Introduction
The Kalotermitidae, also named drywood termites for their high tolerance to dry conditions, is a monophyletic family consistently retrieved as sister to the Neoisoptera in recent molecular and morphological phylogenetic analyses (Engel et al., Reference Engel, Grimaldi and Krishna2009; Bourguignon et al., Reference Bourguignon, Lo, Cameron, Šobotník, Hayashi, Shigenobu, Watanabe, Roisin, Miura and Evans2015; Legendre et al., Reference Legendre, Nel, Svenson, Robillard, Pellens and Grandcolas2015; Bucek et al., Reference Bucek, Šobotník, He, Shi, McMahon, Holmes, Roisin, Lo and Bourguignon2019; Zhao et al., Reference Zhao, Eggleton, Yin, Gao, Shih and Ren2019; Jouault et al., Reference Jouault, Legendre, Grandcolas and Nel2021). Although some discrepancies were observed regarding the divergence time estimates of several isopteran constitutive families, depending on the method or dataset used, molecular- and morphology-based analyses agree for an Early Cretaceous rise of the kalotermitids (Bourguignon et al., Reference Bourguignon, Lo, Cameron, Šobotník, Hayashi, Shigenobu, Watanabe, Roisin, Miura and Evans2015; Bucek et al., Reference Bucek, Šobotník, He, Shi, McMahon, Holmes, Roisin, Lo and Bourguignon2019; Jouault et al., Reference Jouault, Legendre, Grandcolas and Nel2021).
Comprehension of the Kalotermitidae has greatly benefited from the outstanding work of Kumar Krishna who proposed the first phylogenetic hypothesis and described tens of new species and genera, e.g., Bifiditermes Krishna, Reference Krishna1961, Incisitermes Krishna, Reference Krishna1961, Postelectrotermes Krishna, Reference Krishna1961, and the fossil species Incisitermes peritus Engel and Krishna, Reference Engel and Krishna2007 or Cryptotermes glaesarius Engel and Krishna, Reference Engel and Krishna2007.
The kalotermitids have a rather extensive fossil record, with 74 registered occurrences ranging from the Cretaceous to Quaternary periods (Behrensmeyer and Turner, Reference Behrensmeyer and Turner2013). The oldest representative of the family was described from the Brazilian Crato Formation, as Cratokalotermes santanensis Bechly, Reference Bechly, Martill, Bechly and Loveridge2007, and the family seems to have diversified during the mid-Cretaceous, with various species reported from Cenomanian Burmese amber (Cockerell, Reference Cockerell1916, Reference Cockerell1917; Engel et al., Reference Engel, Grimaldi and Krishna2007; Poinar, Reference Poinar2009; Zhao et al., Reference Zhao, Shih, Gao and Ren2021).
Glyptotermes Froggatt, Reference Froggatt1897 is a cosmopolitan genus principally represented in tropical environments and is the most speciose of kalotermitid genera, with at least 128 living species (Krishna et al., Reference Krishna, Grimaldi, Krishna and Engel2013; Scheffrahn, Reference Scheffrahn2021). Glyptotermes is commonly found infesting sound or rotting dry wood, as well as wood scars in live trees (Scheffrahn et al., Reference Scheffrahn, Su and Křeček2001; Engel and Krishna, Reference Engel and Krishna2007). Like all kalotermitids, Glyptotermes reproductives are likely to be trapped in resin during their swarming flight because they commonly land on tree trunks and rarely reach the ground, and in fact the genus is recorded by the numerous wings and winged specimens mentioned from many deposits worldwide (Cowie et al., Reference Cowie, Wood, Barnett, Sands and Black1990; Nel and Paicheler, Reference Nel and Paicheler1993; Engel and Krishna, Reference Engel and Krishna2007; Krishna et al., Reference Krishna, Grimaldi, Krishna and Engel2013; Engel and Kaulfuss, Reference Engel and Kaulfuss2017). As for many extant genera, the fossil record of Glyptotermes remains relatively scarce, with only four species described: G. grimaldii Engel and Krishna, Reference Engel and Krishna2007 and G. paleoliberatus Engel and Krishna, Reference Engel and Krishna2007 from early Miocene Dominican amber; G. shandongianus (Zhang, Reference Zhang1989) from Miocene diatomitic deposits of Shanwang, China (Zhang, Reference Zhang1989; Zhang et al., Reference Zhang, Sun and Zhang1994); and G. pusillus (Heer, Reference Heer1849), from Pleistocene or younger East African copal (Heer, Reference Heer1849; Snyder, Reference Snyder1949; Krishna et al., Reference Krishna, Grimaldi, Krishna and Engel2013). The new specimen described herein represents the first true fossil record of the genus, and one of the very few fossil kalotermitids, from Africa.
Materials and methods
The studied specimen is preserved in a piece of amber originating from a deposit located in the North Shewa Zone of Ethiopia. The exact locality is unknown but is undoubtedly among the four localities reported by Bouju et al. (Reference Bouju, Feldberg, Kaasalainen, Schaefer-Verwimp, Hedenäs, Buck, Wang, Perrichot and Schmidt2021, fig. 2), because the material was accessed through an amber trader from Addis Ababa who obtains amber exclusively from those four localities. Apparently, the amber is dug from the same geological layer in the four deposits. The amber bed corresponds to a siltstone situated within a series of pre-Oligocene to Miocene basalts and ignimbrite (Coulié et al., Reference Coulié, Quidelleur, Gillot, Courtillot, Lefèvre and Chiesa2003; Kieffer et al., Reference Kieffer, Arndt, Lapierre, Bastien, Bosch, Pecher, Yirgu, Ayalew, Weis, Jerram, Keller and Meugniot2004; Belay et al., Reference Belay, Tesfay, Ayalew, Yohannes, Zewdie, Bekele, Tadesse, Demisse and Alemu2009) that are exposed down the gorges of the Jamma and Wenchit rivers or their tributaries (Bouju and Perrichot, Reference Bouju and Perrichot2020). Unfortunately, no radiometric age could be obtained from the adjacent basaltic rocks sampled by two of us in 2019 from the locality of Woll (see Bouju et al., Reference Bouju, Feldberg, Kaasalainen, Schaefer-Verwimp, Hedenäs, Buck, Wang, Perrichot and Schmidt2021, fig. 2). However, the geological data, combined with studies of the amber chemistry, organismic inclusions, and palynomorphs of the amber-bearing sediment, support an early Miocene age for the amber (Bouju and Perrichot, Reference Bouju and Perrichot2020; Bouju et al., Reference Bouju, Feldberg, Kaasalainen, Schaefer-Verwimp, Hedenäs, Buck, Wang, Perrichot and Schmidt2021).
The specimen was found preserved in syninclusion with a tiny chalcid wasp (Pteromalidae?) in a piece of greenish amber. The raw amber piece was ground and polished using thin silicon carbide papers for optimal observation of the insect specimens in frontal, dorsal, and ventral views. The amber piece was ultimately embedded in a block of epoxy resin (Araldite® 2020, Huntsman Advanced Materials, Basel, Switzerland) following the method of Sadowski et al. (Reference Sadowski, Schmidt, Seyfullah, Solórzano-Kraemer, Neumann, Perrichot, Hamann, Milke and Nascimbene2021, fig. 8) for consolidation and long-term conservation. Photographs and examination of the specimen were made using a Leica M205 C stereomicroscope equipped with a Leica DMC4500 digital camera. The series of photographs taken at multiple focal planes were stacked using Helicon Focus 6.7 software (Helicon Soft, Kharkiv, Ukraine). Illustrations were made using Adobe Illustrator and Photoshop CC 2019 software. We follow the morphological terminology and classification of termites as presented by Krishna et al. (Reference Krishna, Grimaldi, Krishna and Engel2013), except for the wing venation that follows Schubnel et al. (Reference Schubnel, Desutter-Grandcolas, Legendre, Prokop, Mazurier, Garrouste, Grandcolas and Nel.2019).
Abbreviations used in the text: AI−AV = antennomeres 1–5; C = costal vein; CuA = anterior cubitus; CuP = claval suture (posterior cubitus); f1–f3 = protibial spurs 1–3; h1–h3 = metatibial spurs 1–3; M = medial vein; m1–m3 = mesotibial spurs 1–3; RA = anterior radius; RP = posterior radius; Sc = subcostal vein.
Repository and institutional abbreviation
The type specimen examined in this study is deposited in the amber collection of the Geological Department and Museum of the Université de Rennes (IGR), France, under registration number IGR.ET2020/017.
Systematic paleontology
Order Blattodea Brunner von Wattenwyl, Reference Brunner von Wattenwyl1882
Infraorder Isoptera Brullé, Reference Brullé1832
Family Kalotermitidae Froggatt, Reference Froggatt1897
Genus Glyptotermes Froggatt, Reference Froggatt1897
Type species
Glyptotermes tuberculatus Froggatt, Reference Froggatt1897 by subsequent designation (Snyder, Reference Snyder1949, p. 45).
Glyptotermes abyssinicus new species
Figures 1–3

Figure 1. Glyptotermes abyssinicus n. sp., holotype IGR.ET2020/017, from early Miocene Ethiopian amber: (1) habitus in dorsal view; (2) habitus in ventral view. Scale bars = 1 mm.
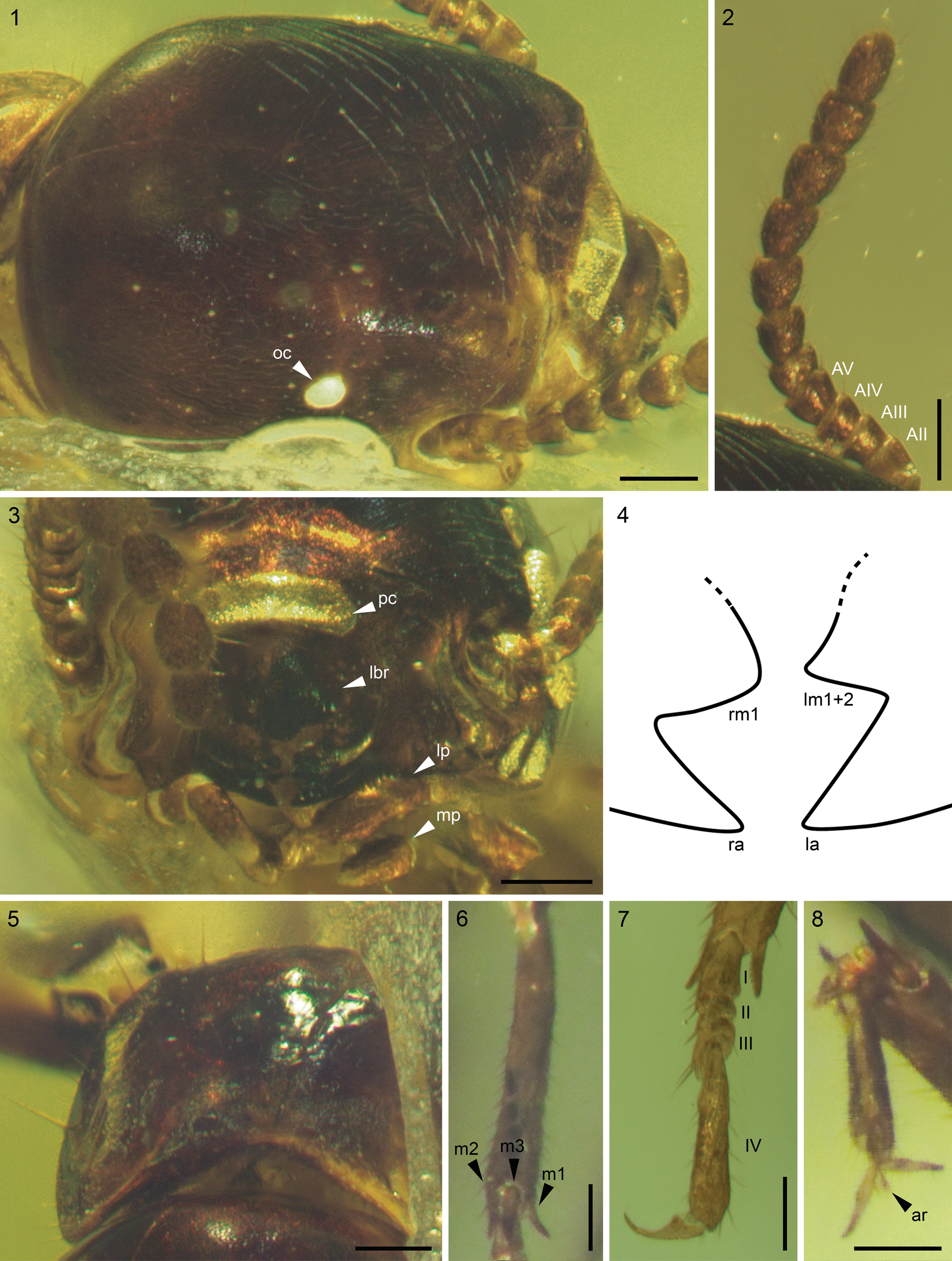
Figure 2. Glyptotermes abyssinicus n. sp., holotype IGR.ET2020/017, from early Miocene Ethiopian amber: (1) head in dorsal view; (2) left antenna in dorsal view; (3) mouthparts in frontal view; (4) diagrammatic line drawing of mandibles in frontal view; (5) pronotum in anterodorsal view; (6) right mesotibia in ventral view; (7) left mesotarsus in ventral view; (8) right protarsus in ventral view. I–IV = tarsomeres 1–4; AII–AV = antennomeres 2–5; ar = arolium; la = left mandible apical tooth; lbr = labrum; lm1+2 = left mandible first + second marginal tooth; lp = labial palp; m1–m3 = mesotibial spurs 1–3; mp = maxillary palp; oc = ocellus; pc = postclypeus; ra = right mandible apical tooth; rm1 = right mandible first marginal tooth. Scale bars = 125 μm (1–3, 5), 100 μm (6–8).

Figure 3. Glyptotermes abyssinicus n. sp., holotype IGR.ET2020/017, from early Miocene Ethiopian amber: (1) right hind- and forewing bases in dorsal view; (2) overlay of right wing scales in (1); (3) left forewing scale in dorsal view; (4) left forewing in dorsal view; (5) explanatory drawing of (4). BS = basal suture; CuA = anterior cubitus; CuP = claval suture (posterior cubitus); fsc = forewing scale; hsc = hind wing scale; M = medial vein; RA = anterior radius; RP = posterior radius; Sc = subcostal vein. Scale bars = 750 μm (4, 5), 250 μm (1–3).
Holotype
One alate specimen, IGR.ET2020/017, in amber from the lower Miocene (16–23 Myr) of the North Shewa Zone, Amhara and Oromia regions, Northwestern Plateau of Ethiopia.
Diagnosis
Head U-shaped in posterodorsal view; eyes small, subcircular and feebly protruding from head laterally; ocelli distinctly separated from eye margin, longest obliquely; antenna 12-segmented, AII longer than AIII, AIII subequal to slightly shorter than AIV; left mandible with apical tooth stout, angle between apical and first + second marginal teeth right to nearly obtuse; right mandible with long, stout apical tooth, angle between apical and first marginal teeth obtuse; forewing scale not fully overlapping hind wing scale; forewing with Sc and RA short, RP long with two anterior branches, M sclerotized, running very close to and parallel to RP to reach wing apex, with faint cross-veins between M and radial sector, CuA with at least eight or nine posterior branches.
Description
Body 3.8 mm long (from tip of labrum to abdomen apex), 7.0 mm with wings (Fig. 1). Head (Fig. 2.1, 2.2) prognathous, 1.1 mm long and 0.65 mm wide excluding compound eyes, with straight sides; anterolateral corners slightly angulate; posterolateral corners rounded; compound eye subcircular, slightly wider than long, ~0.25 mm wide, protruding from head, separated from posterior head margin by more than its length; ocelli white, ovoid (~0.06 mm long and 0.05 mm wide), located above eye midlength, very close to but not touching eye margin; fontanelle absent; antenna (Fig. 2.2) moniliform, with 12 antennomeres, AII longer than AIII, AIII subequal to slightly shorter than AIV, AIV shorter than AV, following antennomeres progressively increasing in length toward apex; left mandible with slightly obtuse to nearly right angle between apical and first + second marginal teeth, third marginal tooth hidden; right mandible with obtuse angle between apical and first marginal teeth; maxillary palps hidden by labial palps in frontal view; labial palps with three segments (Fig. 2.3); labrum ~0.18 mm long and 0.24 mm wide, posterior margin convex, anterior margin straight or nearly so; postclypeus slightly raised medially (Fig. 2.1, 2.3), rectangular, and conspicuously wider than long (~0.24 mm wide and 0.06 mm long).
Pronotum (Fig. 2.5) flat to slightly arched in lateral view; anterior margin slightly concave; sides rounded, slightly narrower than head. Legs slender; profemur ~0.5 mm long, protibia ~0.4 mm long, protarsus at least 0.2 mm long; protibia with three spurs (f1, f2, f3); mesofemur ~0.5 mm long; mesotibia ~0.5 mm long; mesotarsus at least 0.4 mm long; mesotibia with three spurs (m1, m2, m3; Fig. 2.6); metafemur ~0.5 mm long; metatibia ~ 0.8 mm long; metatarsus at least 0.4 mm long; metatibia with three spurs (h1, h2, h3); no additional spine on meso- and metatibiae; tarsi tetramerous, with apical tarsomere as long as or slightly longer than combined length of preceding ones (Fig. 2.7); arolium present (Fig. 2.8).
Wings (Fig. 3.1–3.5) 4.75 mm long (measured from suture to apex), membrane reticulate; forewing scale 0.75 mm long, not overlapping hind wing scale, with humeral margin nearly straight, CuP straight, slightly curved distally, with straight basal suture; forewing with M and CuA veins arising independently from inside the wing scale; Sc short, simple; RA and RP fused basally; RA short; RP long with two anterior branches or fusion point with C; M sclerotized, running to wing apex, close and parallel to RP, with at least one anterior, short branch located near wing apex, and maybe two unsclerotized anterior branches located respectively in basal and apical wing third; CuA unsclerotized, with eight or nine posterior branches and maybe two unsclerotized anterior branches; hind wing not describable; anal lobe absent on both wings.
Abdomen not fully preserved, ~1.4 mm long, with at least six or seven observable segments (Fig. 1.1); abdominal segments apparently widest at midlength. Cerci and styli not visible.
Etymology
The specific epithet refers to the Abyssinian origin of the amber piece, and is to be treated as an adjective.
Remarks
The new fossil does not fit in the early diverged termite families, including the extinct Cratomastotermitidae and Termopsidae, because it combines the following series of derived characters: reduced wing venation (versus more complete, RP with at least several branches, and no costalization of veins in early diverged families), four-segmented tarsi (versus five-segmented, plesiomorphic character), a reduced tibial spur formula 3-3-3 (versus enriched with additional spurs along tibiae), and the head wider than the pronotum (versus smaller than the pronotum). Compared to most of the other families, the fossil lacks a fontanelle (apomorphic character of the Neoisoptera; Krishna et al., Reference Krishna, Grimaldi, Krishna and Engel2013). Following the key to the families based on imago characters by Krishna et al. (Reference Krishna, Grimaldi, Krishna and Engel2013, p. 69), the new fossil keys out in the Kalotermitidae for: hind wing without anal lobe, tarsi four-segmented, antenna 12-segmented, fontanelle absent, forewing scale not overlapping hind wing scale, ocelli present, and pronotum flat. Using the key to the kalotermitid genera of Krishna et al. (Reference Krishna, Grimaldi, Krishna and Engel2013, p. 75, 76), the new fossil species keys out either in Glyptotermes, Calcaritermes Snyder, Reference Snyder1925, or Proneotermes Holmgren, Reference Holmgren1911, depending on character states detailed in the sixth and thirteenth couplets but invisible on the fossil, i.e., the left mandibular portion posterior to the first + second marginal tooth. However, the specimen differs from Calcaritermes and Proneotermes by the 12 antennomeres (versus 13 or 14 in Calcaritermes, 17 or 18 in Proneotermes). Additionally, it differs from Proneotermes but concords with Glyptotermes by the small eye diameter (0.25 mm versus 0.39–0.43 mm in Proneotermes) and the forewing with M as heavily sclerotized as the radial sector, running very close and parallel to the radial sector to reach the apex of the wing, with faint branches between M and radial sector, and radial sector very close to the costal vein.
Glyptotermes abyssinicus n. sp. can be distinguished from the extant Neotropical and African Glyptotermes spp. by the combination of characters stated in the diagnosis. More precisely, G. abyssinicus n. sp. differs from G. adamsoni Krishna and Emerson, Reference Krishna and Emerson1962, G. sicki Krishna and Emerson, Reference Krishna and Emerson1962, and the African G. sinomalatus Krishna and Emerson, Reference Krishna and Emerson1962, by a U-shaped head (versus circular or semicircular in the other species). It differs from other species by the 12-segmented antennae—versus 11-segmented in G. marlatti (Snyder, Reference Snyder1926) and G. planus (Snyder, Reference Snyder1925), or > 12-segmented in G. amplus Scheffrahn et al., Reference Scheffrahn, Su and Křeček2001, G. kawandae Wilkinson, Reference Wilkinson1954, or G. longuisculus Krishna and Emerson, Reference Krishna and Emerson1962.
Glyptotermes abyssinicus n. sp. can also be distinguished from other species by several additional characters. The antennae have AII longer than AIII; versus AII shorter than AIII in G. asperatus (Snyder, Reference Snyder1926), G. ignotus Wilkinson, Reference Wilkinson1959, and G. tuberifer Krishna and Emerson, Reference Krishna and Emerson1962. Measurements of the body, head, and wing length are distinct; G. rotundifrons Krishna and Emerson, Reference Krishna and Emerson1962 and G. suturis (Snyder, Reference Snyder1925) are larger species with wider head and pronotum, and longer forewings; G. contracticornis (Snyder, Reference Snyder1925) and G. longipennis Krishna and Emerson, Reference Krishna and Emerson1962 are about twice as large as G. abyssinicus n. sp. The ocelli are separated from the eye margin, versus ocelli touching the eye margin in G. jurioni Krishna and Emerson, Reference Krishna and Emerson1962, G. parki Krishna and Emerson, Reference Krishna and Emerson1962, G. parvoculatus Krishna and Emerson, Reference Krishna and Emerson1962, and G. truncatus Krishna and Emerson, Reference Krishna and Emerson1962.
Finally, Glyptotermes abyssinicus n. sp. differs from the fossil species G. grimaldii and G. paleoliberatus by a smooth head capsule (versus granulose) and the ocelli separated from the eye margin (versus touching). The old description of G. pusillus from East African copal challenges its comparison with the other Glyptotermes spp. However, it is clear that our specimen does not belong to G. pusillus owing to the differential of temporal range (Pleistocene versus early Miocene).
Discussion
The genus Glyptotermes has a worldwide distribution with a higher diversity in tropical areas (Scheffrahn et al., Reference Scheffrahn, Su and Křeček2001; Krishna et al., Reference Krishna, Grimaldi, Krishna and Engel2013; Debelo and Degaga, Reference Debelo and Degaga2014; Yashiro et al., Reference Yashiro, Takematsu, Ogawa and Matsuura2019; Scheffrahn, Reference Scheffrahn2021). Our current knowledge of the Glyptotermes fossil record, and its correlation with the distribution of the extant representatives of the genus, seems to indicate that Glyptotermes was also widely distributed in the tropical region during the Miocene period and might have experimented successive dispersal events during older geological periods (Krishna and Emerson, Reference Krishna and Emerson1962; Emerson, Reference Emerson1969; Nel and Paicheler, Reference Nel and Paicheler1993; Engel and Krishna, Reference Engel and Krishna2007).
The kalotermitids are generally of particular importance in extant and past ecosystems due to their biology. In fact, they are the only termites that do not need to construct their nest in contact with soil (a direct adaptation for their high tolerance to dryness). The kalotermitids can live exclusively within the wood and participate in its active degradation by consuming the trunk in which they construct their nest. Extant Glyptotermes spp., however, are very tolerant to environmental conditions and can live in swamp forests or mangroves (Johnson et al., Reference Johnson, Lamb, Sands, Shittu, Williams and Wood1980; Tho, Reference Tho1992). According to Scheffrahn (Reference Scheffrahn2021, p. 24), extant Neotropical “Glyptotermes species have a rather high wood moisture requirement and, therefore, are not found in arid parts of the Neotropics.” The same was likely true for the Miocene species newly described here, because this accords with indications of a generally humid, tropical, forest environment with freshwater suggested by the palynomorphs associated with Ethiopian amber as well as by other organismal inclusions in the amber (Bouju and Perrichot, Reference Bouju and Perrichot2020; Bouju et al., Reference Bouju, Feldberg, Kaasalainen, Schaefer-Verwimp, Hedenäs, Buck, Wang, Perrichot and Schmidt2021).
Conclusions
Although Isoptera are relatively well known from the fossil record, numerous fossil species have been described based on compression or imprint fossils of isolated wings, challenging placements, and questioning some systematic attributions (Krishna et al., Reference Krishna, Grimaldi, Krishna and Engel2013). The present description of a nearly complete, three-dimensional Glyptotermes specimen provides elements for more comprehensive, morphology-based phylogenetic studies. Additionally, Glyptotermes abyssinicus n. sp. can be considered as the oldest and first ‘true’ Glyptotermes fossil from Africa. This discovery confirms the presence of the genus since the Miocene period and will allow further inference on the biogeographical history of the genus. The genus Chilgatermes Engel, Pan, and Jacobs, Reference Engel, Pan and Jacobs2013 (Stolotermitidae), described from an Oligocene imprint fossil, was hitherto the only fossil termite known from Ethiopia. Therefore, the description of Glyptotermes abyssinicus n. sp. extends our knowledge of the Isoptera fossil diversity in Ethiopia.
Acknowledgments
We are grateful to Y. Goldman (Bloomfield, Connecticut) who provided the Ethiopian amber piece studied here. We thank A. Zewdalem (Jacksonville, Florida) and B. Teferi (Addis Ababa, Ethiopia) for information and access to the amber deposits of the North Shewa Zone, Ethiopia; and the reviewers and editor for their helpful comments. Partial support for this work was provided by the Tellus-INTERRVIE program of CNRS INSU (project AMBRAFRICA to V. Perrichot, 2019), enabling fieldwork in Ethiopia for V. Bouju and V. Perrichot.






