Introduction
Breast cancer is the most frequent neoplasm in women. Due to improved screening programmes, early diagnosis approach and higher quality of treatments, the overall survival rate has increased in recent years. 1 It is known that 80% of women with breast cancer may benefit from radiation therapy. Reference Poortmans2 Regarding radiotherapy for breast conserving treatment, studies show an improved breast control rate when additional dose to the tumour bed is given after conventional whole breast radiotherapy. Reference Franco, Cante and Sciacero3
For years, breast radiotherapy technique has remained intact: two opposed tangential fields to optimise planning target volume (PTV) coverage while avoiding exposure to ipsilateral lung and other organs at risk (OAR). Nevertheless, planning dynamic techniques such as field in field (FIF) Reference Fournier-bidoz, Kirova, Campana, Dendale and Fourquet4 have evolved into more complex planning and the shortening of overall treatment times with simultaneously delivered boost dose Reference Franco, Cante and Sciacero3 introducing a wide range of possibilities for breast treatment.
Currently, a gold standard boost does not seem to be established [different techniques were evaluated such as intensity-modulated radiation therapy (IMRT), electron boost and non-coplanar photon fields Reference Fournier-bidoz, Kirova, Campana, Dendale and Fourquet4 ]. To overcome potential issues such as adjoining nodes irradiation or additional dose escalation of the tumour bed in a simultaneous integrated boost (SIB) scheme, volumetric-modulated arc therapy (VMAT) is being implemented to whole breast irradiation or for partial arcs in hybrid techniques. Reference Orton, Halle, Chang and Artor5-Reference Vaegler, Scheibner and Hartwig7
Standard of care for boost breast radiotherapy is to administer radiation sequentially to the whole breast and then to the tumour bed. Nevertheless, SIB techniques are currently under study whether they are more or equally effective compared to conventional schemes. Advantages of this method are, for example, the ability to increase boost dose while keeping the remaining volume of breast with an acceptable dose, studied in a VMAT–SIB hypofractionation treatment by Scorsetti et al. Reference Virén, Heikkilä, Myllyoja, Koskela, Lahtinen and Seppälä8 Moreover, this technique reduces visits to the radiotherapy department, therefore making adjuvant radiotherapy a more comfortable process for these patients. Reference Scorsetti, Alongi and Fogliata9 A comparison study of this variety of techniques (tangential fields, IMRT and VMAT) included in an SIB configuration for breast radiotherapy is still being carried out. Reference Nitsche and Hermann10-Reference Aly, Glatting, Jahnke, Wenz and Abo-Madyan12
One of the difficulties in SIB treatment is locating the isocentre position for both boost and breast volumes. Boost conformity could be dependent on this election as the majority of hybrid breast radiotherapy approaches were placed on the breast centre of mass (CoM). Field experts have not reached an agreement in the optimum technique for an SIB breast treatment or boost technique. The aim of this study is to propose a metric Breast Boost Vector (BB Vector), which could help in the election of the isocentre placement in a breast hybrid forward IMRT (fIMRT)–VMAT technique.
Material and Methods
Study setup
A random selection of 22 female patients with breast carcinoma from March 2019 to September 2019 (12 left side and 10 right side), already treated in our Radiation Therapy Department, were included retrospectively in this study. Patients with positive axillary lymph nodes and distant metastasis were excluded.
A volumetric scan was performed with a CT scanner Aquilion LB (Canon Medical Systems, Otawara, Japan) in decubitus supine with a slice thickness of 3 mm. A breast board BreastSTEP (Elekta AB, Stockholm, Sweden) was used for patient immobilisation and comfort. Both arms were positioned above the head in a free breathing technique.
Structure contouring was made by a radiation oncologist specialised in breast radiotherapy following the Radiation Therapy Oncology Group breast cancer atlas Reference Smith, Estoesta and Kader13 as a guideline for delineation of the clinical target volumes (CTVs). CTV breast (CTVbreast) includes the apparent CT glandular breast tissue, and PTV breast (PTVbreast) was created adding 5 mm isotropically to the CTVbreast but limited to 3 mm within the skin surface. The gross tumour volume (GTV) was delineated including the clips placed in the tumour bed during surgery. PTV boost (PTVboost) was generated with the depth, width and height of the tumour bed, including GTV + 1 cm. PTVbreast volume for patients in the study has a mean and standard deviation of 1,184 ± 488 cm3, whereas for PTVboost volume, 92 ± 41 cm3. In addition, OAR were contoured: heart, both lungs and contralateral breast.
For planning evaluation, Eclipse v13.6 software was used (Varian Medical Systems, Palo Alto, CA, USA).
Only one scheme of fractionation was applied to the set of patients. The hypofractionation adopted for SIB treatment in our centre involves a dose of 45·9 Gy to the breast and 51·3 Gy to the tumour bed, simultaneously during 18 sessions (equivalent dose in 2 Gy fractions: EQD2 = 50·5 Gy to breast, 59·2 Gy to tumour bed, with α/β = 3·5 Gy Reference White, Tai and Arthur14 ). The contribution of VMAT therapy to the tumour bed was 5·40 Gy, which is 0·3 Gy per session.
Treatment planning
PTVbreast plan used two 6-MV tangential fields with multi-leaves collimator (MLC) conforming to the PTV, the internal jaws aligned to minimise dose to OAR and exceeding 3 cm beyond contour in external jaws to account for small changes in size and position of the target due to breathing motion. Supplementary FIF was used to achieve desired homogeneity and reduces hot and cold spot areas, resulting in an fIMRT plan (an iterative process through a manual fluence optimisation).
For PTVboost, a VMAT plan was created as the same isocentre as the first plan and comprised a partial arc travelling from internal field to external field angulation with jaws restricted to PTVboost to avoid healthy tissue irradiation. During PTVboost optimisation, the previous PTVbreast plan was used as a base dose plan for keeping OAR and PTVs homogeneity into desired values. Reference Leeuwen, Oei and Crezee15
The hybrid technique convoluted two plan sets resulting in a three dynamic field plan (Figure 1).
Plans were accomplished using Analytical Anisotropic Algorithm with a calculation grid of 2·5 mm, and they were developed for a Varian Clinac 21iX (Varian Medical Systems) equipped with a Millennium 120 MLC and RapidArc technology. The results of both plans were merged into a hybrid plan designed for treatment.
BB vector
In order to analyse the position of the CoM of the tumour bed and to characterise boost position in reference to PTVbreast, we define a metric: BB Vector, which measures the distance between the centres of mass of the two PTV volumes involved.
where (x 1, y 1, z 1) and (x 2, y 2, z 2) are the coordinates (in centimetres) of the CoM of the PTVbreast and PTVboost, respectively. Figure 2 shows a plot of a BB Vector example for a patient of this study.

Figure 1. (a) fIMRT tangentials, (b) VMAT boost, (c) hybrid technique (a) + (b). Red contour: PTVbreast, blue contour: PTVboost.
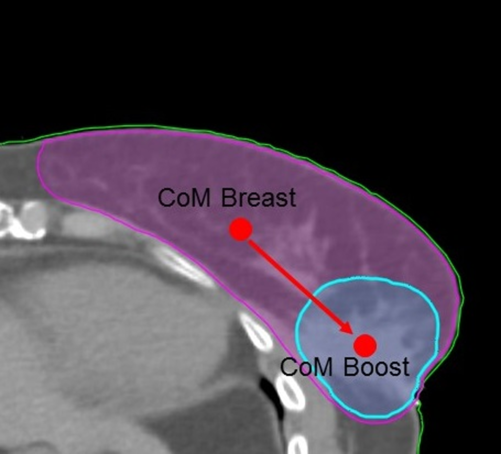
Figure 2. BB Vector: distance from CoM of PTVbreast to CoM of PTVboost.
Variations with isocentre placements and their influence over dose distribution were examined for each patient using hybrid plans with different isocentre locations. One placed at the breast CoM (Iso_CoM_Breast) and another at the boost CoM (Iso_CoM_Boost) (Figure 3).

Figure 3. Schematic presentation of isocentre placements: (a) isocentre at the breast CoM and (b) isocentre at the boost CoM.
Figure 4 shows an example of the hybrid plan isodoses for one of the patients in the study: 55 Gy (107% of the boost prescription dose), 51·3 Gy (100% of the boost prescription dose), 48·7 Gy (95% of the boost prescription dose), 45·9 Gy (100% of the breast prescription dose) and 43·6 Gy (95% of the breast prescription dose).

Figure 4. Isodoses of the SIB technique.
Data analysis
Table 1 shows the metrics analysed in order to evaluate plan quality. Conformity index (CI) and homogeneity index (HI) were defined for the boost treatment, quality index (QI) and heterogeneity index (HeI) for breast and dose index and total monitor units (MU) for the hybrid plan.
Table 1. Metrics

a V 5·4Gy is the volume with a dose of 5·4 Gy, V PTVboost is the volume of the boost and D2% is the dose that receives the 2% of the boost volume.
b D2% is the dose that receives the 2% of the breast volume and D98% is the dose that receives the 98% of the breast volume.
c V 51·3Gy is the volume with a dose of 51·3 Gy, V 45·9Gy is the volume with a dose of 45·9 Gy, MU_Boost are the unit monitors of the arc and MU_Breast are the unit monitors of the fIMRT tangentials.
For evaluating the coverage of PTVboost of the two hybrid plans, relative differences between CI versus BB Vector were represented as
where CI1 was the boost CI for plans centred at the breast CoM and CI2 was the boost CI for plans centred at the boost CoM.
Ipsilateral lung and heart were accounted as OAR. An additional auxiliary organ, free breast of boost (FBB), defined as PTVbreast − PTVboost was analysed to characterise the influence of the boost arc on the hybrid plan. Dose spillage metric was used for FBB evaluation considering the volume of the isodose of 95% of the dose divided by PTVboost volume:
Statistical analysis
Descriptive statistics of the results were presented as the mean values ± standard deviations. The difference between the mean values for the two sets of planning was compared by one-factor analysis of variance analysis using Minitab Statistical software v18.1 (Minitab, LLC, www.minitab.com). Statistically significant findings were assumed for a significance level of p < 0·05.
Results
The values of the BB Vector for the 22 patients under study are shown in Figure 5. The mean value and standard deviation were 4·5 ± 1·6 cm.

Figure 5. Values of the BB vector for the 22 patients under study.
Table 2 presents the evaluation parameters of the RapidArc technique. For VMAT Boost plan, CI and HI were, respectively, 0·85 ± 0·12 and 1·07 ± 0·03 when placing in the Iso_CoM_Breast. Whereas at the Iso_CoM_Boost, metric results were 0·87 ± 0·12 and 1·07 ± 0·03, respectively.
Table 2. Metrics for RapidArc boost

Results and standard deviation of CI and HI.
Figure 6 shows relative variations in CI vs. BB Vector for both hybrid plans (Iso_CoM_Breast and Iso_CoM_Boost) of the 22 patients under study. ΔCI values close to one mean no differences between a boost planned at the boost CoM or at the breast CoM. Whereas for patients with high BB Vector, Figure 6 suggests a correlation between values, showing an enhancement of CI for VMAT plan centred at the boost CoM.

Figure 6. Relative CI variations vs. BB Vector.
Table 3 presents metric parameters of the fIMRT tangentials. QI and HeI were 0·88 ± 0·03 and 1·19 ± 0·04 at the Iso_CoM_Breast. Whereas at the Iso_CoM_Boost, metric results were 0·88 ± 0·02 and 1·18 ± 0·03.
Table 3. Metrics for fIMRT breast

Results and standard deviation of QI and HeI.
Table 4 shows SIB analysis results and OAR dosimetric parameters. Both hybrid plans used the same MUs: 360 ± 10 (Iso_CoM_Breast) and 360 ± 16 (Iso_CoM_Boost). Dose index values are also close between them: 9 ± 4% at the Iso_CoM_Breast plans and 8 ± 4% at the Iso_CoM_Boost plans. The mean dose spillage is about 11% higher at the Iso_CoM_Breast plan, 2·76 ± 0·58 vs. 2·48 ± 0·64.
Table 4. Analysis for hybrid plan comparison

Results with standard deviation were added.
a MUs, DI and dose spillage are used to SIB isocentre comparison.
b OAR dose analysis included V 5Gy, V 15Gy and D mean: mean dose for ipsilateral lung, V 5Gy and D mean for heart and D mean for FBB.
c Heart dose analysed for left-side pathology.
All OAR dose constraints of our protocols were respected in the two techniques. Hybrid plans were able to record V 5Gy < 30%, V 15Gy < 15% and the mean dose < 8 Gy in ipsilateral lung, V 5Gy < 16% and the mean dose < 5 Gy in heart and the mean dose of 46 Gy for FBB. No substantial differences were noted between the two arms.
Discussion
Nowadays, linacs are able to deliver high-dose distributions and mixing techniques with the robustness of conventional radiotherapy and advantages of dynamics techniques. Nevertheless, the implementation of hybrid modalities needs to be simultaneously optimised to avoid possible conflicts. These hybrid approaches for SIB treatments are being investigated to reduce heart dose 16 or in comparison with other techniques. Reference Aly, Glatting, Jahnke, Wenz and Abo-Madyan12,Reference Jöst, Kretschmer and Sabatino17
Although volume contouring is carried out by a single radiation oncologist specialised in breast cancer treatment following international guidelines, extending this study to a wide variety of Radiation Oncology Departments could be interesting to address potential sources of bias. Reference Mourik, Elkhuizen, Minkema and Duppen18
In this study, we analysed isocentre positions when using boost arc for hybrid SIB radiotherapy. Viability of different treatment isocentre placements was also analysed in other studies. Reference Moragues, Pozo and Casals19 Plans treated from the breast CoM may lead to a reduction of boost conformity and overdose PTVbreast with PTVboost dose prescription. Therefore, planning was strongly influenced by the location of PTVboost inside PTVbreast. Outer boosts could be treated differently, an isocentre placed in the PTVboost CoM. In that sense, our BB Vector metric proposal might be a tool for these peripheral boosts. Figure 6 suggests that there is a point when PTVboost conformity in plans centred at the boost CoM starts to increase in comparison with plans centred at the breast CoM. This point starts to be noticeable for patients with high BB Vector, superior to 5 cm.
This hybrid technique is highly based on tangential robustness and slightly on dose delivered to tumour bed (5·4 Gy). Nevertheless, exposing boost to higher doses (16 Gy Reference Armpilia, Antypas, Zygogianni, Balafouta and Sandilos20 ) improves local control despite decreasing the overall treatment time for patient convenience. Hence, in an examination of optimal SIB treatment, VMAT was incorporated into our scheme Reference Byrne, Archibald-Heeren, Hu, Fong, Chong and Teh6 to solve boost issues. This technique was implemented in other studies, for example, SIB treating the whole breast with radiotherapy Reference De Rose, Fogliata and Franceschini21 or a study by Fogliata et al. about dosimetric trade-offs in breast planning with VMAT. Reference Fogliata, Seppälä and Reggiori22 Potential downsides of low-dose bath were limited with this hybrid approach; VMAT is 10·5% of the overall treatment. In addition, the use of inverse planning helps to supply a higher boost CI.
Arc travelling influences PTVboost conformity and overdose to healthy tissue (FBB). In this study, VMAT partial arc was limited into the medial and lateral beam angles. This may become an issue when an isocentre placed at the breast CoM causes a spillage of VMAT boost dose which in turn inhomogeneities into PTVbreast. Moreover, PTVboost location in breast volume influences dose spillage to FBB; consequently, it had made to investigate little modifications in VMAT boost keeping the benefits of this technique. Placing the isocentre close to boost CoM hardly decreases the mean dose to FBB (46·71 ± 0·35 vs. 46·62 ± 0·42). In addition, results of CI and HI showed no significant differences between isocentres (0·85 ± 0·12 vs. 0·87 ± 0·12, p = 0·675; 1·07 ± 0·03 vs. 1·07 ± 0·03, p = 0·929). Dose to OAR was highly dependent in tangential fields. Boost dose is a small percentage of the total prescript SIB dose and can slightly weight in the treatment. Hybrid plans centred at different CoMs show no significant differences in OAR dose analysis.
Considering the study results, it seems that the use of individual BB Vector could help in choosing the optimal technique. It will allow us to distinguish which plans would benefit from a change of isocentre, and with this planning variation, conformity of these marginal boosts will be enhanced without compromising the strength of the hybrid plan. Nonetheless, small size of this sample leads us to conclude that these results should be interpreted with caution and investigated in depth in further studies.
Conclusions
BB Vector has been defined as a metric that helps to modify the choice of the isocentre in hybrid plans for breast cancer treatments.
Two isocentre positions have been evaluated: Iso_CoM_Boost and Iso_CoM_Breast, and we have verified that, when the BB Vector is greater than 5 cm, it could improve PTVboost coverage by placing the isocentre in the Iso_CoM_Boost, while in the rest of the cases, the difference is not noticeable. Regarding metrics and OAR dose analysis, there are no statistical differences between the isocentre approaches.
Acknowledgements
The author would like to thank Sean Cerezo McGee for his constructive criticism on the manuscript.
Conflict of Interest
The authors declare that they have no conflict of interest.













