Background
Dermatofibrosarcoma protuberans (DFSP) is a rare, slow-growing malignant cutaneous tumour, frequently affecting the dermis and subcutis. Most of the DFSP cases present as one or more cutaneous nodules on the trunk or extremities with the head and neck affected less commonly. Less than 5% of these tumours are located on the scalp.Reference Loss and Zeitouni 1 It can appear at any age, most commonly in individuals aged between 20 and 50 years. Microscopically, the tumour is comprised of dense aggregates of spindled cells that form ‘cartwheel’ arrangements.Reference Taylor and Helwig 2 Cluster of differentiation (CD) 34 has been shown in multiple studies to be strongly expressed in cases of DFSP.Reference Kutzner 3 Along with CD34, vimentin is often highly expressed in DFSP as well.
Wide radical excision is the preferred treatment for DFSP. In addition to wide local excision, Mohs micrographic surgery is an alternative option, with a recent meta-analysis establishing their role in preventing recurrences.Reference Ratner, Thomas and Johnson 4 Patients with positive or close surgical margins have an elevated risk of local recurrence after resection alone. The probability of regional or distant metastases is 3%, often with involvement of lymph nodes or the lungs.Reference Kahn, Saxe and Gordon 5 DFSP is radiosensitive and adjuvant radiation therapy is associated with lower local recurrence rate, especially in those with positive margin, incomplete resection or inoperable.Reference Sun, Wang and Huang 6 The European consensus guideline recommends adjuvant radiotherapy to a dose in the range of 60 Gy (conventional fractionation) for resection with microscopic positive margin (R1), gross positive margin (R2) and inoperable disease or with prior multiple tumour recurrences.Reference Saiag, Grob and Lebbe 7
Case Report
A 24-year old female presented with a small, painless nodule around 1 cm in size over right frontal scalp. Initially she had been referred to homoeopathic treatment for a period of over 6–7 months, however, the nodule kept on increasing in size and her local physician advised a computed tomography (CT) scan of brain in September 2015. The CT scan revealed a right frontal scalp soft tissue lesion measuring 2·6×1·6×2·3 cm (Figure 1). Fine-needle aspiration cytology of the scalp swelling in December 2015 reported it as a benign cystic lesion of vascular origin. She then reported to the plastic surgery department of our institution in January 2016 and underwent wide local excision of scalp lesion on 30th January 2016. Histopathology showed features of DFSP with involvement of deep resection margin. Immunohistochemical study confirmed the diagnosis of DFSP, with positive expression of vimentin and weakly positive for CD34. The patient was finally referred to the Department of Radiation Oncology on 24th February 2016. She was planned for high-dose-rate (HDR) brachytherapy using a customised surface mould. The total prescribed dose was 42 Gy to the 100% isodose, delivered in twice daily fractions of 3·5 Gy (350 cGy) each at least 6 hours apart for 12 fractions.

Figure 1 Preoperative computed tomography of the head revealed a 2·5×1·6 cm soft tissue tumour arising in the scalp of right frontal region.
Procedure
The skin over the tumour bed was shaved with a wide margin. The bed was marked over the scar in correlation with preoperative CT images. An area beyond the bed with a 2-cm margin all around was marked as the target area (TA) (Figure 2a). The patient was then immobilised using a three clamp head and neck thermoplastic mask (ORFIT, Orfit Industries NV, Wijnegem, Belgium). The TA was redrawn over the mask (Figure 2b). On the thermoplastic mask layers of dental wax was applied of approximate thickness of 5 mm adequately covering the TA. The mould material (dental wax) was heated in water bath of 60–65°C, and once pliable, it was moulded to patient’s facial and scalp curvatures by gentle pressure. Six parallel grooves were then drawn over the mould converging on the TA with a 1·5 cm separation (Figures 2c and 2d). Care was taken to cover the whole TA with one catheter placed at each edge of the TA (Figure 3). Flexible HDR catheters of adequate length were then fixed to the mould, one on each of these grooves. Additional layers of dental wax were applied to cover the catheters and to give stability to the mould. To ensure proper dose delivery on the borders of target, the number of catheters were selected to cover the whole target volume with the margin (Figures 4a and 4b). The edges of the mould were trimmed and shaped to match the TA. The TA was marked with thin copper wire.

Figure 2 (a–d) Preparation of the thermoplastic mask and the surface mould.

Figure 3 Schematic diagram showing the relation of Tumor Bed, Target Area and the arrangement of plastic catheters.

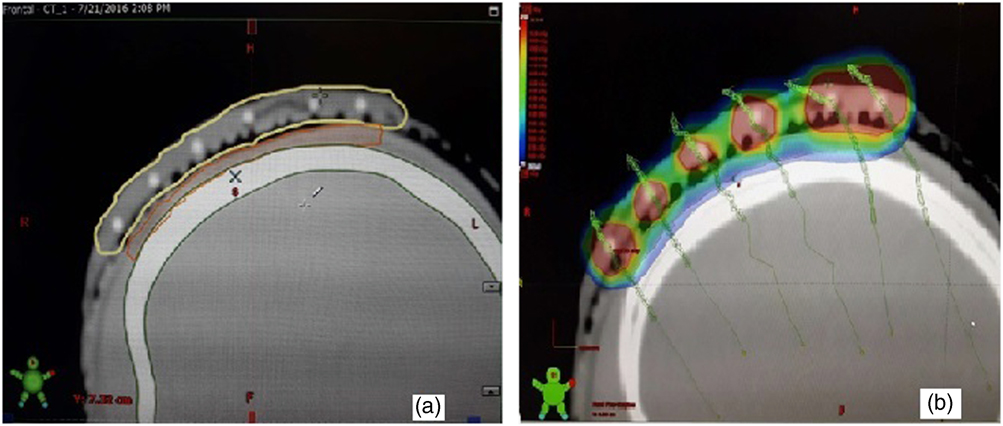
Figure 4 (a, b) The mould (yellow), the target, planning target volume (PTV) (orange) and the underlying bone (green) were contoured separately. The dose colour wash was verified to check adequate coverage. Effort was taken to make the 100% isodose cover the PTV (red) with minimal spillage of 150% on the PTV/skin.
Treatment planning
CT scan (BrillianceCT-16® slice with Accusim Virtual simulation; Philips Health Care Solutions, DA Best, The Netherlands) was performed using thin slice width of 2 mm from vertex to the brow level (120 kv and 250 mAs). After CT simulation the data was transferred to the treatment-planning system (Brachyvision TPS®, Eclipse, Varian Medical Systems, Palo Alto, CA, USA) computer. Then following image reconstruction, the planning target volume (PTV) corresponding to the TA was contoured on the axial slices, while reviewing the same on saggital and coronal images. The skin and the soft tissue of the scalp formed the depth of the target volume. The PTV consisted of the TA and a small additional lateral margin for positional and setup error was delineated11 according to wire markers visible on CT scans. Then after contouring the TA, bone and the mould, dose points were created around the catheters and the dose was prescribed. Following this, volumetric optimisation was performed to make the 100% prescription isodose conform to the target volume. Care was taken to reduce the volume of the target covered by the 150% isodose curve and to ensure the 200% isodose curve did not overlap as far as possible. The total prescribed dose was 42 Gy to the 100% isodose with equivalent dose in 2 Gy (EQD2) of 47·25 Gy10, delivered in twice daily fractions of 3·5 Gy each at least 6 hours apart for 12 fractions. The scheduled duration of treatment was 6 days. Treatment was delivered using Iridium 192 with Gamma Med plus HDR after-loader unit (Varian Medical Systems). We calculated the V100=28.12 cc, V150=4.85 cc, V200=0.98 cc and the Dose Inhomogeneity Index (DHI)=0.82. It was completed on 30 March 2016 and was well tolerated. Following the completion of treatment, the patient was followed up at 4 weeks and thereafter, monthly intervals for the first 3 months and at 3 month intervals up to the 1st year. The patient was assessed clinically for local recurrence, late skin toxicity, regional nodal failure and cosmetic outcome.
Follow-up and toxicity assessment
After 2 weeks of treatment, there was hyperkeratosis and scar formation and patient complained of mild pruritus. At 4th week, there was pus discharge from radiation site. She was advised a course of oral antibiotic along with topical treatment. On follow-up 3 months after brachytherapy, there was no residual disease, good clinical response and cosmesis were achieved. In September 2016, 6 months post radiation, the patient reported to us with hypopigmentation and alopecia (grade I) with locally controlled disease. The patch of hypopigmentation on the anteromedial edge of the target volume corresponded to the overlapping of the 150% isodose with the skin surface, with skin mould interface receiving a total dose of 63 Gy, EQD23Gy of 81·9 Gy (Figure 5a). After 22 months of follow-up, at 3 monthly interval, there was no recurrence and good overall cosmesis (Figure 5b).

Figure 5 (a, b) Follow-up status after 9 months (a). Presence of grade I late skin toxicity (alopecia and hypopigmentation) was observed, with patches of hypopigmentation corresponding to the area of skin receiving >450 cGY per fraction (marked with red dotted line). Follow-up status after 18 months, with appearance of hair re-growth.
Discussion
DFSP is a relatively rare, spindle cell neoplasm of cutaneous origin. DFSP in its classic histologic appearance consists of a proliferation of dermal spindle cells arranged in a storiform or cartwheel pattern. DFSP may at times be difficult to differentiate from other fibrous tumours, including myofibroma, dermatofibroma and fibrosarcoma. Attention to morphologic details and immunohistochemical analysis helps to distinguish DFSP from these other varieties. Fine-needle aspiration should generally be avoided, because often it will not yield a definitive diagnosis,Reference Saiag, Grob and Lebbe 7 as in this case of scalp DFSP. DFSP has been regarded as a fibrous tissue tumour of intermediate malignancy because of its proneness to recurrence rather than metastasis. The probability of regional or distant metastases is <5%, mostly involving the lungs and lymph nodes. The main modality of treatment for this locally infiltrative tumour is wide local excision.Reference Mark, Bailet and Tran 8 Moh’s micrographic surgery is also a valid option with very high local control, however it is laborious and time consuming and is not always suitable for scalp lesions.Reference Huether, Zitelli and Brodland 9 Adequate margin of about 2–3 cm is associated with good local control with surgery alone.Reference Parker and Zitelli 10 Patients with positive or close surgical margins have an increased risk of local recurrence after resection, especially arising from head and neck region. The risk of recurrence is as high as 63% in patients with close or positive margins as compared with 0–13% in cases of negative margins. In cases or areas where repeat surgery for a recurrence is associated with unfavourable cosmetic outcomes, adjuvant radiotherapy is thought to be an effective alternative.Reference Haas, Keus, Loftus, Rutgers, van Coevorden and Bartelink 11 Doses in the range of 50–60 Gy delivered postoperatively for cases with high risk for recurrence has been reported with good long-term local control.Reference Chen, Tu, Lee and Huang 12
Adjuvant radiotherapy for scalp/head and neck DFSP is usually treated with parallel photon beams, a combination of photon and electron beam or with electron beam alone. Scalp being a region with very little subcutaneous tissue, treatment with electrons has the obvious disadvantage of the option of an appropriate low energy selection and dose to underlying bone. Photons also have the disadvantage of appreciable exit dose and dose to underlying brain parenchyma. Customised surface mould HDR brachytherapy with 192Ir sources has the obvious dosimetric advantage of sharp dose fall off and ease of dose conformity achieved by customisation of the mould on curved surfaces. More so, since there is underlying subcutaneous bone in the scalp region, HDR brachytherapy with Ir 192 is an obvious choice as treatment with 4 MeV, 6 MeV electron or 50 kV X-ray leads to much higher dose deposition in the bone.Reference Safigholi, Song and Meigooni 13 Results of customised surface mould brachytherapy for nonmelanomatous skin cancers of the head and neck and scalp region is extremely satisfactory with excellent long-term local control rates (81–92%) and acceptable cosmesis.Reference Guix, Finestres and Tello 14 , Reference Delishaj, Rembielak and Manfredi 15 The use of interstitial HDR brachytherapy in adjuvant setup for soft tissue sarcomas is well documented.Reference Alektiar, Leung, Zelefsky, Healey and Brennan 16 – Reference Alekhteyar, Leung, Brennan and Harrison 18 For superficial and cutaneous soft tissue sarcoma, like angiosarcoma and Kaposi’s sarcoma there have been reports of use of surface brachytherapy and surface applicators with acceptable results.Reference Ozyar and Gurdalli 19 , Reference Evans, Yassa, Podgorsak, Schreiner and Souhami 20 Sanada et al.Reference Sanada, Nakamaya, Irisawa, Okubo, Tsuboi and Tokuuye 21 reported the use of customised surface mould brachytherapy for treatment scalp angiosarcoma with HDR brachytherapy using Ir 192 to a dose upto 60 Gy with acceptable local control, with none of the nine patients experiencing grade IV or more skin toxicity. Mukherji et al. in a recent report has described the use of customised surface mould brachytherapy for boost along with external beam radiotherapy (EBRT) for three patients with dermatosarcoma of the shoulder and trunk region, in a postoperative setting, with positive deep resection margin, with no recurrences at 2 years.Reference Mukherji and Sinnatamby 22 Here we report, probably for the first time, the use of customised surface mould HDR brachytherapy as adjuvant radiotherapy in a cutaneous fibrous soft issue tumour of the scalp with good local control and acceptable cosmesis.
One of the major limitation of this report, apart from the fact that the follow-up duration is of only 22 months, is that the target or PTV delineation was based on a subjective assessment of the patient by the oncologist, based on preoperative CT images and the scar. The dose prescribed was slightly lower than the European consensus recommendations with EBRT for R1 resected tumours, but since the isodose range 100–150% covered the PTV; the effective dose would have been higher.
Conclusion
The present case report advocates on the role of surface mould HDR brachytherapy with 192Ir source, as an alternative option to EBRT in any form, to prevent recurrence in an adjuvant setting for this aggressive cutaneous soft tissue sarcoma. This is especially so in areas or regions like scalp where there is underlying superficial bone and the curvature makes treatment with modalities like electron and photon difficult.
Acknowledgement
The authors sincerely acknowledge all the staff and faculty of the Department of Radiation Oncology, Medical College Kolkata for their support in preparing this case report.