniocaudal mid-plane of the patient and the gantry angle was fixed at 0°. Extended SSD at the central axis was given by the difference of source-to-couch-top-distance (SCTD) which was 225 cm and anteroposterior separation of the patient. The vertical coordinate, thus obtained for FS1, remained the same for abutting fields on either side. The longitudinal coordinate was ±26·5 cm (as given in the example). During treatment, the patient was oriented along the axis of cross-plane, and couch coordinates were over-ridded. After FS1, the gantry was tilted to ±15° and correspondingly the patient was shifted ±(26·5*SCTD/100) cm in the craniocaudal direction (Figures 1a and 1b). Our indigenously developed couch was fixed with a measuring scale and facilitated us with an easy isocentre shifting process. Instead of using the isocentre shift values, the optical field lights could be abutted over the upper surface of the flat couch-top. Perspex sheet of 1·5 cm thickness was used as a beam spoiler to enhance the skin-dose. Opposing fields from the posterior were planned as separate plans in TPS with patient orientation as prone-head-in. The patient was positioned to lay in prone and prone head support was provided during treatment (Figure 1d). Arrangement of radiation fields on an isocentrically mounted linac for field abutment is shown in Figure 5. Plan parameters used for abutting radiation fields are given for paediatric (two fields) and adult (three fields) patients (Table 1). Couch longitudinal shifts calculated using equation (2) were adjusted to account for the radiation penumbra from collimators.

Figure 5. Treatment fields for myeloablative TBI.
Table 1. Treatment plan parameters of abutting radiation fields used in paediatric and adult patients for myeloablative TBI
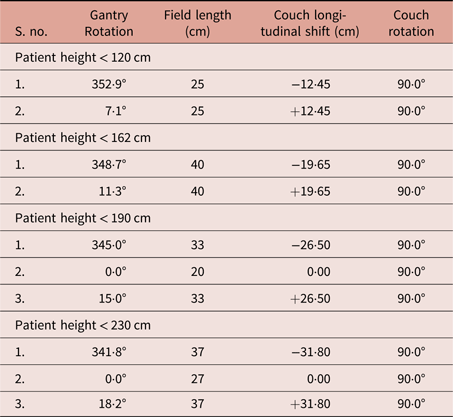
The treatment plan objective was to achieve a mean dose of 12 Gy to PTV and 10 Gy to lungs. The monitor unit (MU) from each field was weighted as per the anteroposterior separation of the patient. The thoracic field (Figure 5a) was sub-divided (Figure 6) and the mean dose to lung was restricted within 10 Gy. As we could not perform image verification, an expert radiologist had verified the lung shielding from optical fields (Figure 7). The dose rate to the lung was kept at ~0·02 Gy/minute (100 MU/minute at isocentre plane) (Figure 5a). However, the dose rate at the isocentre plane was 400 MU/minute for the remaining treatment fields.

Figure 6. Sub-fields to boost the dose which was under-dosed from thoracic field (Figure 5a) to deliver a mean dose of 10 Gy to lungs.

Figure 7. Visual verification of lung shield with the optical field from treatment head by an expert radiologist.
Results
TPS dose validation
Data recorded for TPS dose validation are given in Table 2. Dose with backscatter was also calculated by modelling floor beneath the phantom. The backscatter contribution from the floor did not alter the dose significantly, and the maximum variation observed was an under-dosage of 1·6%. Dose profile of abutting radiation fields from TPS was compared with the auto-radiograph image (Figure 8). Dose profiles were normalised to the dose received at the junction of 5 cm depth. The intensity profile of auto-radiographic images exhibited under-dosage up to 3% at the junction of 5 cm depth and the variation was insignificant at deeper depths (15 cm).
Table 2. Point dose validation between measured and TPS calculated doses/1,000 MUs at 209 cm—SSD


Figure 8. (a) Illuminated auto-radiographs of Gafchromic films on X-ray view box (M/s. Bio-x) at 6500 LUX. (b) Comparison of intensity profile from auto-radiograph and dose profile calculated from TPS.
Myeloablative conditioning
Dose calculated on 46 CT images using abutting radiation field for TBI is summarised in Table 3. Dose received by 95% volume (D95%), 5% volume (D5%), relative volume receiving 12 Gy (V12Gy) and mean dose (Dmean) was recorded for PTV, brain, kidneys and lungs as per earlier report. Reference Fog, Hansen and Kjær-Kristoffersen13 Mean dose to brain and kidneys was 11·46 and 11·80 Gy, respectively. Lungs were partially shielded to keep mean dose to 9·86 Gy. Maximum anteroposterior separation (AP-Sepmax), MU weightage, dose rate and expected treatment time are summarised for different fields (Table 4). Total MU is the summation of MUs from corresponding anteroposterior fields. Average MU delivery time for myeloablative TBI was 16·40 minutes, and real treatment time including treatment set-up for each patient was ~30 minutes/fraction and an additional 15 minutes was taken on the first day of TBI execution. On the first day of treatment, the field borders were marked on the patient and protected using 3M Tegaderm—transparent dressing (M/s. 3M India Ltd., Bangalore, India). Albeit the marking procedure increased the overall treatment time on the first day, it saved more time on consecutive days.
Table 3. Summary of dose received by PTV, brain, kidneys and lungs

Table 4. Influential parameters to reduce treatment time in myeloablative TBI

Clinical experience
Table 5 summarises the dose received by 15 patients who had undergone myeloablative conditioning in our institute. The median dose to the whole body was 12·01 ± 0·28 which corresponds to a deviation of 0·07 ± 2·34% from 12 Gy. The lung was partially shielded to maintain the mean dose to 9·87 ± 0·41 Gy. While shielding the lungs, critical organs such as the heart and liver were also partially shielded and received a mean dose of 10·30 ± 0·42 Gy and 11·14 ± 0·46 Gy, respectively. Upper extremities were irradiated along with the head, neck, thorax and abdomen fields. As the anteroposterior separation of upper extremities was lesser than other parts, on irradiation it received a higher mean dose of 13·48 ± 0·55 Gy (Figure 9). This is the maximum deviation observed in this TBI and over-dosed with 12·34 ± 4·66%. Overall, the dose to the whole body was maintained within ± 5% from this TBI technique.
Table 5. Dose received by 15 patients who underwent myeloablative TBI in our institute


Figure 9. (a) Three-dimensional dose distribution of myeloablative TBI. (b) Dose distribution in frontal view. (c) ‘Island-block’ for shielding lungs using HD120TMMLC.
Discussion
Our study describes a novel, TPS-based planning technique for myeloablative TBI. In our technique, the patient lies on floor and while doing so a particular concern is about the radiation dose increment due to backscatter contribution. However, as per our observations the backscatter contribution from the floor was insignificant by point dose measurement for an extended SSD of 210 cm. The TPS was able to correctly calculate the dose distribution to within a 2% accuracy level. However, discrepancies in dose measurement of up to 7% were observed at 350 cm SSD with open fields. Reference Fog, Hansen and Kjær-Kristoffersen13 Penumbral characteristics during the beam model in TPS played a vital role in accurate dose calculation at the junction of abutting radiation fields and were accurate within 3% for our linac (Figure 8). Hui et al in 2004 have reported a 30% dose inhomogeneity with the use of single large radiation fields at a SSD of 350 cm. Reference Hui, Das, Thomadsen and Henderson14 In TBI, the patient’s anteroposterior separation varies from head to feet, and individualised attenuators are accordingly designed to restrict the dose in-homogeneity to within 5%. Reference Wong, Filippi, Dabaja, Yahalom and Specht1 In our study, radiation fields were abutted to form a single large field and MUs were weighted for multiple sub-fields to achieve dose homogeneity within 5%. The dose received by PTV, brain, kidney and lungs from our study was comparable with the step-and-shoot intensity-modulated radiotherapy (IMRT) technique. Reference Fog, Hansen and Kjær-Kristoffersen13
Use of advanced radiotherapy techniques like IMRT and volumetric modulated arc therapy (VMAT) enhances the dose homogeneity and reduces the doses to critical organs. Reference Hui, Kapatoes and Fowler15–Reference Sun, Cuenca and Itti18 These techniques are delivered isocentrically; hence, the number of fields was increased to irradiate the whole body from head-to-feet. As a consequence of an increase in the number of fields, the MU usage and overall treatment time were increased in our technique. The dose-optimiser takes care of field matching, but involuntary movements of the patient (respiration, cough, isocentre shift, etc.) have an impact on the real-time dose delivery. As the TBI was delivered with anteroposterior opposing fields in this study, the dose-gradient achieved at the junction was similar to TPS calculations (Figure 8) and further requires a verification study to determine dose-gradient at the junctions. Intensity-modulation techniques require laborious planning procedure for TBI to obtain a uniform dose-gradient at the junctions, Reference Hong, Kim and Kim19–Reference Losert, Shpani and Kießling24 as compared to open field techniques. Reference Fog, Hansen and Kjær-Kristoffersen13 The superimposition of dose from adjacent fields or arcs at the junction deteriorates the dose delivered to the patient in helical-tomotherapy and VMAT for TBI.
Treatment length in TBI is several times larger than the available treatment FSs in Linacs. The challenge was either inevitable adjoining beams/arcs in IMRT or VMAT techniques or uncomfortable patient position with bent legs in open opposing field techniques. In helical-tomotherapy, 135 cm is the maximum treatment length which could be treated continuously and the patient had to be rotated 180° (with feet-in position) for further treatment. Reference Hong, Kim and Kim19 VMAT requires several adjoining arcs having up to 9 isocentres, and the patient had to be rotated with the feet-in position for part of the treatment. Reference Aydogan, Yeginer, Kavak, Fan, Radosevich and Gwe-Ya20–Reference Losert, Shpani and Kießling24 Adjoining fields were eliminated by a single large open field, Reference Fog, Hansen and Kjær-Kristoffersen13 but the patient had to bend their legs and accommodate themselves within 165 cm treatment field (at 350 cm—SSD); treatment prolonged for more than 1 hour. In this study, we explained the tactics to abut two fields for paediatric patients with height up to 162 cm and three fields for adult patients up to 200 cm (at 210 cm—SSD) and the treatment time was ~30 minutes. This is the first time in TBI, an adult patient could remain in a fully stretched comfortable position and the adjacent radiation fields are abutted perfectly.
The dose rate on which the TBI is delivered plays a vital role in optimising treatment time and toxicity pertained to dose rate. Interstitial pneumonitis is the major cause of mortality up to 50% in myeloablative TBI. Reference Gao, Weisdorf, DeFor, Ehler and Dusenbery3 The toxicity for lung pneumonitis was reduced with 8–10 Gy mean dose to lung, and the dose was delivered at a rate of 0·015 Gy/minute. Reference Wong, Filippi, Dabaja, Yahalom and Specht1,Reference Gao, Weisdorf, DeFor, Ehler and Dusenbery3 TBI requires more MU usage, and dose rates of 0·015 Gy/minute will increase the overall treatment time hugely. In our TBI treatments, the mean dose to lung was kept ≤10 Gy and delivered at a dose rate of 100 MU/minute at isocentre (100 cm), which corresponds to ~0·02 Gy/minute at treatment plane (~210 cm). As under-dosage to sanctuary sites would relapse the disease, no other organs were shielded in our study. Myeloablative conditioning poses a high risk of permanent sterilisation, and patients desirous of child bearing in the future was advised to opt for semen banking, prior to initiation of treatment. Radiation dermatitis (Grade-2) was observed in only one patient during our initial experience. The Perspex plate was placed only on alternate fractions for remaining patients and radiation dermatitis (Grade-1) was observed.
Conclusion
Our abutting radiation field technique for myeloablative TBI is feasible in any linac bunker. This novel technique to treat a patient at SSD of 2·1 m reduced the overall treatment time in TBI and dose calculation by TPS was more accurate. Radiation fields were adjoined as easy as any other available TBI technique. The dose was homogeneously delivered using multiple sub-fields. ‘Island-blocking’ is feasible in this technique using MLC. The dose rate was decreased to reduce lung toxicity and increased elsewhere to reduce overall treatment time. Our technique is simple, quick, less cumbersome and requires minimal additional equipment to deliver TBI on an isocentrically mounted linac by laying the patient on the floor. In general, TBI requires laborious planning procedures and spacious linac bunkers; this novel technique has the potential to change these facts to myths.
Acknowledgements
The authors are grateful to the Head of Jaypee Hospital, Noida, India for constantly supporting our academic and research pursuits. We are especially thankful to Dr. Sudarsan De, Director-Cancer Care and the team of radiotherapists (Ms. Mousuminath Sharma, Ms. Jyotsana Pant and Mr. Sachin Sharma).
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All procedures in studies involving human participants were performed in accordance with the ethical standards of our Institutional Review Board (IRB) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.

















