Introduction
Oesophageal cancer is the 13th most common cancer in the UK and the 8th most common in men.1 It is linked to older age, male sex, smoking and excess alcohol consumption. Historically, adenocarcinoma has been the predominant type in the UK and is increasing in incidence.Reference Vizcaino, Moreno, Lambert and Parkin2 Squamous cell carcinoma is the more common type in other areas such as Asia and the Middle East.Reference Leichman and Thomas3 In 2009, the 5-year survival for oesophageal cancer was 12·5%.1 Commonly, patients will present with symptoms of locoregional diseases or metastases. The best chance of a curative treatment is achieved by surgery with peri-operative oncological treatment; however, many patients are not suitable due to higher stage of disease, co-morbidities, poor physiological function or a poor World Health Organisation (WHO) performance status.Reference Allum, Stenning, Bancewicz, Clark and Langley4 If they do not have metastatic disease, then they may be suitable for definitive radical chemoradiotherapy.Reference Allum, Blazeby and Griffin5
Dysphagia is common in patients presenting with oesophageal malignancy and can be exacerbated during radiotherapy due to treatment-related oesophagitis. It improves slowly and variably during or after radiotherapy treatment due to tumour shrinkage. However, if the patient is only able to swallow liquids at presentation, it is likely that oral nutrition will become problematic during treatment. Furthermore, an inability to swallow will affect the patient’s nutritional status and their quality of life (QOL).Reference Lagergren, Avery and Hughes6 It is common for patients to lose weight during chemoradiotherapy for oesophageal carcinoma. In part, this is due to side effects of treatment such as chemotherapy-induced nausea and vomiting (CINV), radiation oesophagitis-related pain or worsening dysphagia. This malnourishment can delay the healing of irradiated tissues further perpetuating a prolongation of symptoms from chemo-radiation toxicity. In patients receiving pelvic radiotherapy, it is known that presence of malnourishment before or during treatment leads to increased treatment toxicity and decreased chance of cure.Reference Nitenberg and Raynard7 It has been shown that improved nutritional intake can improve QOL in patients with gastrointestinal (GI) tract cancer undergoing radiotherapy.Reference Ravasco, Monteiro-Grillo and Camilo8
Several options exist to maintain nutrition during radiotherapy. Long-term nasogastric tubes are used by some centres, but these are cosmetically unacceptable to some patients and have a significant complication rate.Reference Mura, Bertino, Occhini, Mevio, Scelsi and Benazzo9 These can also frequently dislodge requiring repeated replacement. Percutaneous endoscopic gastrostomy may not be technically feasible due to tumour structuring and there is a small theoretical risk of seeding of the tumour during the insertion technique. Radiologically inserted gastrostomy (RIG) is the preferred option for many patients as a means of optimising nutrition and has been shown to be safe in these patients.Reference Lowe, Laasch and Stephenson10 However, patients often dislike these feeding tubes and there is concern that they should not be placed in patients who may have go on to have a radical oesophagectomy with gastric pull-up surgery. However, evidence for this is lacking.Reference Bhatti, Rizvi and Waheed11 Furthermore, gastrostomy tubes negate the enjoyment of eating with the social aspect which goes along with this.Reference Brotherton, Abbott and Aggett12 In patients with poor survival, this may adversely affect the QOL. Patients who are planned to have surgery may have a feeding jejunostomy inserted at the time of staging laparoscopy, which can be used for enteral feeding. A recent study in oesophageal cancer patients undergoing chemoradiotherapy or surgery showed no benefit to enteral feeding tube placement versus none.Reference Starr, Davis, Ayala-Peacock, Blackstock and Levine13 However, this study examined biochemical ‘nutritional parameters’ such as serum albumin rather than anthropometric measurements or QOL.
Self-expanding metal stents (SEMS) are one option to achieve luminal patency and maintain oral nutrition. They can provide immediate relief of malignant dysphagia.Reference Sarper, Oz, Cihangir, Demircan and Isin14 However, they have a high rate of locoregional side effects and may be poorly tolerated, requiring a covering membrane to allow removal in symptomatic patients, or patients treated with curative intent.Reference Song, Lee and Seo15, Reference Jones and Griffiths16 Their role is controversial when planning to treat with radical radiotherapy. The metal can cause radiological artefacts due to scatter effects of the diagnostic radiation on the radiotherapy planning CT scan, which can make planning the radiotherapy treatment difficult. Also, there is a theoretical risk of perturbation of the radiation dose by the air gap which the stent produces.Reference Abu Dayyeh, Vandamme, Miller and Baron17, Reference Li, Chibani, Greenwald and Suntharalingam18 This is less likely to be the case with biodegradable stents as they produce less radial force. Furthermore, the synthetic material used in such stents has an in vivo tissue equivalent electron density which is applied adjacent to the tumour thus allowing good build-up of radiation dose where needed. Finally, as the tumour shrinks, covered SEMS can migrate or slip into the stomach and would have to be retrieved which would be traumatic for the patient and may lead to treatment interruption. This is less of a concern with biodegradable stents which will biodegrade quickly in an acidic environment. Therefore, SEMS are usually reserved for palliation.Reference Allum, Blazeby and Griffin5
The SX-Ella biodegradable (BD) stent (Ella-CS, Hradec Kralove, Czech Republic) is the only biodegradable stent available in EuropeReference Mullan, O’Dwyer and Laasch19 and can be seen in Figure 1. It is licensed for treatment of benign oesophageal strictures. These stents have been used off-label successfully for the temporary treatment of benign and malignant strictures throughout the GI tract.Reference Stivaros, Williams, Senger, Wilbraham and Laasch20–Reference Griffiths, Gregory, Pursnani, Ward and Stockwell25 SX-Ella BD stents are braided from a polydioxanone monofilament which was originally developed as a surgical suture material. The stents are radiolucent with seven radiopaque markers and flared ends which prevent migration (Figure 2). SX-Ella BD stents are loaded by hand into the delivery system prior to use (at which point significant elongation occurs, as seen with braided metal stents). The stents are available in diameters of 18–25 mm and lengths of 60–135 mm. The 28-Fr delivery system is designed for use in the oesophagus and the tortuous access route requires an ultra-stiff guide wire. The manufacturer reports that the radial expansion force of an 18-mm diameter BD stent is approximately 62% of the force exerted by the Wallflex nitinol stent (Boston-Scientific, St. Albans, UK) (166 g vs. 269 g, respectively); the force of the BD stent is also reduced in larger sizes. The radial force is maintained for 6–8 weeks. Biodegradable stents become noticeably disintegrated after 3 months and usually have disappeared after 4 months. This process is accelerated in an acidic environment and therefore patients should be prescribed proton pump inhibitors.Reference Stivaros, Williams, Senger, Wilbraham and Laasch20 The costs of biodegradable stents and metal stents are comparable.

Figure 1. Biodegradable stent and delivery system.
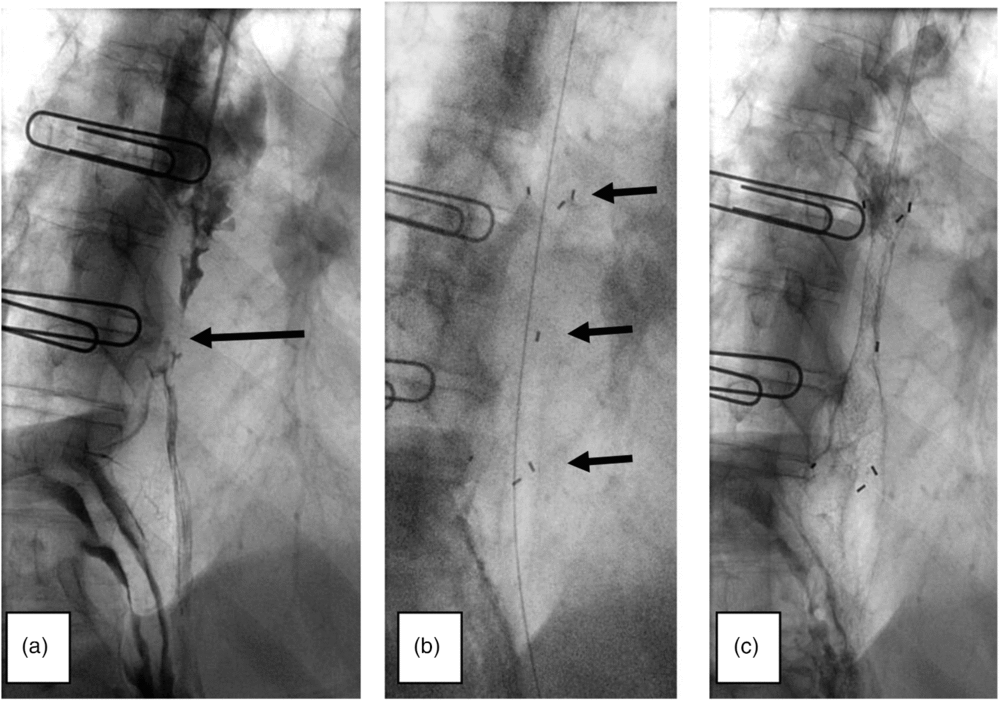
Figure 2. Fluoroscopically guided biodegradable stent insertion. (a) Arrow indicates malignant stricture identified upon injection of contrast medium. (b) Arrows indicate top, middle and bottom portions of stent which cross the extent of stricture. (c) Further injection of contrast then outlines stent.
Currently, BD stents are licensed for use in benign oesophageal strictures. Their use in malignant strictures remains unclear. They have been used at our centre as a treatment for malignant dysphagia, to maintain a degree of luminal patency and bridge patients through chemoradiotherapy without the need to subsequently remove the stent. Intuitive advantages include decreased radial force, decreased dose perturbation, decreased image scatter on RT planning CT scan and stability. They may have an added benefit in holding the oesophageal lumen open to some degree which may help to prevent fibrotic stricturing although this is theoretical. At our centre, they have often been combined with RIG insertion to improve nutritional status whilst also allowing the patient to maintain the psychosocial aspects of being able to swallow liquids and semi-solid diet throughout treatment.
The aim of this study was to determine the clinical effectiveness of BD stents for this indication and furthermore to establish the complication and re-intervention rates associated with their use.
Methods
This was a retrospective, observational study. Local approval was granted by the clinical audit department. Informed consent to each procedure was obtained on a patient–by-patient basis including explanation of the off-licence use of this type of stent. Clinical data including demographics, disease characteristics and therapy received were obtained retrospectively from the electronic records of 22 patients who had biodegradable stents inserted pre-radiotherapy treatment for oesophageal malignancy between 2008 and 2013. Further clinical data regarding O’Rourke score, weight, complications and re-intervention were collected from case notes. Procedural complications or failures were recorded. Complications occurring within the immediate 2-week period were recorded as were episodes of re-intervention required within 4 months of stent insertion. Patients were followed-up very closely in the year following radical radiotherapy and therefore any subsequent intervention was likely to be detected by reviewing the case notes. However, in order to avoid missing any episodes of re-intervention, the Computerised Radiology Information System (CRIS) system was also reviewed for radiological intervention which may have occurred at other centres.
Method of stent placement
The method of stent placement at this centre has been described elsewhere (Stivaros et al.Reference Mullan, O’Dwyer and Laasch19).
Results
Twenty-two patients underwent a biodegradable stent insertion prior to radiotherapy between February 2008 and March 2013. The patient demographics are shown in Table 1. The patients had a performance status of 0–2 with the exception of one patient with a performance status of 3.
Table 1. Patient demographics (n = 22)

The biodegradable stent was inserted prior to the radiotherapy planning scan in all patients. This CT scan was performed within 1 week of stent insertion in 15 patients and 1–2 weeks following stent insertion in five patients. Patients were asked to contact the nutritional support clinic or the clinical team in the event of any complications. One patient had treatment delayed due to recurrent stent obstruction and toxicity secondary to neoadjuvant chemotherapy for a synchronous primary and the planning scan occurred 37 days after stent insertion. One patient has no planning CT scan recorded on the Picture Archiving and Communication System (PACS). The mean time from stent insertion to commencement of radiotherapy was 17 days.
Nineteen patients received a radical course of radiotherapy delivered; 15 patients received a dose of 55 Gy prescribed in 20 daily fractions, whereas four of these patients had concurrent chemotherapy with a dose of 50 Gy in 25 daily fractions. One patient with borderline fitness commenced a radical course of treatment but only tolerated three fractions due to treatment toxicity. Two patients received a palliative course of 30 Gy in ten fractions.
All stents were inserted under continuous fluoroscopic control by two experienced radiologists. Two patients required two stents in one procedure, with a single stent placed in 20/22 patients. Three patients required pre-dilatation in the same procedure as stent placement and one stent was post-dilated within the same procedure. Stents were placed with the radiopaque metal markers overlying the epicentre of the tumour mass. Five patients had RIGs placed as a combined procedure, or as a separate procedure a few days before stenting. These patients had an initial modified O’Rourke dysphagia score of 3–5. There were no procedural complications.
Fourteen patients (64%) had modified O’Rourke dysphagia score recorded 1–3 weeks following stent insertion. This improved in eight patients, remained the same in four and deteriorated in two patients. Pre-stent insertion, the mean O’Rourke score was 3.5 (median 3, range 2–5). This improved to a mean score of 2.8 (median 3, range 1–4) 1–3 weeks following stent insertion. Only six patients (27%) had weight recorded 1–3 weeks following treatment although the majority had it recorded at baseline. This decreased in four patients and increased in two patients. No other anthropometric measurements were examined. The patients had a mean survival of 238 days (median 172, range 68–1114) after stenting.
There were no immediate procedure-related complications such as slippage of the stent or perforation; therefore, there was a 100% technical success rate. Complication data were available for all patients. Complications occurred in seven patients (32%) in an immediate 2-week period, including: pain (2), dysphagia requiring dilatation (1), food obstruction not requiring intervention (1), food obstruction requiring intervention (2) and upper GI bleed not requiring intervention (1). There were no major or life-threatening complications. Table 2 outlines the complications within 2 weeks and the re-intervention required in all patients.
Table 2. Complications within 2 weeks, re-intervention and initiation of supplemental enteral feeding (n = 11)

Re-intervention was required in 18% (four patients) within a 4-month period. This was balloon dilatation in two patients, dilatation then stent in one patient and SEMS insertion in one patient. The mean time to re-intervention was 27 days (median 8, range 7–86). Patients 3, 6 and 14 had complications which led to early intervention (within 2 weeks). Supplemental enteral feeding was required in five patients (this does not include the aforementioned patients who had an RIG inserted prior to or at the time of BD stent insertion). The route for this additional feeding was nasogastric (2), naso-gastric then RIG (1) and RIG (2).
Discussion
This novel use of the biodegradable stent which is licenced only in benign oesophageal stricturing disease provides a viable option for maintaining enteral feeding during (chemo-)radiotherapy for oesophageal malignancy. This may have benefits in terms of QOL with the potential avoidance of NG feeding. Unlike SEMS, biodegradable stents do not provide the same degree of relief from dysphagia. Instead, they maintain or improve luminal patency prior to radiotherapy to allow patients some degree of oral intake. The lower radial force which they produce may provide less luminal expansion than SEMS but appears to limit the intra-oesophageal air gap which can be seen on planning CT. Furthermore, they are thought to produce less radiation dose enhancement due to back-scatter effects than SEMS.Reference Abu Dayyeh, Vandamme, Miller and Baron17 The major advantage of these stents over SEMs for this temporary indication is that they do not require a second intervention to remove after treatment and if they do migrate they can be left in situ to dissolve.
Modified O’Rourke score was available for 64% of the patients 1–3 weeks after the stent was inserted. This time point was chosen as an ideal time to derive benefit of the stent ideally before any worsening of dysphagia secondary to treatment-related oesophagitis. This improved or remained stable in 12 of the 14 patients in whom it was measured at this time point. The two patients in whom the O’Rourke score deteriorated, radiotherapy treatment commenced 12 and 14 days after stent insertion. Therefore, treatment toxicity was unlikely to be the cause of this deterioration in their swallowing. One of these patients developed food obstruction at 8 days post-stent requiring oesophageal dilation. The other did not subsequently require intervention. Thus, prompt commencement of radiotherapy is suggested following insertion of the stent, and careful patient education and specialist nutritionalist guidance on the consistency of foods to be taken.
The lack of anthropometric data available for analysis potentially reflects the lack of a dietetic outpatient service at this tertiary centre. Patients are seen by a dietician while they are admitted on the ward at the time of stent insertion to screen for re-feeding risk and advice regarding ‘post-stent diet’. However, patients undergoing radiotherapy are not formally subsequently seen by an out-patient based dietician during their treatment period unless they are re-admitted with complications. The degree of community dietician support was found to be variable.
In this study, there was a complication rate of 32% (seven patients) within 2 weeks. In four patients who experienced complications, only conservative treatment was required while the remainder required initial oesophageal dilatation with or without subsequent stent or RIG insertion. Two of the three patients who required dilatation had food bolus obstruction. It is not unexpected that a certain proportion of patients will need subsequent dilatation of the stent, given the low radial force exerted by the material. There were no major complications which compares favourably with a rate of 3.4% in one study examining the use of RIGs in a similar patient group.Reference Tessier, Piessen, Briez, Boschetto, Sergent and Mariette26 In another study which examined the use of insertion of SEMs with or without palliative radiotherapy, there was a ‘major complication’ rate of 35% including upper GI bleeding, respiratory distress secondary to increased radial force and recurrent dysphagia.Reference Javed, Pal and Dash27
The mean time to re-intervention was over 6 weeks which fits with the expected lifetime of the stent. The re-intervention rate was 40.9% within 4 months of stent insertion which is comparable to a re-intervention rate of 40% in one study examining the use of SEMs in palliation of malignant dysphagia secondary to oesophageal malignancy.Reference Shenfine, McNamee, Steen, Bond and Griffin28
This study had several limitations. First, due to its retrospective nature, data regarding complications and re-intervention at other centres may have been missed if not recorded in the hospital case notes or electronic notes. Every effort was taken to detect admission to, or intervention delivered at other sites and it is felt that these data are accurate. Generally, these patients are followed-up closely in the immediate 6-month period following treatment with such events documented in clinic letters. Furthermore, a regional on-line system for recording radiological events was screened for intervention delivered elsewhere.
Another limitation of this work was the lack of measurement of QOL. It is known that dysphagia is only symptom of these patients and it does not necessarily correlate with QOL. BD stents may enhance QOL by allowing the patient to maintain a degree of oral intake. However, this should be measured in the context of prospective studies.
Finally, it is difficult to know when is the best time point to measure objective markers such as weight and dysphagia score. During radiotherapy treatment, swallowing may deteriorate due to treatment-related oesophagitis. There may be a resulting deterioration in anthropometric measurements also. However, after 4 weeks of radiotherapy treatment, the stent will have begun to lose its radial force and start to biodegrade. Therefore, measuring ‘efficacy’ of the stent is challenging.
Conclusion
There is no single technique which is perfect for optimising nutritional status in these patients. However, we propose that biodegradable oesophageal stents are safe and may have benefit over SEMS in terms of less potential airway compression, radiotherapy planning and dose delivery. They do not improve dysphagia score in all patients however and therefore we recommend that they are to be placed alongside an RIG in a combined procedure prior to radiotherapy planning. This should allow a degree of oral feeding whilst maintaining calorie intake enterally. Patient preference should also be taken into account when deciding on a ‘package’ of care to ensure nutritional intake during radiotherapy.
Research is ongoing in the Phase 2 Biostent study to establish the feasibility of using biodegradable stents in patients undergoing palliative radiotherapy for oesophageal malignancy. It remains to be seen whether biodegradable stents are efficacious for this indication in this cohort of patients. Further studies focusing on the efficacy of palliating dysphagia, defining the rate of re-intervention, symptom response, nutritional status and health-related QOL are required, as is research into the role of biodegradable stents (and concomitant RIG insertion) with radical (chemo-)radiotherapy patients who have advanced grades of dysphagia. Future goals would be to consider a randomised controlled trial of biodegradable stent and palliative RT against current standard of SEMS alone for palliation of oesophageal cancer-related dysphagia.
Acknowledgements
None
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None






