Introduction
In this study, the methods used for deriving target volumes in external beam radiotherapy are reviewed and the implications of technological developments, in imaging and treatment delivery, on these margins and their determination are considered. There is a wealth of new and emerging methods of target volume delineation leading to adjusted margins. This article aims to review and compare these methods and to discuss their relative merits. It is however noted that ultimately the applicability of these methods can only be validated through well-controlled clinical trials.
Implementation of the International Commission on Radiation Units and Measurements (ICRU) margin recommendations1,2 has been aided by improvements in radiotherapy imaging leading to better definition and delineation of the visible tumour volume. Microscopic disease spread, however, remains undetectable through imaging techniques. The application of image-guided radiotherapy (IGRT) techniques has led to the reduction in setup and organ-motion–related uncertainties, thus also leading to better target volume definition.
Methods based on clinical target volume (CTV) dose coverage probabilities, biological considerations and physical considerations have been used to determine radiotherapy target volumes. In these methods statistical,Reference Price and Moore3 simulation,Reference Killoran, Cooy, Gladstone, Welte and Beard4,Reference Mageras, Fuks and Leibel5 direct measurementReference Ekberg, Holmberg, Wittgren, Landberg and Bjelkengren6,Reference Beltran, Herman and Davis7 and artificial intelligence techniquesReference Waschek, Levegrun, van Kampen, Glesner, Engenhart-Cabillic and Schlegel8,Reference Caudrelier, Vial and Gibon9 have been applied to derive formulations and recipes that combine the treatment uncertainties to deduce margin sizes for use in treatment planning. Similar techniques have been used to derive margins around organs at risk.Reference McKenzie, van Herk and Meijnheer10,Reference Muren, Ekerold, Kvinnsland, Karlsdottir and Dahl11
The increasing use of intensity-modulated radiotherapy (IMRT) which allows improved dose conformity to target volumes and offers the possibility of dose escalation requires further accuracy in the definition of the volumes to be treated due to the steep dose gradients around the target volumes to spare the normal tissues and critical organs.Reference Marks and Ma12 The risk of inadequate target volume coverage due to spatial uncertainties may be more significant. The introduction of IGRT and other new imaging techniques in radiotherapy facilitate a review of the margins in current clinical use as more information regarding the treatment uncertainties becomes available.
The magnitude of set up errors can be reduced by the use of electronic portal imaging protocols and immobilisation techniques.Reference De Boer, van Os, Jansen and Heijmen13,Reference Erridge, Seppenwoolde and Muller14 The use of IGRT techniques provides information to monitor and reduce the uncertainty in setup and organ motion errors, which both consist of a random and a systematic component. This is because any errors that are present at treatment can be identified and corrected for through imaging and compensating movements of the patient position. Random errors lead to a blurring of the dose distribution while systematic errors cause a shift of the dose distribution.Reference van Herk15 The reduction in setup and organ motion uncertainties should potentially lead to the reduction in the size of the planning target volume (PTV) and hence provide the potential for dose escalation and further healthy tissue sparing. The use of imaging before treatment can also be used for adaptation of the margins and treatments plans to optimise the dose delivered to the tumour and organs at risk thus potentially improving treatment outcomes.
The efficacy of the proposed concept of adaptive radiotherapy requires the use of clinical trials to validate this new technique. Current trials in radiotherapy are mainly focused on comparing conventional and IMRT techniques in dose escalation regimes; however, the adaptation of these treatments as a result of the additional information from IGRT techniques remains to be fully justified through radiotherapy clinical trials.
Radiotherapy Target Volumes And The Icru Formulations
Target volume margins are used to account for microscopic disease spread as well as the various uncertainties encountered from the imaging stage and throughout the treatment phase in radiotherapy.1, 2 The main uncertainties which can lead to under-dosage of the target volumes and loss of tumour control are observer delineation errors, setup errors and organ motion.Reference van Herk15 These uncertainties lead to the definition of the target volumes as shown in Figure 1.
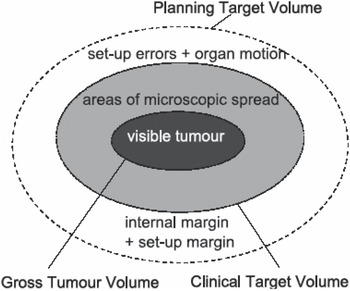
Figure 1. Definition of target volumes in radiotherapy.
GTV determination
The gross tumour volume (GTV) outline is based on the visible or palpable extent and location of the tumour. Delineation errors in outlining the GTV margin result due to images of poor spatial resolution, intra- and inter-observer variations in target volume perception as well as the use of different imaging and treatment planning protocols.Reference Rasch, Steenbakkers and van Herk16,Reference Gao, Wilkins, Eapen, Morash, Wassef and Gerig17
Since the publication of these ICRU Reports significant technological advances, especially in radiotherapy imaging, have increased the ability to reduce further some of the uncertainties in target volume definition. The use of multimodality imaging, including computed tomography (CT), positron emission tomography (PET) and magnetic resonance imaging (MRI) have led to the potential for improved localisation of the tumour volume and hence better definition of the GTV. Important physiological and functional information about the tumour can now be obtained from the application of PET and MRI imaging in radiotherapy.Reference Ling, Humm and Larson18
To aid diagnosis image fusion techniques using CT and MRI images or CT and PET images are also used in radiotherapy planning. The use of MRI has the advantage of providing excellent soft tissue contrast resolution compared with CT; however, the lack of electron density information, poor visibility of bony anatomy and potential image distortion mean that it is seldom used on its own for treatment planning purposes. The combination of PET and CT imaging for radiotherapy planning produces a biological target volume incorporating all the structural and functional informationReference Mac Manus and Hicks19 without the need for secondary image registration.
CTV determination
The GTV and surrounding areas of known or potential microscopic spread of the disease constitute the CTV. There are presently no imaging methods to show accurately the microscopic spread of the disease leading to the CTV definition. This results in the uncertainty in determining the extent of the CTV margin for most tumour sites. Thus inter- and intra-observer delineation uncertainties in structure outlining still remain and need to be quantified and incorporated in the margin derivation.
For some treatment sites, such as in the head and neck region, the use of pathological and imaging information has resulted in proposals and guidelines for the delineation of the CTV and the involved nodes.Reference Grégoire, Coche, Cosnard and Reychler20–Reference Nowak, Wijers, Lagerwaard and Levendag22 The use of a common atlas to aid in outlining the various node levels is also proposed. Such guidelines, though currently not available for all treatment sites, should assist in standardising CTV margin delineation; however, the experience and judgement of clinicians is still fundamental to the accurate delineation.
PTV determination
The CTV is enclosed by the PTV to incorporate the uncertainties in patient setup and organ motion, a geometrical concept expanded in ICRU's Report 62.2 This report recommends that PTV margin sizes can be derived from the total standard deviation (SDTot) which is calculated from the quadrature sum of the mean standard deviations of the systematic errors (Σ) and the random errors (σ),
This recommendation has been generally found to be invalidReference Stroom and Heijmen23 because it assumes that systematic and random uncertainties have equal effects on a patient's dose distribution. Other margin recipes have therefore needed to be developed.Reference Stroom, de Boer, Huizenga and Vissier24,Reference van Herk, Remeijer, Rasch and Lebesque25 Both ICRU 50 and ICRU 62 argue against the linear addition of uncertainties as this would lead to an excessively large PTV which would compromise the tolerance of surrounding normal tissues.
Advances in IGRT techniques, including the use of MV- and kV-Cone Beam CT (CBCT) imaging, have led to the potential for monitoring and reducing the uncertainty in setup errors and inter-fraction organ motion. Similar advances in the use of motion modelling, respiratory gating and four-dimensional CT (4DCT) imaging have led to the potential reduction in the uncertainty in intra-fraction organ motion.Reference Meijer, Rasch, Remeijer and Lebescue26 There is thus the potential to reduce the PTV margin sizes and escalate the dose to the tumour and therefore improving treatment outcomes.
ICRU margin limitations
With the introduction of new complex treatment techniques, including IMRT, the ICRU margin formulations have largely continued to be used for deriving margins for planning purposes.Reference Samuelsson, Mercke and Johansson27 The original ICRU margin recommendations1,2 have limitations in that they do not address the issues of overlapping volumes between target volume and organs at risk or the non-rigid tumour transformations that occur during a course of radiotherapy. They also do not address how tumour delineation uncertainties should be incorporated into treatment margins. They do not provide details on how margins around organs at risk should be derived. Other margin recipes have been suggestedReference Stroom and Heijmen23–Reference van Herk, Remeijer, Rasch and Lebesque25 which take some of the above issues into consideration in their formulations.
ICRU Report 7128 refines the volume recommendations from ICRU 50 and 62, and provides further examples to clarify these concepts. In this report the recommendation for the inclusion of delineation errors in quadrature with the other systematic errors for deriving the PTV margin is given. The use of margin recipes is included with the acknowledgement that the standard deviation of the systematic errors is about three times that of the random errors. A method is also proposed for deriving margins around organs at risk. An element of uncertainty still remains on the applicability of these margin recommendations in new radiotherapy techniques including IMRT, IGRT and adaptive radiotherapy.
METHODS FOR THE DETERMINATION OF TARGET VOLUMES
Radiotherapy treatment target volume margins have been derived mainly based on dose-coverage probabilities, physical considerations as well as biological considerations. Margin recipes have been proposed from some of these derivations and can be used to deduce margins at individual radiotherapy centres using measured preparation and treatment execution errors.
Margins based on dose coverage probability
Analytical derivations of treatment margins based on the probability of correct target dose coverage were performed by Stroom et al.Reference Stroom, de Boer, Huizenga and Vissier24 and by van Herk et al.,Reference van Herk, Remeijer, Rasch and Lebesque25 where margin recipes were proposed. The impact of random and systematic errors were quantified in terms of the dose delivered to the CTV and dose–volume histograms were calculated and used to determine the effect of the uncertainties. In van Herk et al.'s formulation convolution of the dose distribution with random errors was first performed to obtain a blurred dose distribution. This dose distribution was then shifted using the systematic error to obtain the dose distribution in the CTV. The probability distribution of the systematic errors was finally used to compute the dose distribution received by each fraction of the patient population. A margin recipe was derived based on the condition that for 90% of the patients the minimum dose should be at least 95% (see Table 1).
Table 1. Margin recipes based on dose coverage probability.

Stroom et al. used 12 parameters describing random and systematic deviations in their margin derivation. The patient's CTV was described as a 3D matrix using the treatment room coordinate system. This matrix was convolved using the probability distributions of the random and systematic errors. The influence of these errors on the dose to the CTV was deduced using dose–probability histograms and a margin recipe was derived. This derivation was based on the condition that 99% of the CTV should get at least 95% of the dose.
Both formulations came to the conclusion that the standard deviation for the systematic errors is at least three times larger than that for the random errors.
The inclusion of breathing motion in margin recipes was proposed using a mathematical approach by McKenzie.Reference McKenzie29 The approach combines the dose distribution from a generalised cyclical breathing motion with an idealised step function. The study proposes that breathing motion should be added linearly to the quadrature sum of the other errors. On the other hand, van Herk et al.Reference van Herk, Witte, van der Geer, Schneider and Lebesque30 combined respiration motion with the standard deviations of penumbra and random errors in quadrature. They found that respiration induced motion of up to 1 cm amplitude could be approximated by a Gaussian distribution. Larger respiration motion however requires the use of asymmetric margins.
The use of a Monte Carlo technique to simulate the effects of organ motion and setup errors relative to a fixed dose distribution was first considered by Killoran et al.Reference Killoran, Cooy, Gladstone, Welte and Beard4 The simulation used the Box-Muller technique to sample translations and rotations of organ motion and setup errors from Gaussian distributions. The combined effect of organ motion and setup errors was applied as a shift of the anatomy relative to the static dose distribution. This was repeated for all treatment fractions comprising the treatment course. Each complete course of treatment was simulated 100 times to quantify the range of possibilities. For the example of the prostate it was found that a 10 mm CTV to PTV margin combined with a 5 mm dosimetric margin provided adequate coverage to the CTV when treatment uncertainties were incorporated.
In our previous workReference Mzenda, Hosseini-Ashrafi, Palmer, Liu and Brown31 we used a Monte Carlo technique to simulate the effects of organ motion and setup errors on the dose delivered to three treatment sites, a prostate case, a lung case, and a head and neck case. The input to the simulation was based on portal imaging data obtained from the use of fiducial markers. Displacements of the target volumes and organs at risk with respect to the static planned dose distribution were then performed using the simulated data. The margin sizes used at our centre for treatment planning were found to be adequate in achieving the required target dose coverage where the criterion that 99% of the CTV should get 95% of the dose was used.
Using a set of planning and treatment CT scans, a simulation method was also used by Mageras et al.Reference Mageras, Fuks and Leibel5 to design non-uniform margins with the aim of maximising target coverage and normal tissue sparing. A generic data set of CT scans was used to deduce differences due to organ motion, and these differences were then applied to the patient being planned. The setup error, consisting of a systematic and a random component, was randomly sampled from the frequency distribution of setup errors. The Box-Muller technique was applied for sampling Gaussian distributions and a numerical technique was used for arbitrary shaped distributions. The method was applied to five prostate plans and the results indicated an increase in the seminal vesicle tissue control probability (TCP) when this technique was used; however, no change was noted for the prostate TCP and the rectal wall normal tissue complication probability (NTCP).
In practice, the most common technique for deducing PTV margins for use by individual radiotherapy centres is the use of portal imaging to deduce the organ motion and setup errors which are then combined using one of the published margin recipes to derive the required margins. Numerous studies using either offline or online portal imaging protocols have been undertaken to deduce margins for different tumour sites. For example, the effects of organ motion and setup errors for lung cancer patients was investigated by Ekberg et al.Reference Ekberg, Holmberg, Wittgren, Landberg and Bjelkengren6 with the aim of determining the PTV margin sizes to be used. Fluoroscopy was used to study the tumour movement while an electronic portal imaging device was used to study setup errors. The total standard deviation of these errors was then calculated in quadrature and the PTV margin was calculated using a nominal probability factor for the CTV coverage. Taking into account the measured errors and allowing for unquantified uncertainties a total PTV margin of 11 mm in the transverse plane and 15 mm cranially and caudally was derived. Beltran et al.Reference Beltran, Herman and Davis7 used four localisation methods, i.e. skin markers, pelvic bone anatomy and gold seeds (with and without a 5 mm action level threshold), to determine PTV margins for the prostate. Margin calculations were then performed for each of the four methods using the margin recipe proposed by van Herk et al.Reference van Herk, Remeijer, Rasch and Lebesque25 (see Table 1).
The BIR publication32 on geometrical uncertainties provides comprehensive practical guidance and recommendations on how to determine treatment uncertainties and geometric margins around target volumes and organs at risk for different cancer sites. These recommendations are also briefly mentioned in ICRU Report 71.28 In our studyReference Mzenda, Hosseini-Ashrafi, Palmer, Liu and Brown33 we proposed the use of a novel Gaussian mixture modelling technique to quantify and include the doctor's delineation error into the CTV margin determination. This results in a blurred CTV margin which is then used to derive the PTV margin for use in radiotherapy treatment planning.
Margins based on biological and physical considerations
In a separate study, van Herk et al.Reference van Herk, Witte, van der Geer, Schneider and Lebesque30 investigated the biological and physical fractionation effects of random errors, and assumed systematic errors to be zero. The standard deviations of penumbra, random errors and organ motion were combined in quadrature and used to displace a dose distribution for a number of simulated fractions. The total dose was corrected for the biologic effects of fractionation. It was found that the limited number of fractions causes an uncertainty in the isodose levels of the total dose equal to the standard deviation of the random errors divided by the square root of the number of fractions.
The use of fuzzy logic for the determination of target volumes in radiotherapy was initially performed by Waschek et al.Reference Waschek, Levegrun, van Kampen, Glesner, Engenhart-Cabillic and Schlegel8 The aim of their work was to use the experience and knowledge of clinicians to deduce the CTV margin by incorporating the region comprising the microscopic spread of the disease which leads to diagnostic uncertainty into the target volume definition. A minimal CTV definitely containing the tumour was outlined as well as a maximal CTV outside of which there was no tumour spread. The region of diagnostic uncertainty in between these two volumes was then processed using a knowledge-based fuzzy system to determine the optimum extent of the CTV. The knowledge-based system was based on the expected increase in NTCP and the gain in TCP for each voxel, if included in the CTV, where the relationship between NTCP and TCP was based on the knowledge of experienced clinicians. The fuzzy system was applied to test phantoms and to clinical cases. From the phantom results it was deduced that using this system could lead to sparing of critical structures. From the clinical cases it was found that the size of the fuzziness region and the variation in target volumes defined by the clinicians were in the same range. This work, however, did not consider the effects of organ motion and setup errors which lead to the definition of the PTV.
In most margin recipes, organ motion and setup errors are represented by rigid body translations, thus organ deformation is generally ignored. Price and MooreReference Price and Moore3 introduced a statistical shape model that may be used to account for organ deformation in margin determination. Data from CBCT scans were used to account for uncertainties due to observer delineation errors, organ motion and setup errors. They created a point distribution model from corresponding surface points from a set of rectal delineations on the CBCT images. Coverage probability matrices were generated from the point distribution models. Their technique takes into account organ shape deformations.
A Markov chain model was proposed by ÇetinReference Çetin34 to deduce patient specific CTV to PTV margins. To construct the model planning CT scans were first obtained, followed by a few repeat CT scans at different time intervals. The transition probability matrix representing patient setup error and organ motion was derived from the reference and successive CT scans. The initial stationary distribution of the tumour was obtained using a Markov chain and then the knapsack problem approach was applied to maximise the probability of tumour coverage and span. In this analysis the tumour was visualised in terms of area and the planning CT was divided into small cells. A recipe was provided for calculating the CTV to PTV margin; however, no clinical validation of this formulation was performed in the study.
Caudrelier et al.Reference Caudrelier, Vial and Gibon9 applied a fuzzy logic method to reduce the inaccuracies due to 2D contour definition and its associated segmentation methods which are used in the creation of the 3D target volume. Using MRI images, a 2D minimum region definitely containing the object and a maximum region definitely not containing the object were delineated. The fuzzy values for the degree of membership for each voxel between the two contours were processed using possibility distribution functions, taking into account the slice position and profile. Smaller target volumes were obtained using the fuzzy logic method compared to the classical method and a small decrease in intra- and inter-observer variations was also noticed when using the fuzzy logic method.
The design of patient-specific margins has been implemented in a number of lung tumour studiesReference Sixel, Ruschin, Tirona and Cheung35–Reference Allen, Siracuse, Hayman and Balter37 since target motion in this case is most likely to result in geographic miss. Rietzel et al.Reference Rietzel, Liu and Doppke38 used 4DCT to design patient-specific margins for 10 lung patients. 4DCT as well as free breathing standard helical CT scans were acquired for these patients. The same observer delineated GTV margins on the 4DCT data as well as on the helical CT scans. An 8 mm GTV to CTV margin was grown. The PTV margin was then calculated by the root of the sum of the internal margin (IM) and the setup margin (SM). This resulted in PTV margins ranging from 15.0 to 19.9 mm, depending on the SM size and the inter- and intra-fractional motion components of the IM. IMs could be reduced from 10 to 5 mm, hence the PTV margin size could be reduced from 20 mm for a standard helical CT scan to 15 mm based on 4DCT data.
MARGIN DETERMINATION FOR ORGANS AT RISK
The ICRU recommends the drawing of margins around organs at risk to produce planning organ at risk volumes (PRVs) which include the effects of movement and setup errors. However there is no recommended procedure from their publications on how to determine these margins.
McKenzie et al. proposed an algorithm for drawing margins around organs at risk when dose levels used around these margins would cause unacceptable complications.Reference McKenzie, van Herk and Meijnheer10 For large parallel organs at risk a margin of 1.3Σ was shown to be sufficient to account for systematic errors. For small parallel structures and for serial structures where the blurring caused by random errors will affect the risk of complications a margin of 0.5σ was recommended for addition to the margin of 1.3Σ for systematic uncertainties.
Muren et al.Reference Muren, Ekerold, Kvinnsland, Karlsdottir and Dahl11 applied this formulation to clinical measurements of rectum displacements using repeat CT scans. This was compared to a direct empirical method where the margins around the planning scan rectum were deduced to encompass the observed rectum displacements. The margins derived using McKenzie et al.'s formulation were substantially smaller than those derived using the empirical method. This difference was attributed mainly to the measurement techniques used to find the rectal displacements as well as the fact that the empirical margins were based on internal rectal motion only.
The use of a PRV margin is found to be especially critical for serial organs where damage to a small section results in severe clinical complications. In instances where the PRV is found to overlap with the PTV the ICRU 62 recommendation is to modify the PTV according to the presence of organs at risk and the dose prescription.
IMRT MARGIN REQUIREMENTS
The question of the applicability and validity of using the ICRU margins for IMRT will continue to be asked as use of this technique continues to grow in radiotherapy. This is particularly so as the effects of patient positioning and organ motion errors have been found to be more pronounced in IMRT compared to conventional radiotherapyReference Convery and Rosenbloom39, Reference Xing, Lin and Donaldson40 due to the tighter dose conformity and high dose gradients around the target volumes.
For head and neck treatments the effect on IMRT dose distributions of directly using the ICRU margins was assessed by Samuelsson et al.Reference Samuelsson, Mercke and Johansson27 A 5 mm margin was used around the CTV and around the spinal cord for three patients. IMRT plans were then generated and the effects of the setup errors were simulated by shifting the isocentre and recalculating the dose distributions without altering the field fluence distributions. The effects of random errors were not considered in this study. No differences were found between the two treatment techniques when ICRU margins were used; however, the sensitivity to the setup errors for the target volume depends on the quality of the treatment plan while the effect on the organs at risk depends on the sharpness of the dose gradients around these organs.
In another head and neck IMRT studyReference Astreinidou, Bel, Raaijmakers, Terhaard and Lagendijk41 the inclusion of random errors for displacement, translational and rotational deviations was performed to evaluate the adequacy of PTV margins. It was deduced that for this IMRT solution PTV margins of 1.5 mm and 3.0 mm were required to account for random uncertainties with standard deviations of 2 and 4 mm, respectively, for the primary CTV margin. For the case of an elective CTV where the goal of treatment was a V95 of at least 99% larger PTV margins were required.
A number of techniques are employed for IMRT to reduce the IM because a smaller PTV margin often results in improved sparing of the organs at risk.Reference Nuyttens42 These techniques include immobilisation, the use of online and offline correction protocols, breathing control and tracking techniques. The tighter dose conformity to the PTV in IMRT makes it imperative to consider the use of a PRV around organs at risk, especially where these are in close proximity to the PTV. Overlapping margins between the PTV and PRV tend to result in complicated trade-offs in IMRT treatment plan optimisation.
INFLUENCE OF IGRT ON MARGINS
The use of IGRT techniques can lead to the quantification and reduction in setup errors and organ motion uncertainty, hence the PTV margin size can be reduced. This can potentially lead to a reduction in the dose to normal tissues and will allow for dose escalation and improvements in tumour control. However, clinical trials are still required to validate this assumption.Reference Ling, Yorke and Fuks43 The use of IGRT will still not lead to the elimination of all geometric uncertainties.Reference van Herk, Witte, van der Geer, Schneider and Lebesque30 This is mainly due to the delay between imaging, treatment planning and treatment; limitations on the accuracy of the correction protocols as well as observer errors.
The use of IGRT does not result in the reduction of delineation uncertainties, and these contribute significantly to the PTV margin size. Thus when PTV margin reduction and dose escalation are proposed, it is important to account for and consider the effects of delineation errors. Also, the available time in busy radiotherapy centres makes online corrections for all patients impractical, hence geometric uncertainties still remain.
Patient-specific IGRT measured variables can be used to modify the patient setup and treatment plan, leading to the concept of adaptive radiotherapy. The use of 4D-imaging techniques such as CBCT and 4D gating prior to treatment provides information about the tumour position just before treatment. Such information can be used to re-optimise the margin sizes used and allows for patient re-planning. The benefits and feasibility of this re-planning process need to be balanced against other requirements in busy radiotherapy departments. Automation tools can be used to minimise the time and data handling requirements leading to margin re-delineation and re-planning. However, the increased clinical benefit of this re-planning can only be fully validated by the use of clinical trials.
CLINICAL TRIALS
The use of clinical trials in radiotherapy is essential for assessing and validating the efficacy of new techniques. In the UK trials comparing conventional and new IMRT techniques are ongoing to look at improvements in tumour control and the reduction in side effects for treatment sites including prostate (CHHiP), head and neck (PARSPORT and COSTAR) as well as dose escalation for breast treatment (IMPORT). Published recommendations from the RT01 trialReference Griffiths, Stanley and Sydes44 should result in the standardisation and reproducibility in patient setup for the pelvic region.
The combined use of IMRT and IGRT has also been assessed in the PROFIT clinical trials conducted in both Australia and Canada. In a randomised Phase II trial a prescription dose of 60 Gy in 20 fractions for localised prostate cancer was assessed using IMRT and implanted fiducial markers.Reference Martin, Rosewall and Bayley45 The results showed low rates of late bladder and rectal toxicity. The follow-up phase III trial looks to assess a similar combination of IMRT and IGRT for localised prostate cancer using an escalated dose of 78 Gy in 39 fractions.
Clinical trials are still required to assess other aspects of IGRT and new techniques in radiotherapy, particularly the use of adaptive radiotherapy. It is recommended that well-structured clinical trials be devised to assess the efficacy of the use of techniques such as biological imaging, inter- and intra-fraction organ motion corrections and setup errors in radiotherapy, incorporating margin reduction and dose escalation. Such trials would also assess the effects of the additional dose delivered in IGRT techniques such as CBCT. Any treatment margin recommendations would then be based on the clinical findings from these trials.
CONCLUSIONS
The derivation of treatment margins in radiotherapy continues to benefit from the introduction of new techniques, particularly in radiotherapy imaging. The ICRU recommendations remain fundamental to the margin derivation process and have been revised to include recent findings where margin recipes have been proposed. It is recommended that these revised recommendations should be used by individual radiotherapy centres for deriving treatment margins from measured treatment uncertainties.
The same principles are applicable for deriving margins for use in IMRT treatment; however, in this case it is recommended that PRV margins should be used around organs at risk which are in close proximity to steep dose gradients. More recently, IGRT has provided the means for monitoring, and controlling the accuracy of dose delivery through adaptive radiotherapy, and has also provided the opportunity for quantification and adjustment of setup and organ motion margins. Such a proposition should lead to improved treatment outcomes.
It is recommended that clinical trials should be conducted to investigate fully the efficacy of these new techniques, particularly in radiotherapy image-guidance and adaptive radiotherapy. Results of these would validate the current clinically used margins and allow for recommendations on their continued use.
Acknowledgement
This work is kindly sponsored by the Medical Physics Department of Portsmouth Hospitals NHS Trust.




