Introduction
Across the world, gastric cancer remains an important cause of cancer-related mortality, with approximately 1,313,000 new cases annually and an estimated 819,000 deaths, making it the third-leading cause of cancer-related deaths.Reference Fitzmaurice, Allen and Barber1 The strategy for the treatment of stomach cancer is still controversial.Reference Kamran, Hong and Wo2 Adjuvant therapies, such as chemotherapy, radiotherapy (RT) and chemoradiotherapy, have been used in clinical applications for years and proved to be effective in the stomach cancer.Reference Boda-Heggemann, Hofheinz and Weiss3,Reference Oblak, Vidmar, Anderluh, Velenik and Jeromen4 Radiation therapy, generally administered with concomitant fluoropyrimidine-based chemotherapy, is also indicated for locally confined gastric cancer that either is not technically resectable or occurs in medically inoperable patients.Reference Perez, Brady, Halperin and Wazwe5 Anatomically, stomach is located in close proximity to critical organs such as kidneys, heart, liver and spinal cord. Adjuvant RT of stomach cancer, doses placed by treatment planning system (TPS) on critical organs and target volumes have importance in terms of the control and toxicity. Traditionally, adjuvant radiation has been used with either two-dimensional (2D) or three-dimensional conformal RT (3D-CRT). Intensity-modulated RT (IMRT) differs from 3D planning through the delivery of radiation dose by partitioning a radiation field into multiple smaller fields of various shapes and sizes and varying the dose intensity between each area. This is performed with either dynamic IMRT (the multi-leaf collimators (MLCs) move in and out of the radiation beam path during treatment) or ‘step-and-shoot’ IMRT (MLCs change the radiation field shape while the beam is turned off).Reference Bortfeld, Kahler and Waldron6,Reference Xia and Verhey7 Either method is effective at conforming radiation dose to the target structures while avoiding dose to normal tissue. It has been discussed in many studies which 3D-CRT or IMRT is better for gastric cancer RT.Reference Chung, Lee, Park, Lu and Xia8–Reference Dahele, Skinner, Schultz, Cardoso, Bell and Ung12
The purpose of the present study was to undertake a dosimetric comparison of three different RT techniques as 3D-CRT, WB-CRT and IMRT with respect to the doses received by the planning target volume (PTV) and organs at risk (OARs) including the right kidney, left kidney, liver, heart and spinal cord, dose homogeneity index (DHI), conformity indexes (CIs) and total monitor unit (MU) counts required for the treatment. Therefore, our objective was to examine the best dose to target volume and the most appropriate treatment planning method for maximum protection of OARs for the RT treatment of gastric cancer using dosimetric analysis and to evaluate which treatment plan was best for the gastric cancer.
Methods and Materials
Patient selection
Ten consecutive patients with histologically confirmed stomach cancer were selected for the study. All patients were presented with a supine position with a chest board used to rest their arms over their heads. Computed tomography (CT) planning scans were performed using a CT scanner with a 3 mm slice thickness. The data obtained from CT were transferred to the TPS (Eclipse, version 15.1; Varian Medical System Inc., Palo Alto, CA, USA).
Target volumes and OARs
All clinical target volumes (CTVs) were contoured according to recommendations of consensus guidelines by the same radiation oncologist using the Varian Eclipse Operation version 15.1 treatment planning software.Reference Xia and Verhey7 CTV included all regions of potential microscopic disease, and PTV was generated by expanding the CTV 10 mm isotropically. Normal tissues including the right kidney, left kidney, liver, heart and spinal cord were contoured as OARs in the TPS. The combined left and right kidney’s OAR volumes were considered the kidneys OAR volume.
Treatment planning
The treatment plans were generated using 3D-CRT, WB-CRT and IMRT techniques. All plans were carried out using the Eclipse TPS (Varian Medical Systems, Inc., Palo Alto, CA, USA). The prescribed dose, which was defined as the mean dose in the PTV, was 45 Gy in 25 fractions at 1·8 Gy per fraction. The 100% of the PTVs were covered by 95% of the prescribed dose. For 3D-CRT, treatment plans were generated using four-field box technique using 18-MV photons that were used to treat the PTV. 3D-CRT plans were created using one anterior, one posterior and two lateral fields. Irregular beam portals of the four field plans were shaped with MLCs and manually optimised using the beam’s eye view technique. Beams were individually optimally weighted to provide the best PTV coverage. For WB-CRT, four coplanar radiation fields with angles of 0˚, 90˚, 180˚ and 270˚ covering the target volumes with physical wedges and MLC leaves were generated to achieve the best plan. For IMRT plans, five non-coplanar fields (gantry angles: 0°, 40°, 100°, 285° and 320°) were generated with a sliding window technique with inverse planning. IMRT planning was performed using 6 MV photons designed to treat. Anisotropic analytical algorithm dose distributions were calculated after optimisation with inverse planning. All the plans were normalised to target the mean dose. The plan accepting criteria were set that at least 95% of the prescribed dose should cover the target volume.
Dosimetric measurements
Dosimetric evaluation of doses to OARs was performed using quantitative analysis of normal tissue effects in clinics (QUANTEC) parameters.Reference Chung, Lee, Park, Lu and Xia8 Maximum and mean dose (Dmax and Dmean), HI, CI, MU and delivery time were compared for the PTV. For OAR, the values of interest in this study including Dmax, Dmean, V13Gy, V20Gy and V28Gy for the right, left and total kidneys, Dmax, Dmean, V20Gy and V30Gy for the liver, Dmax, Dmean, V30Gy for the heart and Dmax for the spinal cord were compared. DHI was defined according to the International Commission on Radiation Units and Measurements Report 83.13 Dose homogeneity in the PTV for all techniques was compared by means of the DHI.Reference Yoon, Park and Shin14
In this formula, D98 is the maximum dose absorbed in that 2% of the PTV least irradiated, D2 is the minimum dose absorbed in that 2% of the PTV most irradiated. CI is defined by a ratio of reference isodose volume to target volume of PTV.Reference Serarslan, Okumus, Gursel, Meydan, Dastan and Aksu15 The value of CI varies between 0 and 1, and a value closer to one indicates better conformity of dose to the PTV. The 95% isodose volume was taken as the reference volume of the PTV.
Statistical analysis
All statistical analyses were performed using SPSS software (Ver. 16.0; SPSS Inc., Chicago, IL, USA). Paired, two-tailed Wilcoxon signed-rank tests were used for comparisons. A value of p < 0·05 was considered to be statistically significant.
Results
The dosimetric comparison for PTV coverage, HI, CI, MU and delivery time is tabulated in Table 1. According to the results from the study, average mean doses for PTV were 45·36 ± 0·41, 45·21 ± 0·35 and 45·47 ± 0·39 for 3D-CRT, WB-CRT and IMRT, respectively. For the three techniques, desirable PTV coverage, with 95% of the prescribed dose, is covering at least 95%. Figure 1 shows the evaluation of the 3D-CRT plan with IMRT plan and Figure 2 shows the evaluation of the WB-CRT plan with IMRT plan. There was not any significant difference with respect to minimum and mean doses of the PTV. The CI values were 0·87 ± 0·14 for 3D-CRT, 0·89 ± 0·13 for WB-CRT and 0·97 ± 0·11 for IMRT. When compared to IMRT with the 3D-CRT and WB-CRT plans, IMRT was significantly closer to a value of 1 (p < 0·05). Concerning the calculated dose, HI value for 3D-CRT was 0·09 ± 0·01, for WB-CRT was 0·17 ± 0·20 and for IMRT was 0·08 ± 0·02. The smaller HI means more homogeneous dose distribution to PTV, so IMRT plans gave significant results than other plans. The MU counts required for 3D-CRT, WB-CRT and IMRT plans were 191·60 ± 5·89, 208·70 ± 8·94 and 875·50 ± 60·21, respectively. 3D-CRT technique delivers fewer MUs compared to other plans (p < 0·05). The statistical dosimetric comparison for OARs was given in Table 2. Dose–volume histograms obtained from plans were showed for target volumes and critical structure (Figure 3). The mean and V13 doses for the right kidney were significantly lower in 3D-CRT plans than WB-CRT and IMRT (p < 0·05). When we compared the mean, maximum, V13, V20 and V28 doses for the left kidney, all of them were in favour of IMRT techniques (p < 0·05). Regarding the total kidneys doses in patients, all kidney parameters, including kidneys Dmean, V13, V20 and V28 were significantly lower in IMRT plans (p < 0·05). The liver dose in patients, all liver parameters, including heart Dmean, Dmax, V20 and V30 was significantly lower in IMRT plans than 3D-CRT and WB-CRT (p < 0·05). When we compared the heart dose in patients with gastric cancer, heart Dmean and V30 were significantly lower in IMRT techniques (p = 0·000). For the spinal cord, the average maximum doses were 31·91 ± 5·43, 32·97 ± 1·24 and 27·49 ± 2·02 for 3D-CRT, WB-CRT and IMRT techniques, respectively. When IMRT plans were compared with 3D-CRT and WB-CRT, there was a statistically significant difference in maximum doses to the spinal cord for the IMRT (p < 0·05). Moreover, concerning the lung mean dose tended to be lower in the IMRT technique than 3D-CRT and WB-CRT (p < 0·05).
Table 1. Dosimetric results for planning target volume

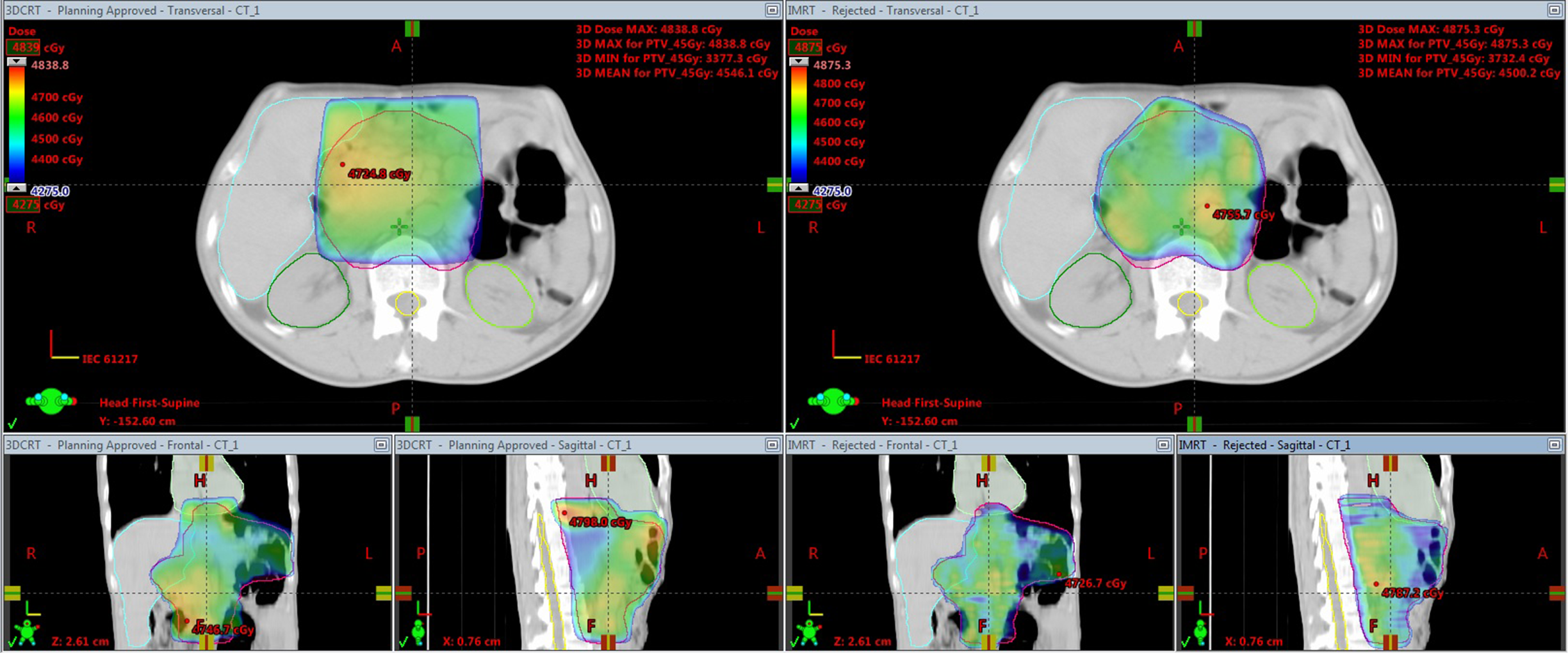
Figure 1. The comparison of dose distribution between IMRT plan with 3D-CRT plan.

Figure 2. The comparison of dose distribution between IMRT plan with WB-CRT plan.
Table 2. Dosimetric results for planning target volume in locally advanced stomach cancer


Figure 3. Dose–volume histogram comparison of a patient red: PTV, dark green: kidneys, light green: heart, yellow: spinal cord, blue: liver ![]() : WB-CRT techniques
: WB-CRT techniques ![]() : IMRT technique and
: IMRT technique and ![]() : 3D-CRT techniques.
: 3D-CRT techniques.
Discussion
In this study, the dosimetric advantages were evaluated in 3D-CRT, WB-CRT and IMRT for stomach cancer. Dosimetric points were performed to evaluate the dose to the target volume and normal tissues. According to the results obtained in this study, we found similar PTV coverage in all treatment techniques. When the dosimetric analysis is examined, particular emphasis was placed on improving the sparing of kidneys, liver, heart and spinal cord. The kidneys are very important organs, which are at risk in stomach cancer RT because of the radiosensitivity of renal tissue. In the present study, dose to total kidney was evaluated as mean, maximum, V13, V20 and V28. For the total kidney, compared with IMRT to 3D-CRT and WB-CRT resulted in significantly lower in all parameters for IMRT. For the stomach cancer RT, numerous studies have compared different RT methods. Wang and colleagues compared the dosimetric advantages in 3D-CRT, IMRT and single-arc VMAT treatment plans.Reference Wang, Li, Zhang, Bai, Xu, Wei and Gong9 Their results suggested that IMRT and VMAT methods showed similar target dose, but were superior to 3D-CRT method. They also suggested that IMRT and VMAT treatment plans performed an advantage with respect to OAR sparing compared to 3D-CRT plans. In another study, Serarslan and colleagues compared the dosimetric differences using physical WB-CRT, field-in-field IMRT and IMRT.Reference Serarslan, Okumus, Gursel, Meydan, Dastan and Aksu15 Techniques were evaluated with respect to expected target volume coverage and the dose to OAR. They suggested that IMRT was superior to WB-CRT (p = 0·005 for the V20 for the right kidney; p = 0·001 for the D-mean in the left kidney; p = 0·023 for the V20 for the left kidney). Zhang and colleagues compared the dosimetric advantage of 3D-CRT, IMRT and double-arc VMAT in gastric cancer. They found that VMAT and IMRT treatment plans achieved superior PTV coverage compared to 3D-CRT.Reference Zhang, Liang and Han16 In 2010, Minn and colleagues were published the first report on the results of a comparison of the dynamic techniques (IMRT) and CRT for post-operative gastric cancer.Reference Minn, Hsu and La17 They found that the V20 dose for kidneys as a single organ was lower for the IMRT plans compared to 3D-CRT plans. It is found that IMRT method is statistically more advantageous with respect to V13, V20, V28 and mean dose. IMRT improves kidney sparing. Our study results were overall consistent with the above studies. Radiation-induced liver disease has been a critical issue for patients with primary liver cancer treated with radiation. Because of the large radiation volume required for stomach cancer, it is very important to protect the liver from radiation injury when using RT to treat gastric cancer. Dosimetric parameters, such as the mean liver dose and V30, are predictors of increased toxicity risk during partial liver radiation.Reference Kim, Kim and Park18,Reference Xu, Zhu and Zhao19 In our study, we found that IMRT technique was the most consistent in allowing the lowest mean and max dose to the liver compared to 3D-CRT and WB-CRT techniques. IMRT significantly improved the sparing of the mean irradiated volume of the liver. Similar results were found for liver in volume-based criteria V30. Minn and colleagues compared the dosimetric advantage using IMRT versus 3D-CRT for stomach cancer and they found that IMRT techniques better protected the liver. The stomach is located on the left side of the body as is the heart, so the cardiac radiation dose administered in RT of stomach cancer is of importance. According to the results obtained with the study, we found that all parameters of heart dose (Dmean and V30) were significantly lower for IMRT treatment plans. The highlight of our results showed that IMRT achieved the most favourable dose improvements with respect to heart sparing in stomach cancer.
Based on the dosimetric results in this study, for stomach cancer, there is a potential clinical advantage for IMRT technique compared to 3D-CRT and WB-CRT. IMRT technique was found to be the effective to reduction dose in kidney, liver, heart and spinal cord. It is seen that IMRT technique is statistically more advantageous compared to 3D-CRT and WB-CRT RT plans with respect to OARs. In addition, IMRT provides superior dose conformity and homogeneity. All three techniques could be used in such treatments to achieve comparable PTV coverage and sparing of the OARs. Since different clinical requirements apply to the different RT treatments, the choice of approach should be determined on an individual basis. In the current study, there have been several limitations. This is a dosimetric study, and it does not include vital aspects required for clinical use. The number of patients used for comparison was limited to ten; this may be improved in the next study to obtain a better sample.
Conclusion
Three advanced RT techniques were compared in this dosimetric study. It is seen that IMRT plans are statistically more advantageous against 3D-CRT and WB-CRT plans at the RT technique to be applied the patients for stomach cancer. Among three treatment methods, IMRT might reduce the kidney, liver, heart and spinal cord doses. IMRT plans improved the conformity and homogeneity of the dose distribution in the target volume and provided sufficient PTV coverage. Based on compared target dose distribution and estimated risks for OARs, IMRT might be the appropriate technique for stomach cancer.
Acknowledgements
None.
Conflict of Interest
The authors have no conflicts of interest.








