Introduction
The Turpan-Hami Basin is located in the eastern part of the Xinjiang Uygur Autonomous Region, NW China, which is divided into the western Turpan Depression and the eastern Hami Depression. The Jurassic flora in the Xishanyao Formation of the Hami Depression is one of the representatives of Middle Jurassic floras of China. The earliest report about this flora came in 1981 in regional geological survey reports as a species list (WGRSCXUAR, 1981), followed by a brief description of ex situ and in situ cuticles (S.-J. Wang et al., Reference Wang, Tang, Zhang and Yu1994; Zhang et al., Reference Zhang, Jin, Wu and Wang1997). After that, for nearly two decades, brief systematic studiess gradually culminated in a list of nearly 90 species (Shang et al., Reference Shang, Fu, Hou and Deng1999; Deng et al., Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and Fang2010). More detailed descriptions appeared only recently, including a new conifer species with cones (Wang et al., Reference Wang, Wang, Chen, Deng, Wang, Li and Sun2015), a new species of Ginkgo bearing male cones (Wang et al., Reference Wang, Sun, Jin, Chen, Chen, Deng, Yang and Sun2017), and a new thalloid liverwort (Li et al., Reference Li, Wang, Chen, Deng, Wang, Dong and Sun2016). In this paper, we provide a detailed report on both macromorphological and cuticular features of more than 40 Nilssoniopteris specimens among thousands of fossils collected by SHD and his colleagues during the past two decades. We also review all known Jurassic species of Nilssoniopteris and establish two new species from the flora: N. hamiensis Zhao and Deng, n. sp. and N. crassiaxis Zhao and Deng, n. sp. Prior to our study, no cycadophytes from this flora had been studied by means of macromorphology and epidermal anatomy, and among the reported Jurassic Nilssoniopteris species previously reported from North China (Zhang et al., Reference Zhang, Zhou and Huang1976; Wang, Reference Wang1984; Li et al., Reference Li, He, Wu, Mei and Li1988; Sun and Shen, Reference Sun and Shen1988; Sun and Yang, Reference Sun and Yang1988), few had been observed by SEM. Thus, this study represents the first comprehensive macro- and micro-morphological and anatomical observation of Nilssoniopteris from the Xishanyao Formation.
The genus Nilssoniopteris Nathorst, Reference Nathorst1909 is one of the common genera among the Mesozoic Bennettitalean leaf fossils, with about 84 species reported, most from the Northern Hemisphere with a few scattered records in the Southern Hemisphere (e.g. Harris, Reference Harris1932b; Carpentier, Reference Carpentier1938; Samylina, Reference Samylina1963; Krassilov, Reference Krassilovl967; Ash, Reference Ash1978; Kiritchkova, Reference Kiritchkova1985; Daniel, Reference Daniel1989; Zhou, Reference Zhou1989; Watson and Sincock, Reference Watson and Sincock1992; Deng, Reference Deng1995; Kvaček, Reference Kvaček1995; Boyd, Reference Boyd2000; Pott et al., Reference Pott, Krings and Kerp2007; Yamada et al., Reference Yamada, Legrand and Nishida2009). This genus first occurred in Upper Triassic beds (Pott et al., Reference Pott, Krings and Kerp2007) and extended into the Late Cretaceous (Daniel, Reference Daniel1989; Kvaček, Reference Kvaček1995), with Middle Jurassic and Early Cretaceous times representing two peaks in its evolutionary history. Before the present study, 43 species of Nilssoniopteris had been reported from the Jurassic (Supplementary Data Set and references therein), and the establishment of the two new species in this study from the Middle Jurassic of Hami, Xinjiang, China further extends the paleogeographic distribution of Jurassic Nilssoniopteris in the Northern Hemisphere.
Geological setting, material and methods
Since the late 1990s, SHD and his colleagues have been systematically collecting plant fossils at several coal mines near Hami City, Xinjiang Uygur Autonomous Region, NW China, and the plant fossils described in this study are from the Sandaoling Opencast Coal Mine, ~90 km west of Hami City (Fig. 1). Gymnospermous fossil leaves collected from those mines often occur as compressions with well-preserved cuticles that are readily applied for precise identification.

Figure 1 Location of the Sandaoling Coal Mine and the stratigraphic column.
The Jurassic strata exposed in the Hami Depression comprise only the Lower Jurassic Badaowan Formation and Sangonghe Formation, as well as the Middle Jurassic Xishanyao Formation, which is disconformably covered by the Late Cretaceous–Paleocene Shanshan Group. The upper part of the Middle Jurassic and the Upper Jurassic have been eroded. The formation consists of grayish white, grayish green, or light red fine-grained sandstone and siltstone alternating with grayish to dark gray mudstone intercalated with conglomerate and coal seams or beds (Fig. 1). Plant fossils from the Xishanyao Formation yielded an Aalenian to Bajocian age (Deng et al., Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and Fang2010).
The specimens studied here are preserved as impressions and compressions. The hand specimens were first photographed using a Canon EOS 5D Mark III digital camera. When clean cuticles were needed, pieces of leaf fossil compressions were removed using a blade, then dissolved in hydrofluoric acid. The isolated fossil pieces were then macerated in sodium hypochlorite for oxidation until they became yellow in color. The cuticles then were washed in distilled water and treated with drops of ammonia to remove impurities before being thoroughly rinsed with distilled water. The adaxial and abaxial clean cuticles were separated by a silver needle under a light microscope. Cuticles were mounted on microscope slides for observation and photographing with a Leica DMLB light microscope equipped with a Leica DFC450C digital camera, or mounted on scanning electron microscope (SEM) sample stubs, coated with gold, and then observed and photographed through a TESCAN VEGA II LMU SEM.
Repository and institutional abbreviation
All illustrated specimens are deposited in the Research Institute of Petroleum Exploration and Development (SDL), Beijing, China.
Systematic paleontology
Order Bennettitales Engler, Reference Engler1892
Family unknown
Genus Nilssoniopteris Nathorst, Reference Nathorst1909
Type species
Nilssoniopteris solitaria (Phillips, Reference Phillips1829) Cleal and Rees, Reference Cleal and Rees2003 from White Nab near Scarborough (Yorkshire), Yorkshire, UK (Pott and Van Konijnenburg-van Cittert, Reference Thomas and Bancroftin press).
Diagnosis
Emended from Pott et al. (Reference Pott, Krings and Kerp2007) and Pott and Mcloughlin (Reference Pott and Mcloughlin2009). Leaves falling from stem at maturity, petiolate. Lamina mostly entire-margined, or undulate, lobed, to completely dissected down to the rachis in the middle portion with at least basal or apical portion entire-margined, attached laterally or to adaxial surface of midrib, leaving part of the upper surface of the midrib exposed. Veins simple or forked, or occasionally anastomosed, ending at margin. Cuticle with syndetocheilic stomata; epidermal cell walls straight or usually sinuous.
Remarks
The morphogenus Nilssoniopteris was established by Nathorst (Reference Nathorst1909) to accommodate European Jurassic cycadophyte leaves with an entire margin, without designating a type species (but assigned only one species, N. tenuinervis, to the newly erected genus). Soon afterwards, Thomas and Bancroft (Reference Thomas and Bancroft1913) suggested that this generic name should be abandoned. Later, Harris (Reference Harris1932a) recognized the need for establishing a genus of Taeniopteris-type leaves with bennettitalean cuticle features and proposed the new name Taeniozamites. However, Florin (Reference Florin1933a) noted that the genus Nilssoniopteris should be restored with precedence over Taenizamites. Harris (Reference Harris1937) endorsed this view, and in his 1969 paper (Harris, Reference Harris1969) gave the first diagnosis of Nilssoniopteris based on the original description of Nathorst (Reference Nathorst1909). Watson and Sincock (Reference Watson and Sincock1992) slightly emended the diagnosis by adding the strap leaf shape. Boyd (Reference Boyd2000) expanded the range of leaf forms to include lobed leaves, while Pott et al. (Reference Pott, Krings and Kerp2007) further emended the diagnosis to include leaves that are completely dissected down to the rachis. In addition, after reviewing the macro- and micro-morphological features of the mid-Mesozoic Laurasian bennettitalean leaf morphogenera, Pott and Mcloughlin (Reference Pott and Mcloughlin2009) further specified the lamina of Nilssoniopteris to be of four types: (1) entire margined; (2) having incompletely segmented (or “dissected”) lobes in the middle, but with basal and/or apical portions entire-margined; (3) having a completely but irregularly segmented middle portion, but with apical and/or basal portions entire-margined; and (4) having a (completely) and regularly segmented middle portion, but with apical and/or basal portions entire-margined. To summarize, the middle portion of the lamina of Nilssoniopteris can be of almost any shape that a bennetitalean lamina may have (from entire-margined, to incompletely dissected/lobed, completely and irregularly segmented, as well as completely and regularly segmented), as long as at least the apical and/or basal portions are entire-margined. Bennetitalean leaves with other shapes, particularly those whose whole blades are regularly segmented, were assigned by the authors to Anomozamites or Pterophyllum, which can be further separated by whether or not the leaflets (or lobes/segments) are up to two times as long as broad and whether the leaves are impari-pinnate or pari-pinnate. As all of our present specimens are entire-margined and readily assignable to Nilssoniopteris, therefore we tentatively simply follow the diagnosis of Pott et al. (Reference Pott, Krings and Kerp2007) and Pott and Mcloughlin (Reference Pott and Mcloughlin2009) to which we add the emendation, particularly of venation pattern.
In previous diagnoses, the veins of Nilssoniopteris were noted as being free, simple or forked, ending at the margin. Accordingly, when Zhou (Reference Zhou1978) described several taeniopterioid laminae from the Upper Triassic of Fujian, China (with veins sometimes bifurcated and then merging again or with two neighboring veins merging to form one vein, which are different from the simple veins traditionally described for Nilssoniopteris and Nilssonia, the marginal veins described in Yabeiella, and the forking and anastomosing secondary veins of Danaeopsis), he erected a new genus and a new species, Mironeura dakengensis, with unknown reproductive structures and cuticle features, to accommodate these fossils (Fig. 2.5). Later, more fossils that resemble Nilssoniopteris in macromorphology and cuticular features but with forked and merged veins were reported under the genus Nilssoniopteris, including Nilssoniopteris beyrichii from the Lower Cretaceous of Huolinhe, Inner Mongolia, China (Deng, Reference Deng1995), Nilssoniopteris neimenguensis from the Lower Jurassic of Xilinhot, Inner Mongolia, China (Deng et al., Reference Deng, Zhao, Lu, Shang, Fan, Li, Dong and Liu2017b), and Nilssoniopteris zirabensis from the Lower Jurassic of Iran (Schweitzer and Kirchner, Reference Schweitzer and Kirchner2003) (Fig. 2.1–2.3). This type of vein anastomosis was also reported in entire-margined Nilssonia having cycadalean-type cuticles, such as Nilssonia dictyophylla from the Lower Cretaceous of the outer zone of Japan (Kimura and Okubo, Reference Kimura and Okubo1985) (Fig. 2.4) and Nilssonia taeniopterides from the Upper Triassic–Lower Jurassic of Queensland, Australia (Pattemore, Reference Pattemore2016). These special veins are also found in our Xinjiang samples, especially in Nilssoniopteris crassiaxis n. sp., which fork and then merge, or merge with the adjacent veins. Due to the fact that this anastomosing venation is shared by both the bennettitalean Nilssoniopteris and the cycadalean Nilssonia, we see no logical reason to establish a new taxon solely on the basis of these anastomosing veins. We thus add the anastomosing venation to the diagnoses of these two genera, and accordingly, suggest abandoning the genus name Mironeura Zhou, Reference Zhou1978. Because no cuticular features were provided, we suggest following the notion of van Konijnenburg-van Cittert et al. (2017) to transfer M. dakengensis Zhou, Reference Zhou1978 to the genus Taeniopteris.

Figure 2 Line drawings of species with anastomosing veins in Nilssonia and Nilssoniopteris. (1) Nilssoniopteris beyrichii (Deng, Reference Deng1995); (2) Nilssoniopteris neimenguensis (Deng et al., Reference Deng, Zhao, Lu, Shang, Fan, Li, Dong and Liu2017b); (3) Nilssoniopteris zirabensis (Schweitzer and Kirchner, Reference Schweitzer and Kirchner2003); (4) Nilssonia dictyophylla (Kimura and Okubo, Reference Kimura and Okubo1985); (5) Mironeura dakengensis (Zhou, Reference Zhou1978), which should be more correctly transferred to Taeniopteris.
Nilssoniopteris hamiensis Zhao and Deng, new species

Figure 3 Nilssoniopteris hamiensis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1) A narrowly elliptic leaf with a retuse apex and a slightly obtuse base, SDL-1-133; (2) an oblanceolate leaf with a cuneate base, SDL-98-L12; (3) an oblanceovate leaf with a retuse apex and a slightly obtuse-rounded base, SDL-98-0208. Scale bars=1 cm.
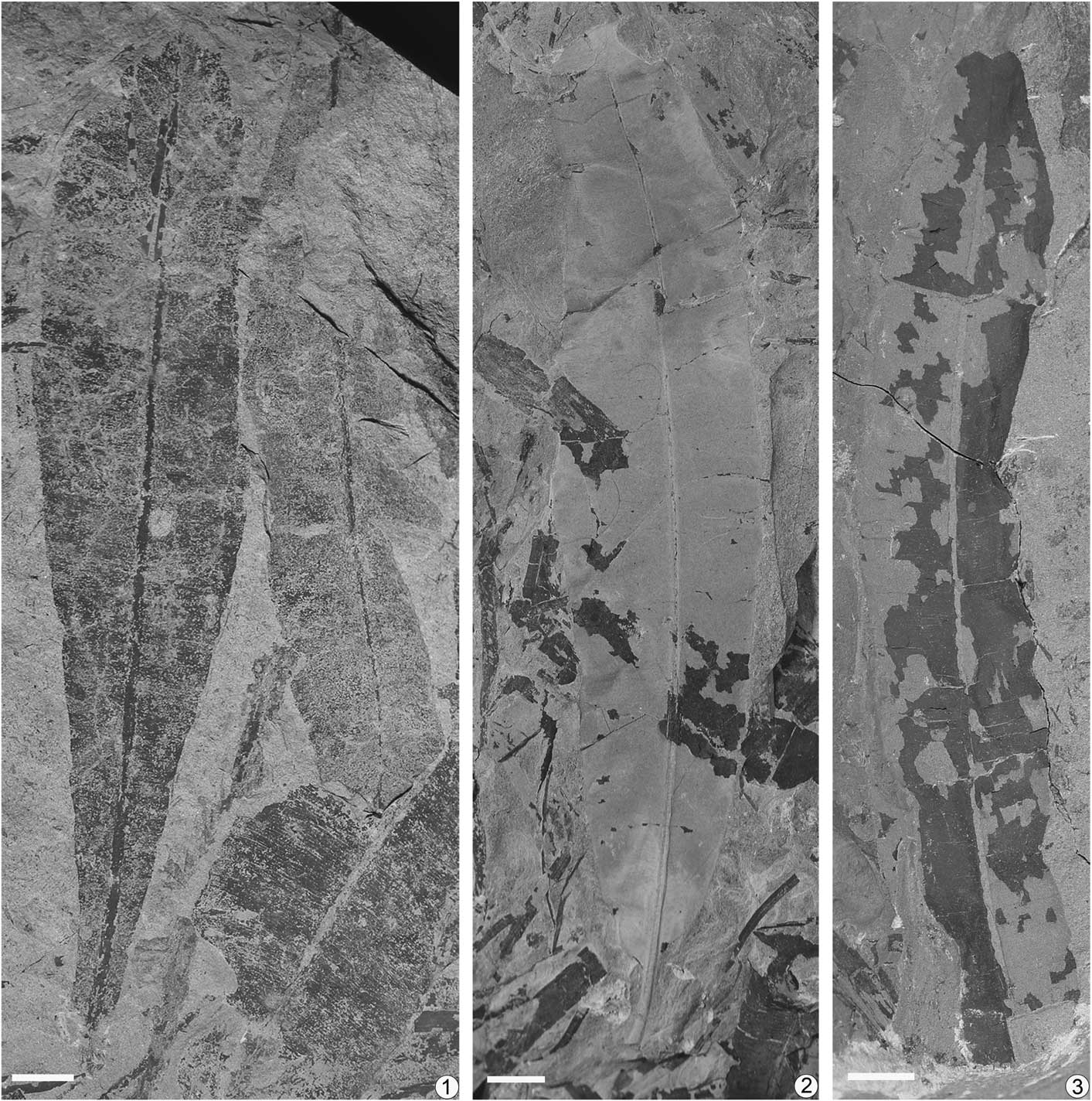
Figure 4 Nilssoniopteris hamiensis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1) A narrowly oblong leaf with a slightly obtuse base and other fragments, SDL-98-L12; (2) a narrowly oblong leaf with a truncated apex and an obtuse-rounded base, SDL-99-065; (3) a narrowly oblong leaf with a retuse apex, SDL-99-025. Scale bars=1 cm.

Figure 5 Nilssoniopteris hamiensis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1) An oblanceovate leaf with a short pointed apex and a cuneate base, SDL-98-4-05; (2) details of two midribs; transverse wrinkles are shown on the impression of the adaxial side and a longitudinal groove shown on the impression of the abaxial side, SDL-99-070; (3) a fragment on the left and a young leaf with narrowly oblong in outline and a short pointed apex on the right, SDL-98-0208; (4) a fragment with a retuse apex, SDL-99-080; (5) two young leaves with elliptical outline, SDL-98-0208; (6) a leaf with an obtuse-rounded base, SDL-99-037; (7) middle part of a leaf, SDL-98-4-02; (8) details of veins, showing a vein fork once and then anastomose (indicated by the arrow), SDL-99-045; (9) details of veins, showing two adjacent veins merge to be one vein and then disjoin again (indicated by the arrow), SDL-99-045; (10) details of veins, showing two adjacent veins merge to be one vein and then disjoin again (indicated by arrows), SDL-99-070; (11) a young leaf with narrowly oblong in outline, SDL-99-075; (12) lower part of a leaf with a rounded base, SDL-99-075. Scale bars=5 mm in (8, 9) and=1 cm in the others.

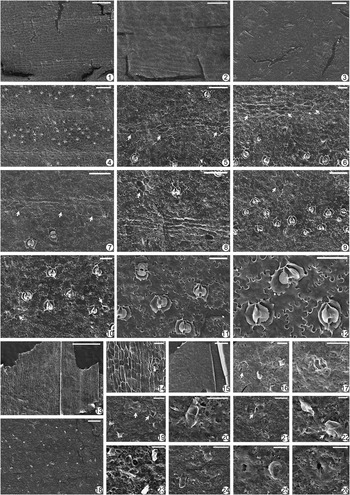
Figure 6 Nilssoniopteris hamiensis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1–3) Adaxial cuticle: (1) areas along veins and between veins (the cuticle was from the specimen shown in Fig. 5.7); scale bar=500 μm; (2) epidermal cells and trichome bases along and between veins (the cuticle was from the specimen shown in Fig. 5.4); scale bar=200 μm; (3) epidermal cells and trichome bases (the cuticle was from the specimen shown in Fig. 4.2); scale bar=100 μm; (4–13) abaxial cuticle: (4) epidermal areas along two veins and between veins (the cuticle was from the specimen shown in Fig. 3.3); scale bar=500 μm; (5) epidermal areas along two veins and between veins (the cuticle was from the specimen shown in Fig. 4.2); scale bar=200 μm; (6) epidermal areas along three veins and between veins (the cuticle was from the specimen shown in Fig. 4.3); scale bar=200 μm; (7) stomata between veins and trichome bases along and between veins (the cuticle was from the specimen shown in Fig. 3.3); scale bar=200 μm; (8) stomata between veins and trichome bases along and between veins (the cuticle was from the leaf shown in Fig. 5.3 [a]); scale bar=100 μm; (9) three stomota and one trichome base; scale bar=50 μm; (10) one stoma and two trichome bases; scale base=50 μm; (11) two stomota and one trichome base; scale bar=50 μm; (12) one stoma and one trichome base; scale bar=50 μm; (13) one stoma and one trichome base; scale bar=50 μm; (14, 15) adaxial cuticle of the midrib: (14) epidermal cells and trichome bases of the central area of a lamina (notice the epidermal cells and about two rows of stomata on each side of the midrib); scale bar=200 μm; (15) enlargement of stomata (indicated by white arrows) and trichome bases (by black arrows); scale bar=100 μm; (16) abaxial cuticle on the midrib, showing epidermal cells and trichome bases, and stomata on one side (indicated by the white arrow); scale bar=200 μm.

Figure 7 Nilssoniopteris hamiensis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China: (1, 2) internal view of adaxial cuticle; (the cuticles were from the specimen shown in Fig. 5.7); (1) epidermal areas along veins and between areas; scale bar=100 μm; (2) epidermal cells between veins; scale bar=50 μm; (3) external view of adaxial cuticle, showing trichome bases of adaxial cuticle (the cuticle was from the specimen shown in Fig. 5.7); scale bar=50 μm; (4–12) internal view of abaxial cuticle (the cuticles were from the leaf shown in Fig. 5.3 [a]): (4) areas along a vein and distribution of stomata; scale bar=100 μm; (5) one rectangular stoma; scale bar=20 μm; (6) two stomata; scale bar=20 μm; (7) one stoma with sunken guard cells and cutinized inner margins of guard cells; scale bar=20 μm; (8) one round stoma and one rectangular stoma from side view; scale bar=20 μm; (9) two stomata and one trichome base (indicated by the black arrow); scale bar=50 μm; (10) three stomata and one trichome base consisting of two cells (indicated by the black arrow); scale bar=50 μm; (11) three stomata and one trichome base consisting of three cells (indicated by the black arrow); scale bar=50 μm; (12) three stomata and two trichome bases consisting of four cells (indicated by black arrows); scale bar=50 μm; (13) internal view of adaxial cuticle on the midrib, showing epidermal cells and about two rows of stomata (indicated by white arrows); scale bar=100 μm; (14) external view of adaxial cuticle on the midrib, showing trichome bases on the central area (indicated by black arrows) and rows of stomata on one side (by white arrows); scale bar=100 μm; (15) internal view of abaxial cuticle on the midrib, showing epidermal cells and one row of stomata (indicated by white arrows); scale bar=100 μm; (16) external view of abaxial cuticle on the midrib, showing trichome bases; scale bar=100 μm; (17–20) external view of abaxial cuticle; (17) areas along a vein and trichome bases along and between veins; scale bar=100 μm; (18) two trichome bases; scale bar=50 μm; (19) one trichome base and one stoma (indicated by the white arrow); scale bar=20 μm; (20) one stoma; scale bar=10 μm.

Figure 8 Nilssoniopteris hamiensis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1–6) Variations of leaf shapes; scale bar=5 cm; (7, 8) two young leaves; scale bar=2 cm; (9) enlargement from Figure 5.10 to show anastomosed veins; scale bar=1 cm; (10) details of veins; scale bar=1 cm; (11) 1 mm2 abaxial epidermis of the fragment shown in Figure 5.3 (a), showing orientation of stomata (“ø”) and distribution of trichome bases (“x”); (12) 1 mm2 abaxial epidermis of the narrowly oblong leaf shown in Figure 4.3, showing orientation of stomata (“ø”) and distribution of trichome bases (“x”).
2010 Nilssoniopteris vittata; Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and FangDeng et al., p. 93, fig. 4.11C.
Holotype
SDL-99-0208, from the Middle Jurassic Xishanyao Formation at Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China (Fig. 3.3).
Paratypes
SDL-98-L12 (Fig. 3.2), SDL-98-4-05 (Fig. 5.1), SDL-99-065 (Fig. 4.2), and SDL-99-025 (Fig. 4.3)
Diagnosis
Leaves petiolate, entire-margined, varied in leaf shape from narrowly oblong to oblanceovate. Lower part of lamina gradually narrowing down to petiole, ending in cuneate, slightly obtuse, obtuse-rounded, or rounded leaf base. Leaf apex retuse, truncated, or short pointed. Midrib straight, with lamina laterally attached. Secondary veins slender, simple or forked once or twice, occasionally a few veins merging. Young leaves narrowly oblong to elliptic in shape. Lamina hypostomatic. Epidermal cells of adaxial surface along veins and between veins not obviously different from each other. Epidermal cells square, rectangular, or more or less isodiametric, with anticlinal walls finely sinuous. Unicellular trichome bases present on veins and between veins. Epidermal areas of abaxial surface along veins and between veins obviously different from each other. Epidermal cells along veins rectangular or elongated, between veins irregularly shaped, with anticlinal walls wavy. Numerous trichome bases consisting of 1–4 cells of varied shapes occurring along veins and between veins. Stomata syndetocheilic, irregularly orientated. Epidermal cells on both surfaces of midrib having straight anticlinal walls, with trichome bases and stomata present.
Occurrence
The Xishanyao Formation at Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China.
Description
This species is represented by ~25 leaf-bearing specimens in our collection. The leaves are entire-margined and varied in leaf shape (Fig. 8.1–8.6). They are mainly narrowly elliptic, oblanceolate, and oblanceovate, with some narrowly oblong. Leaves are up to 17 cm long. The narrowly elliptic to oblanceovate leaves are 4–6 cm wide (Fig. 3.1–3.3); narrowly oblong leaves are 2.5–4 cm wide (Fig. 4.1–4.3). In the narrowly elliptic to oblanceovate leaves, the lamina gradually narrows from the widest part towards the base, then contracts to a slightly obtuse (Fig. 3.1), obtuse-rounded (Fig. 3.3), or rounded leaf base (Fig. 5.12), or the lamina tapers to a cuneate base (Figs. 3.2, 5.1). In the narrowly oblong leaves, both sides of the widest part run nearly parallel for a distance, gradually narrowing downwards (Fig. 4.1–4.3). All laminae narrow from the widest part towards the apex slightly, and then contract quickly near the top to form a retuse apex 1–3 mm deep (Figs. 3.1, 3.3, 4.3), or to have a pointed end 4 mm high (Fig. 5.1), or to form a truncated apex (Fig. 4.2).
The midrib is straight, having a width of 1.5–2.5 mm. The midrib is ridged on the abaxial surface but relatively flat on the adaxial surface, sometimes shows irregularly transverse wrinkles (Fig. 5.2). The wrinkles can be obvious or not depending on bedrock grain size. The lamina is attached laterally to the midrib and does not cover it. The petiole is 0.7–1.5 cm long and 2–3 mm thick.
The secondary veins are slender and even inconspicuous in most specimens. They arise from the midrib at angles of 80°–90°. The veins are simple or forked once or twice, and the location of bifurcation is not fixed. In a few specimens, a few veins fork and then merge to form a closed loop (Fig. 5.8), or two adjacent veins merge and then disjoin again (Figs. 5.9, 5.10, 8.9). Vein density varies at different distances from midrib in the same leaf and differs among different leaves. In some leaves, vein density increases from ~16 per cm near the midrib to ~23 per cm near the margin; while in others, it can increase from ~20 per cm near the midrib to ~34 per cm near the margin (Fig. 8.10). Vein density of narrowly oblong leaves is generally higher than that of other leaf shapes.
Some young leaves are preserved along with the above-mentioned mature leaves. They show variety in their macromorphology (Fig. 8.7, 8.8). For example, a narrowly oblong young leaf is 6.5 cm long and 2 cm wide with its apex short pointed (Fig. 5.3 [b]) while another narrowly oblong young leaf is 7 cm long and 2 cm wide, having a retuse apex (Fig. 5.11). Two elliptic young leaves are ~4.5 cm long and 2.5 cm wide (Fig. 5.5). One of them (Fig. 5.5 [left]) shows sparse veins having a vein density of 11 per cm near the midrib while the other (Fig. 5.5 [right]) has a vein density of 19 per cm near the midrib. Because these young leaves: (1) often are preserved together with each other, (2) often are preserved together with mature leaf specimens of N. hamiensis n. sp. (3) have their leaf morphology showing gradual but continuous changes, and (4) exhibit cuticular features similar to those of the mature leaves of N. hamiensis n. sp. we interpret them to be young leaves of the current new species.
The adaxial cuticle is thin. Epidermal cells along veins are not different from those between veins in some leaves under a transmitted-light microscope (Fig. 6.2), although in some other leaves, they might be slightly different (Figs. 6.1, 7.1). The epidermal areas along veins are ~40–80 μm wide, composed of ~2–4 rows of rectangular or elongated cells while the epidermal areas between veins are 300–650 μm wide, consisting of square, rectangular, or more or less isodiametric cells. In addition, cells between veins are generally shorter and wider than those along the veins. Epidermal cell outlines are distinctly recognizable and mostly regular in arrangement. Their anticlinal walls are wavy to finely sinuous (Figs. 6.2, 7.2). Stomata are absent. Trichome bases, consisting of a single oval or round cell with a thickened ring, occur along veins and between veins (Figs. 6.2, 6.3, 7.3). The density of trichome bases differs among leaves.
The abaxial cuticle is nearly equal in thickness to the adaxial one. Epidermal areas along veins are obviously different from those between veins (Fig. 6.4–6.6): those along veins are 70–100 μm wide, composed of about 2–4 rows of rectangular or elongated cells while those between veins are 300–740 μm wide, consisting of irregularly shaped cells. Under a transmitted-light microscope the epidermal cell outlines are clear and the anticlinal walls of these cells are generally wavy.
Laminae are hypostomatic. Stomata are scattered mainly in the areas between veins on the abaxial surface. Stomatal bands are obviously wider than bands along veins without stomata (Figs. 6.7, 8.11, 8.12). The density range of stomata is 25–55 per mm2: 25–35 per mm2 of narrowly elliptic to oblanceovate leaves (Figs. 6.4, 8.11) and 40–55 per mm2 of narrowly oblong leaves (Figs. 6.5, 8.12). Stomata are irregularly oriented, being parallel, vertical, or oblique to veins (Figs. 6.7, 6.8, 7.4). They are syndetocheilic, mainly near-rectangular to elliptic in shape and 25–30 × 50–60 μm in size (Figs. 6.9, 6.10, 6.13, 7.5, 7.6). Sometimes they can be nearly square to rounded, ~35 µm in diameter (Figs. 6.11, 7.8). From the external view, subsidiary cells are flat (Fig. 7.19, 7.20). The two polar anticlinal walls of subsidiary cells are usually parallel, and the exceptional polar anticlinal walls extend outwards to form an angular shape. The two distal anticlinal walls are usually semicircular in shape. Guard cells are sunken below subsidiary cells with cutinized inner wall thickenings (Fig. 7.5, 7.7).
Numerous trichome bases of 1–4 cells of varied shapes occur along veins and between veins of the abaxial surface. Trichome bases are rounded or elliptic (Fig. 7.18, 7.19) and are 20–50 μm in diameter. The unicellular trichome bases may or may not have thickened anticlinal walls. Multicellular trichome bases consisting of 2–4 cells (Fig. 7.10–7.12) have their anticlinal walls sometimes ridged and sometimes strongly cutinized and surrounded by radial thickenings on the periclinal walls. The internal sculpture of anticlinal walls of trichome base cells is usually similar to that of the neighboring epidermal cells under SEM, although sometimes the anticlinal walls of the former can be slightly more thickened and straighter than those of the ordinary epidermal cells. The density range of trichome bases is 12–40 per mm2: 12–25 per mm2 of narrowly elliptic to oblanceovate leaves (Fig. 8.11) and ~35 per mm2 of narrowly oblong leaves (Fig. 8.12).
On the middle portion of the adaxial surface of the midrib, cells are near square or more or less isodiametric, with many scattered trichome bases (Fig. 6.14). Towards both sides, epidermal cells on the midrib become increasingly elongated (Fig. 7.13). Two files of stomata can be observed along each side of midrib (Figs. 6.14, 6.15, 7.13, 7.14). Cells on the abaxial surface of the midrib are rectangular or elongated and are longitudinally arranged, with many scattered trichome bases. One file of stomata is distributed on one side of the midrib (Figs. 6.16, 7.15, 7.16). Anticlinal walls of cells on both surfaces of the midrib are straight.
Etymology
The specific epithet “hamiensis” is a latinization of the Chinese Pinyin name of the discovery location, Hami City.
Remarks
Based on the entire-margined laminae, sinuous anticlinal walls of epidermal cells, and syndetocheilic stomata, the present specimens can be undoubtedly assigned to the genus Nilssoniopteris. One of the leaves in the present collection was previously assigned to N. vittata without detailed cuticular features (Deng et al., Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and Fang2010). However, newly prepared cuticles not only allowed us to align the specimen with others, but separate this set of specimens from all known species. Besides some macromorphological differences, our specimens are mainly distinguishable from known species in the following combined suite of characteristics: (1) they have anticlinal walls of their cells finely sinuous on the adaxial epidermis; (2) they have unicellular trichome bases, oval or round, on the adaxial epidermis; and (3) they have numerous trichome bases of 1–4 cells with various shapes on the abaxial epidermis.
The Supplementary Data Set displays the main comparison of our two new species with all 43 Jurassic species worldwide. Among those 43 species, the one most similar to the present new species in leaf form, leaf size, cuticular feature, and geological age is N. major, which is an important bennettitalean species from the Middle Jurassic flora of Yorkshire, England (Harris, Reference Harris1969). For example, cuticular analysis of Harris’s specimens demonstrated that its trichome bases are also usually present on the abaxial epidermis, and the number of their basal cells is 1–4, similar to those of our specimens. The densities of stomata and trichome bases of Harris’s specimens are similar to those of our narrowly elliptic and oblanceovate leaves. Leaf form and leaf size of Harris’s mature and young leaves resemble those of our mature narrowly elliptic and oblanceovate leaves and our young narrowly oblong leaves. In addition, line drawings of Harris’s specimens also show vein anastomosis. However, our specimens are different from those of N. major in the following ways: (1) the lower parts of Harris’s ovate-lanceolate leaves narrow slightly and then end abruptly, whereas in our narrowly elliptic and oblanceovate leaves they more commonly taper gradually towards the base; (2) oblancelate and narrowly oblong leaves are lacking in Harris’s specimens, but common in our collection; (3) the leaf margins of N. major have minute teeth, which are not observed in our specimens; (4) the density of secondary veins of N. major is 6–12 per cm, which is considerably lower than that of most of our specimens; and (5) trichome bases are lacing on the adaxial epidermis of N. major while existing on that surface of our specimens. These differences provide us the confidence to separate our specimens from N. major.
Nilssoniopteris vittata, described by Harris (Reference Harris1969) from Yorkshire (now referred to as N. solitaria by Cleal and Rees, Reference Cleal and Rees2003; Cleal et al., Reference Cleal, Rees, Zijlstra and Cantrill2006; Van Konijnenburg-van Cittert et al., Reference Van Konijnenburg-van Cittert, Pott, Cleal and Zijlstra2017; Pott and Van Konijnenburg-van Cittert, Reference Pott and Van Konijnenburg-van Cittertin press), is another species showing some similarities to our specimens. However: (1) in N. solitaria, the leaves are linear-lanceolate in outline and rarely up to 3 cm wide, which is much narrower than most of our specimens; (2) the anticlinal walls of adaxial epidermal cells of N. solitaria are coarsely sinuous (ridges extend from folds almost to middle of cell), which are different from the finely sinuous walls of most cells of our specimens; and (3) trichome bases on the abaxial epidermis of N. solitaria are composed of 1–2 cells, while our specimens have 1–4 cells. These differences prevent us from allocating our specimens to N. solitaria.
One of the typical features of our specimens is the presence of numerous and conspicuous trichome bases of 1–4 cells with various shapes on the abaxial epidermis. Similar trichome bases have been reported in N. glandulosa (Florin, Reference Florin1933b) and Nilssoniopteris sp. b (Lundblad, Reference Lundblad1950) from the Early Jurassic Lias flora of Sweden. Florin’s leaves lack apices and bases and are 1–4.5 cm wide, which were considered to be linear-lanceolate to lanceolate. The secondary vein density of leaves that are l–2.5 cm wide is 17–22 per cm (Florin, Reference Florin1933b, pl. 1, figs. 4, 6) and 13 per cm in leaves ~4 cm wide (Florin, Reference Florin1933b, pl. 1, fig. 2). They are respectively lower than that of our narrowly oblong leaves and that of our narrowly elliptic to oblanceovate leaves. In addition, the number of trichome basal cells of N. glandulosa is up to 5–6 (Florin, Reference Florin1933b, pl. 2, figs. 2, 3, text-fig. 5), which is different from 1–4 of our species. Lundblad’s specimen (Nilssoniopteris sp. b, Lundblad, Reference Lundblad1950) is only preserved with the apical portion of a leaf. Although its trichome bases bear a certain resemblance to ours, based on the figures in Lundblad (Reference Lundblad1950), its blunt-rounded laminar apex is clearly different from the retuse, truncated, or short pointed apex of our specimens.
Another species that is more or less comparable with the present species is N. kokalensis from the Early Jurassic of western Kazakhstan (Kiritchkova, Reference Kiritchkova2000; Kiritchkova and Nosova, Reference Kiritchkova and Nosova2012), which also possesses numerous trichome bases having cutinized thickenings on the abaxial epidermis. However, its stomatal bands are slightly narrower than nonstomatal bands, its stomata are smaller in size than ours, and its adaxial epidermis lacks trichome bases. These features make them different from our leaves.
In addition, the stomatal densities of our narrowly elliptic to oblanceovate leaves (25–35 per mm2) and the narrowly oblong leaves (40–55 per mm2) are quite different from each other. Due to the fact that: (1) these two leaf shapes have continuous intermediate shaped specimens in the flora, (2) they sometimes are even preserved on the same hand specimen, and (3) they share almost all micromorphological and anatomical characteristics, we interpret them to belong to the same species. The obvious stomatal density difference might be the result of different ecological adaptations, which might have been caused by their different positions on the same plant or different plants growing under different microenvironments.
Nilssoniopteris crassiaxis Zhao and Deng, new species

Figure 9 Nilssoniopteris crassiaxis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1) Apical portion of a leaf, SDL-98-4-11; (2) apical portion of a leaf, SDL-98-268; (3) lower part of a leaf, SDL-98-409; (4) enlargement of a midrib, showing transverse wrinkles, SDL-98-L7; (5) enlargement of the midrib from Figure 9.6, showing the exposed midrib, SDL-98-4-13; (6) near the widest part of a leaf, SDL-98-4-13; (7) veins fork and then merge, or fork and then merge with the adjacent vein (indicated by white arrows), SDL-98-L2; (8) veins merge (indicated by white arrows), SDL-98-L2; (9) veins fork, then merge, and then fork again (indicated by white arrows), SDL-98-4-10; (10) part of a leaf, SDL-98-L14; (11) lower part of a leaf and a petiole, SDL-98-L1; (12) a young leaf, SDL-07-85; (13) a leaf on the left and a leaf on the right, SDL-98-L2. Scale bars=5 mm (4, 5), 2 mm (7–9) and 1 cm in the others.

Figure 10 Nilssoniopteris crassiaxis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1, 2) Adaxial cuticle; the cuticles were from the specimen shown in Figurew 9.10; (1) epidermal areas along veins and between veins; notice veins fork once or anastomose (indicated by white arrows); scale bar=500 μm; (2) areas along a vein; scale bar=100 μm; (3–8) abaxial cuticle; (3, 4) the cuticles were from the leaf shown in Figure 9.13 (right); (5) the cuticle was from the specimen shown in Figure 9.2; (6–8) the cuticles were from the specimen shown in Figure 9.10; (3) stomata between veins and trichome bases along veins and between veins; scale bar=500 μm; (4) distribution of stomata and trichome bases; scale bar=200 μm; (5) three trichome bases along veins and one stoma; scale bar=50 μm; (6) three stomata and one trichome base; scale bar=50 μm; (7) several stomata and trichome bases; scale bar=50 μm; (8) stomata between veins and trichome bases along veins and between veins; scale bar=100 μm; (9) abaxial cuticle on the midrib, showing epidermal cells and trichome bases; scale bar=500 μm.

Figure 11 Nilssoniopteris crassiaxis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1, 2) Internal view of adaxial cuticle; (1) epidermal areas along two veins and between veins; the cuticle was from the specimen shown in Figure 9.9; scale bar=200 μm; (2) epidermal cells along and between veins; the cuticle was from the specimen shown in Figure 9.3; scale bar=100 μm; (3) external view of adaxial cuticle; the cuticle was from the leaf shown in Figure 9.9; scale bar=100 μm; (4–12) internal view of abaxial cuticle; the cuticles were from the leaf shown in Figure 9.13 (right); (4) areas along two veins and between veins; scale bar=200 μm; (5) trichome bases consisting of one or two cells along veins (indicated by white arrows); scale bar=100 μm; (6) trichome bases consisting of two or three cells along veins (indicated by white arrows); scale bar=50 μm; (7) trichome bases consisting of one or three cells along veins (indicated by white arrows); scale bar=100 μm; (8) one trichome base along veins and one trichome base consisting of three cells between veins (indicated by the white arrow); scale bar=100 μm; (9) stomata and trichome bases consisting of two cells between veins (indicated by the white arrow); scale bar=100 μm; (10) stomata and trichome bases between veins (indicated by white arrows); scale bar=50 μm; (11) several stomata; scale bar=50 μm; (12) three stomata and sunken guard cells; scale bar=50 μm; (13, 14) internal view of abaxial cuticle on the midrib; (13) epidermal cells; scale bar=500 μm; (14) one trichome base and one stoma; scale bar=50 μm; (15–17) external view of abaxial cuticle on the midrib; (15) many trichome bases; scale bar=500 μm; (16) One multicellular trichome base (indicated by the white arrow) and several other trichome bases; scale bar=50 μm; (17) one trichome base consisting of two cells; scale bar=50 μm; (18–26) external view of abaxial cuticle; the cuticles were from the leaf shown in Figure 9.13 (right); (18) many trichome bases; scale bar=200 μm; (19) two trichome bases consisting of two cells (indicated by white arrows) and some other trichome bases; scale bar=50 μm; (20) one trichome base consisting of two cells; scale bar=50 μm; (21) one trichome base consisting of three cells (indicated by the white arrow) and another trichome base; scale bar=50 μm; (22) one trichome base consisting of three cells (indicated by the white arrow) and another trichome base; scale bar=20 μm; (23) several trichome bases. Scale bar=50 μm; (24) two trichomes; scale bar=50 μm; (25) one stoma and one trichome base; scale bar=20 μm; (26) one stoma; scale bar=20 μm.

Figure 12 Nilssoniopteris crassiaxis Zhao and Deng, n. sp. from the Xishanyao Formation of the Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China. (1) Reconstruction of a whole leaf based on Figure 9.1, 9.3, 9.6, 9.10, 9.11, and 9.13; scale bar=5 cm; (2) details of veins; scale bar=1 cm; (3) 1 mm2 abaxial epidermis of the leaf shown in Figure 9.13 (right), showing orientation of stomata (“ø”); (4) 1 mm2 abaxial epidermis of the leaf shown in Figure 9.3, showing orientation of stomata (“ø”); (5) 1 mm2 abaxial epidermis of the leaf shown in Figure 9.13 (right), showing distribution of trichome bases (“x”).
1911 Taeniopteris vittata; Reference SewardSeward, p. 16, pl. 3, figs. 30, 31.
Holotype
SDL-98-L2, from the Middle Jurassic Xishanyao Formation at Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China (Fig. 9.13).
Paratypes
SDL-98-4-11 (Fig. 9.1), SDL-98-409 (Fig. 9.3), SDL-98-4-13 (Fig. 9.6), SDL-98-L14 (Fig. 9.10), and SDL-98-L1 (Fig. 9.11).
Diagnosis
Leaves petiolate, entire-margined, long strap-shaped, but with the widest part at the apical portion. Petiole stout. Lamina gradually and very slowly narrowing from the widest part to the base while narrowing towards the apex more quickly. Leaf base cuneate, apex likely obtuse to obtuse-rounded. Midrib at leaf base stout, accounting for one-third to one-half width of leaf base, tapering gradually towards leaf apex. Midrib surface bearing irregular transverse corrugations. Lamina attached to the adaxial side of midrib but leaving the central part exposed. Secondary veins thin, conspicuous, simple or forked once, twice, or even three times, with a few veins forking and then merging and a few others merging with their neighboring veins. Leaf hypostomatic. Epidermal areas along veins and between veins distinct on both surfaces. Epidermal cells on the adaxial surface square, rectangular, or more or less isodiametric, with anticlinal walls sinuous, U-shaped, or Ω-shaped. Epidermal cells along veins on the abaxial surface rectangular or elongated, between veins irregularly shaped, with anticlinal walls strongly sinuous, U- or Ω-shaped. Stomata syndetocheilic, mostly vertical or oblique to veins but occasionally parallel to veins. Trichome bases consisting of 1–3 cells occurring numerously along veins and between veins of the abaxial epidermis. Epidermal cells on the abaxial surface of midrib having straight anticlinal walls, with numerous trichome bases present.
Occurrence
The Xishanyao Formation at Sandaoling Coal Mine, Xinjiang Uygur Autonomous Region, China.
Description
This new species is represented by ~20 leaf-bearing specimens in our collection. The leaves are long strap-shaped, entire-margined, and petiolate. Among our collection, the longest leaf reaches a length of ~22 cm, but with only the lower region of the leaf preserved (Fig. 9.3). Based on the shape of the preserved part, the full length might be ~50 cm. Its widest preserved part is at the apical portion of the leaf, which reaches up to 7.5 cm wide (Fig. 9.1, 9.2). The lamina gradually and very slowly narrows from the widest part down to the petiole (Fig. 9.3, 9.6, 9.10, 9.13), and the width of the leaf is ~4.5 cm at a distance of ~22 cm from the base and ~2 cm at a distance of ~6 cm from the base (Fig. 9.3). The lamina tapers to a cuneate base (Fig. 9.3, 9.11). The lamina narrows from the widest part towards the apex more quickly than towards the base, from 6.6 cm wide to 3.5 cm wide at a distance of ~6 cm (Fig. 9.2). The petiole is stout, ~3 cm long and 9 mm wide (Fig. 9.11). The midribs of these specimens are also stout with a width of 5–8 mm at the leaf base, accounting for one-third or even one-half of the leaf base width (Fig. 9.3). The midrib width gradually decreases acropetally. Irregular transverse corrugations are always observed on the midrib on the impressions (Fig. 9.4). The lamina is attached to the adaxial side of the midrib, but leaves the central part of the midrib exposed. The exposed width is 1–2.5 mm, wider below and thinner above, with the thinnest near the widest part of the leaf (Fig. 9.5). A young leaf is preserved (Fig. 9.12). The lamina is ~3.2 cm long and 6.5 mm wide, with an obtuse to obtuse-rounded apex. Its midrib is 0.7–1 mm wide.
The secondary veins are thin and conspicuous, arising from the midrib at almost right angles, except that in the apical laminar part the angles are ~80°. Veins are simple or forked once, twice, or even three times, and the location of bifurcation is not fixed. In addition, a few veins fork and then merge to form a closed loop (Figs. 9.7–9.9, 12.2), or fork again after forming a closed loop (Figs. 9.9, 12.2), or merge with neighboring veins (Figs. 10.1, 12.2), or fork and then merge with neighboring veins (Figs. 9.7, 12.2). The density of veins near the leaf base can be ~18–20 per cm (Fig. 9.3 [lower]) or up to 25 per cm (Fig. 9.11). Above the base, the density of veins is 15–17 per cm near the midrib, becoming denser towards the margins due to their bifurcation; the wider the lamina, the denser the veins. For example, vein density near the leaf margin is ~21 per cm (Fig. 9.3 [upper]), 27 per cm (Figs. 9.6, 9.10, 9.13 [right], 12.2), and ~35 per cm (Fig. 9.2) acropetally.
The adaxial cuticle is moderately thick and can be readily divided into areas along the veins and areas between veins. Those along the veins are 75–100 μm wide, marked by ~3 rows of rectangular or quadrilateral cells (Figs. 10.1, 10.2, 11.1, 11.2). Areas between veins are 360–500 μm wide, with epidermal cells square, rectangular, or more or less isodiametric in shape and regular in arrangement. Cell outlines are clear, with their anticlinal walls sinuous, U-, or even Ω-shaped (Fig. 11.2). Trichome bases are absent from this surface.
The abaxial cuticle is thicker than the adaxial one. Epidermal areas along veins and between veins are distinct from each other. The epidermal areas along veins are ~95 μm wide, composed of 3–4 rows of rectangular or elongated cells. Those between veins are 360–450 μm wide, consisting of irregular epidermal cells with anticlinal walls strongly sinuous, U-, or Ω-shaped (Fig. 11.7–11.12). Trichome bases are also always present along the veins and between veins, densely or sparsely arranged at irregular distances (Figs. 10.3, 10.4, 11.5–11.10), with a density of ~33 per mm2 (Fig. 12.5). Some bases consist of a single rounded cell (Fig. 11.5, 11.7, 11.10), some of two rectangular or triangle cells (Fig. 11.5, 11.9, 11.19, 11.20), and some of three cells (Fig. 11.6–11.8, 11.21). Little difference is seen between these basal cells and the neighboring epidermal cells on the internal surface under SEM. Generally, the anticlinal walls of these basal cells are straighter and slightly more cutinized than those of the ordinary epidermal cells.
Leaves are hypostomatic. Stomata form stomatal bands (~270–360 μm wide) between veins, which are approximately equal to or slightly wider than nonstomatal ones (~200–280 μm wide) (Fig. 11.4). Stomata density is ~35–50 per mm2 (Fig. 12.3, 12.4). The stomata are syndetocheilic, nearly rounded (~43 μm in diameter) or nearly rectangular (31–40 × 57–65 μm in size) (Fig. 11.9–11.12). Most of the stomata are vertical or oblique to veins, with a few parallel to veins (Fig. 12.3, 12.4). Guard cells are sunken below two subsidiary cells. Under SEM, on the internal surface of the cuticle, some of the subsidiary cells show smooth outer edges while some others have toothed outer edges. Sometimes subsidiary cells are laterally surrounded by one or two encircling cells (Fig. 11.10–11.12). Viewed externally, two subsidiary cells, without papillae, are flat and not overly close to each other (Fig. 11.25, 11.26).
Epidermal cells on the abaxial surface of midrib are of several rows of rectangular or rhomboid cells that are longitudinally arranged, with their anticlinal walls straight (Figs. 10.9, 11.13). Trichome bases are numerously present (Figs. 10.9, 11.15), which is observed from the external side to contain two or five cells (Fig. 11.16, 11.17). Stomata are rarely seen on the midrib. So far, only one stoma has been observed (Fig. 11.14).
Etymology
Specific epithet is taken from the Latin crass, strong, and axis, midrib.
Remarks
Based on the features of entire-margined leaves, sinuous anticlinal walls of epidermal cells, and the syndetocheilic stomata, the present specimens should be readily assigned to Nilssoniopteris. A suite of other characteristics distinguishes the current set of specimens from all known species of the genus; they are: (1) macromorphology: the species has broad midrib of the leaf, especially that near the leaf base, with the ratio of midrib width to leaf base width as high as one-third to one-half; (2) venation pattern: besides the simple or forked veins, this species has anastomosing veins to form closed loops or even forwards-backwards turning fork shapes; and (3) cuticular feature: the species has trichome bases of 1–3 cells commonly occurring on the abaxial epidermis.
Seward (Reference Seward1911) assigned two specimens from the Middle Jurassic of the Junggar Basin, Xinjiang to Taeniopteris vittata, although he noticed the differences between those specimens and the type ones of the species. The macromorphological features of Seward’s specimens correspond exactly with those of the present specimens. Although their cuticular features remain unknown, given that this type of our specimens collected from the Middle Jurassic of Xinjiang has their cuticular features confirmed to be bennettitalean, we are confident about including Seward’s specimens in our new species.
Among other Jurassic species (Supplementary Data Set), the one most similar to our new species in leaf macromorphology and cuticular features is N. vulgaris from the Middle Jurassic of Georgia (Doludenko and Svanidze, Reference Doludenko and Svanidze1969). In leaf morphology, leaves of both species are long strap-shaped with cuneate leaf base and the ratio of midrib width to leaf base width is high. In adaxial cuticular features, leaves of both species have epidermal areas along the veins being composed of 2–4 rows of cells. In the abaxial cuticular features, stomatal and nonstomatal bands are of almost equal width, trichome bases of 1–3 cells often occur along veins, and cells of the midrib are rectangular. However, the leaf of N. vulgaris shows a tendency to narrow upwards from the leaf width of ~2.3 cm (Doludenko and Svanidze, Reference Doludenko and Svanidze1969, pl. 44, fig. 3), which is a maximum leaf width that is far less than that of our specimens. In addition, the general appearance of the Georgian leaves is smaller and narrower, with much more slender petioles. Moreover, the long axis of the oval-shaped stomata of N. vulgaris is generally >70 μm long, which is much longer than ours.
Nilssoniopteris musafolia from the Early Jurassic of Dorud, Iran (Barnard, Reference Barnard1965) is a species showing the widest (up to 8 mm) midrib among Jurassic species hitherto described. However, its cuticular features are different from those of our specimens in that: (1) their anticlinal walls of epidermal cells of both surfaces are all straight and (2) their stomata on the abaxial epidermis are oriented only transversely to veins.
Nilssoniopteris karataviensis from the Early Jurassic of Karatau, Kazakhstan (Doludenko and Orlovskaya, Reference Doludenko and Orlovskaya1976) has comparable external features. Its leaves are also long strap-shaped, with a full length up to 36 cm. Its midrib is also broad (4–6 mm wide) in the lower region of the leaf, which decreases towards the top of leaf to 1 mm wide. It is also different from the present species in several characteristics: (1) its maximum laminar width is only 3.2 cm, while that of our specimens can be more than 7 cm; and (2) its lamina gradually narrows towards both the apex and the base, whereas our specimens have their widest part near the apical portion of the leaf and the lamina narrows towards the apex much more quickly than towards the base (i.e., Karatau’s leaves are much narrower and with both laminar margins more parallel than our specimens). Another species having a similar distinction is N. longifolius from the Middle Jurassic of Baotou, Inner Mongolia, China (Zhang et al., Reference Zhang, Zhou and Huang1976). Considering both the morphological differences and different geological localities, we separate our specimens from these two species.
When our two new species are compared, more obvious differences are noticed as the following: in leaf macromorphology, N. crassiaxis n. sp. has much longer leaf length, which can be up to 50 cm (compared with the 17 cm of N. hamiensis n. sp.), thicker laminae, and broader midrib, particularly in the middle and lower portions of the leaf. In venation, secondary veins of N. crassiaxis n. sp. are more clearly observable and vein anastomosing is more common, more conspicuous, and more complicated than in N. hamiensis n. sp. In cuticular features, in N. crassiaxis n. sp., trichome bases are absent on the adaxial epidermis, anticlinal cell walls of the abaxial epidermis are strongly sinuous (U- or even Ω-shaped), trichome bases on the abaxial epidermis have 1–3 cells, stomata occupy a part of the areas between veins and with more regular orientation, which are all very different from those of N. hamiensis n. sp., whose trichome bases are obviously present on the adaxial epidermis, anticlinal cell walls of the abaxial epidermis are wavy to finely sinuous, trichome bases possess 1–4 cells, stomata occupy nearly the entire areas between veins, and their orientation is more irregular.
Distribution of the Jurassic Nilssoniopteris and their paleoclimatic implications
We summarized and analyzed geographical distributions of all 45 Jurassic species of Nilssoniopteris (including the current two new species) in the Supplementary Data Set and Figure 13. According to our analysis, during the Early Jurassic, 19 Nilssoniopteris species have been reported from Europe in Sweden (Florin, Reference Florin1933b; Lundblad, Reference Lundblad1950); Central Asia in Iran (Barnard, Reference Barnard1965; Schweitzer and Kirchner, Reference Schweitzer and Kirchner2003), Kazakhstan (Kiritchkova, Reference Kiritchkova1973, Reference Kiritchkova2000; Dolundenko and Orlovskaya, 1976), and the Caspian Basin (Kiritchkova and Nosova, Reference Kiritchkova and Nosova2012); and East Asia (China) in Inner Mongolia (Deng et al., Reference Deng, Zhao, Lu, Shang, Fan, Li, Dong and Liu2017b) and Hubei Province (Sun and Yang, Reference Sun and Yang1988). Among them, 14 species, the most abundant, are from Central Asia.

Figure 13 Jurassic stratigraphic and geographical distribution of Nilssoniopteris (base maps are from Boucot et al., Reference Boucot, Chen, Scotese and Fan2009): (1) one species; (2) two or three species; (3) four to five species; (4) more than six species.
The number of Middle Jurassic species increased to 27 with their geographic distribution also expanded. They were reported from Europe in Andøya, Norway (Manum et al., Reference Manum, Bose and Vigran1991), Yorkshire, England (Harris, Reference Harris1969), and Peski locality, European Russia (Gordenko, Reference Gordenko2008); Central Asia in many locations (Doludenko and Svanidze, Reference Doludenko and Svanidze1969; Markovitch, Reference Markovitch1971; Vassilevskaja et al., Reference Vassilevskaja, Iminov, Loseva and Mogutcheva1972; Gomolitzky, Reference Gomolitzky1974; Barnard and Miller, Reference Barnard and Miller1976; Dolundenko and Orlovskaya, 1976; Kiritchkova and Nosova, Reference Kiritchkova and Nosova2012); and East Asia, mainly in North China, including Inner Mongolia, Hebei Province, Gansu Province, Shanxi Province, Henan Province, the Qaidam Basin of Qinghai Province, and Hami of the Xinjiang Uygur Autonomous Region (Zhang et al., Reference Zhang, Zhou and Huang1976; Wang, Reference Wang1984; Li et al., Reference Li, He, Wu, Mei and Li1988; Sun and Shen, Reference Sun and Shen1988; Barale et al., Reference Barale, Thévenard and Zhou1998; this paper).
In the Late Jurassic, Nilssoniopteris had a rather limited geographical distribution, recorded only in Spitsbergen in the Northern Hemisphere (Nathorst, Reference Nathorst1897) and in India in the Southern Hemisphere (Bose and Baneriji, Reference Bose and Banerji1984), with the number of species dramatically decreased to only two.
In general, from the Early to Middle Jurassic, the number of species of Nilssoniopteris rose and the distribution range expanded; while entering into the Late Jurassic, both the number of species and distribution areas remarkably declined.
Vakhrameev (Reference Vakhrameev1991) divided the Jurassic climatic zones into the moderate-warm climate belt of the Northern Hemisphere (Siberian region), the subtropical belt of the Northern Hemisphere (Euro–Sinian region), the tropical belt (Equatorial region), and the subtropical belt of the Southern Hemisphere (Austral region) on the basis of global phytogeographic regions. Accordingly, phytogeographic regions of the Early–Middle Jurassic in China have been divided into the North China Phytogeographical Region and the South China Phytogeographical Region, the former of which belongs to Vakhrameev’s Siberian region and the latter to Vakhrameev’s Euro–Sinian region (Wu, Reference Wu1983; S.-E. Wang et al., Reference Wang, Tang, Zhang and Yu1994; Deng et al., Reference Deng, Lu, Fan, Fang, Li and Liu2012, Reference Deng, Lu, Zhao, Fan, Wang, Yang, Li and Sun2017a). The above-mentioned geographical analysis of Nilssoniopteris confirmed that plants of this genus not only grew in subtropical regions as living cycads do, but also were abundant in warm climatic regions.
The species list of the Middle Jurassic flora from Sandaoling in Hami, Xinjiang shows that the flora belongs to the Conioperis-Phoenicopsis flora, with Filicopsida and Ginkgoposida as the dominating components, Cycadopsida and Coniferopsida next most abundant, and Sphenophyllales, Lycophyta, and Bryophyta least dominant (Deng et al., Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and Fang2010). In the flora, deciduous plants (e.g., Ginkgoales, Czekanowskiales, and Pinaceae) as indicators of seasonal warm climate and Osmundaceae as an indicator of humid warm climate occupied dominant positions, indicating a warm and humid climate in general (Deng et al., Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and Fang2010, Reference Deng, Lu, Zhao, Fan, Wang, Yang, Li and Sun2017a). Lacustrine bivalves discovered from the same stratigraphic layer are composed of Ferganoconcha and Unio, which are adaptive to warm and cool climates (Yu et al., Reference Yu, Mizuno and Wang1993; Chen, Reference Chen2003; Deng et al., Reference Deng, Lu, Zhao, Fan, Wang, Yang, Li and Sun2017a). In addition, the presently studied strata are mainly characterized by fluvial, lacustrine, and swamp facies sandstone–mudstone, with developed coal beds and dark mudstones, which also indicate a humid climate. As a result, the paleoclimatic condition of the two new Nilssoniopteris species in the present flora further corroborates the conclusion that Nilssoniopteris can flourish in a warm temperate zone in addition to subtropical–tropical zones.
The presence and density of trichome bases have long been used to indicate a dry and hot climate (Watson and Alvin, Reference Watson and Alvin1996). Interestingly, because the abaxial surface of some of our current specimens of Nilssoniopteris has abundant trichome bases, it may suggest that there existed dry and hot microenvironments within the generally humid and warm climate. The wide range of stomatal density of variously shaped leaves of the species N. hamiensis n. sp. can be viewed as additional evidence in support of various microenvironments in one flora.
Conclusions
We describe two new Nilssoniopteris species, N. hamiensis Zhao and Deng, n. sp. and N. crassiaxis Zhao and Deng, n. sp., based on more than 40 specimens collected from the Middle Jurassic Xishanyao Formation of Sandaoling, Hami, Xinjiang, NW China. The study of their leaf morphology and cuticular features represents the first time that cycadophyte plant fossils have been characterized in detail from this formation.
Our specimens from Xinjiang, China confirmed the existence and elaborated the degree of variation of anastomosing veins of Nilssoniopteris, and an emended diagnosis is thus provided for this genus.
We summarized the geographical distributions of all 45 Jurassic species of Nilssoniopteris and discovered that during the Jurassic time the genus first experienced a diversification and distribution expansion, which was followed by a sudden decline in both diversity and geographic range in the Late Jurassic. Our brief paleoclimatic reconstruction suggests that Nilssoniopteris might have been growing not only in subtropical regions, as the living cycads, but also in warm climatic regions, probably under different hydrological/humidity conditions.
Acknowledgments
This study was jointly supported by the National Natural Science Foundation of China (NSFC: 40372021), the Key Research Program of PetroChina (2014A-0216), the International Geological Correlation Program (IGCP-632), and the State Key Basic Research Program of China (2012CB822003). We thank Q.-Z. Hou from Petrochina Tuha Oilfield Company for his help in field work, X.-D. Liu from Research Institute of Petroleum Exploration and Development, Petrochina for her assistance in SEM operation, and A. Kiritchkova for providing rare literature. H. Yang from Bryant University provided fruitful discussions and invaluable suggestions. We also thank M.E. Popa and C. Pott for their constructive review comments and suggestions for the manuscript.
Accessibility of supplemental data
Data are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.0bs64