Introduction
Limestones rich in tiny phosphatic steinkerns occur in Upper Ordovician rocks of the Cincinnati Arch region of Kentucky, Ohio, and Indiana. These phosphoritic layers have been informally referred to as ‘Cyclora beds’ after the dominant fossils from most localities—tiny partial internal molds, or teilsteinkerns (Dattilo et al., Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016) of gastropods that have traditionally but informally been classified as Cyclora Hall, Reference Hall1845. The small fossils are preserved in carbonate fluorapatite and the accumulation of tiny fossils was likely taphonomic rather than ecologic (Dattilo et al., Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016). Fossils occur in high densities in these rocks, and this study demonstrates that the fidelity of apatite preservation is exceptional. The grain size of the minerals that formed internal coatings is sufficiently small to preserve sub-micrometer details of fine-order microstructural units. Phosphatization is particularly important for characterizing original aragonitic shell microstructures because aragonite (the dominant mineral in the Mollusca) has nearly entirely been replaced inside shells from before the Carboniferous (Runnegar, Reference Runnegar1985).
Some information about original aragonite textures in early Paleozoic shells has been provided by inclusions within small regions of mostly replaced shells (Balthasar et al., Reference Balthasar, Cusack, Faryma, Chung, Holmer, Jin, Percival and Popov2011) and relict/ghost textures in replaced shells (e.g., Carter and Tevesz, Reference Carter and Tevesz1978; Drushchits et al., Reference Drushchits, Doguzhayeva and Korinevskiy1979). Where phosphate imprints have an advantage is in providing excellent second- and third-order constituents (e.g., tablets, fibers, blades) within first-order textures (e.g., laminar) that are essential in identifying fossil shell microstructures. Such details are provided by phosphatic micro-steinkerns, as well as phosphate replacement of shell organics (Mutvei, Reference Mutvei1983, Reference Mutvei2002; Vendrasco et al., Reference Vendrasco, Checa, Heimbrock and Baumann2013).
Information on mollusk shell microstructures has been recovered from similar phosphatic fossils from the Cambrian, but the rate of secondary phosphatization declined significantly after the middle Cambrian (Porter, Reference Porter2004). Because of this, shell microstructure data are limited for late Cambrian to Devonian mollusks. The Cyclora beds thus help fill a gap in knowledge about the early evolution of shell microstructures in the Mollusca.
Mollusks in these shell beds show a clear preponderance of aragonite, in contrast with the mixture of inferred calcite and aragonite among Cambrian taxa. In the Cambrian bivalves Pojetaia Jell, Reference Jell1980 and Fordilla Barrande, Reference Barrande1881, the inner shell layer consisted of semi- to well-ordered aragonitic blades. Aragonitic blades also occur in modern monoplacophorans as well as the Cambrian fossils Anabarella Vostokova, Reference Vostokova1962 and Watsonella Grabau, Reference Grabau1900 (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a). This type of shell microstructure could easily be a precursor to nacre. Horizontally arranged laminae of fibers were common in early Cambrian mollusks, typically classified as lamello-fibrillar (Feng and Sun, Reference Feng and Sun2003), and the elongate nature of the crystals indicates an aragonitic composition. Most of the remaining Cambrian mollusk shell microstructure data comes from other helcionellids such as Mellopegma Runnegar and Jell, Reference Runnegar and Jell1976, and Bemella Rozanov et al., Reference Rozanov, Missarzhevsky, Volkova, Voronova, Krylov, Keller, Korolyuk, Lendzion, Mikhnyar, Pykhova and Sidorov1969, coiled forms such as Aldanella Vostokova, Reference Vostokova1962 and Pelagiella Matthew, Reference Matthew1895, and others such as the pseudo-bivalved Pseudomyona Pojeta and Runnegar, Reference Pojeta and Runnegar1976 and Eotebenna Runnegar and Jell, Reference Runnegar and Jell1976. Among these Cambrian mollusks with shell microstructure data, an inner shell layer of laminar calcite (foliated calcite, calcitic semi-nacre, or similar) was common (Vendrasco and Checa, Reference Vendrasco and Checa2015; Vendrasco et al., Reference Vendrasco, Kouchinsky, Porter and Fernandez2011b), in contrast to the higher occurrence of aragonite among younger mollusks.
Nacre occurs in the Late Ordovician cephalopod Isorthoceras Flower, Reference Flower1962 (Mutvei, Reference Mutvei1983; Vendrasco et al., Reference Vendrasco, Checa, Heimbrock and Baumann2013). Most reports of nacre in older fossils have not yet been conclusively determined (e.g., the apparent nacre in Scenella Billings, Reference Billings1872 shown by Erben et al. (Reference Erben, Flajs and Siehl1968) could be any type of laminar shell microstructure), or have been shown to be a different shell microstructure instead. Nevertheless laminar shell microstructures in at least a few Cambrian mollusks bear similarities to nacre-like microstructures in modern monoplacophorans, in particular Yochelcionella snorkorum Vendrasco et al., Reference Vendrasco, Porter, Kouchinsky, Li and Fernandez2010 from the middle Cambrian of Australia (see below).
The fossils described herein provide the most detailed record of Ordovician mollusk shell microstructures, as well as exceptional details on the earliest cases of undisputed nacre. This information is essential in understanding the early evolution of shell microstructures in the Mollusca and its relation to the accelerating escalation between mollusks and their predators through the Cambrian Radiation and Great Ordovician Biodiversification Event.
Materials and methods
Rock samples were dissolved in 10% acetic acid, macerates were sorted, and specimens mounted on Scanning Electron Microscope (SEM) stubs. The stubs were first examined via a Phenom Pro SEM at the University of Granada, then coated with carbon (Hitachi UHS evaporator) for FE-SEM observation (Zeiss Leo Gemini 1530 and Zeiss Auriga Cross-Beam Station) at the Center of Scientific Instrumentation at the University of Granada. Measurements were made with ImageJ (Rasband, Reference Rasband1997–2018).
Repository and institutional abbreviation
All specimens figured in this study and additional fossils from the same sedimentary beds are deposited at the Los Angeles County Museum of Natural History, Invertebrate Paleontology collections (LACMIP).
Fossils shown herein were extracted from rocks from the following localities:
Kope Formation, Kentucky, north of E. Alexandria Pike, 38.993°N, 84.401°W; LACMIP Locality 41957.
‘Arnheim’ Formation, Kentucky, along Florence Freedom Road, 38.982°N, 84.638°W; LACMIP Locality 41958.
Fairview Formation, East Fork Lake spillway, Bethel, Ohio, 39.029°N, 84.158°W; LACMIP Locality 41959.
Point Pleasant Formation, road cut along Kentucky (KY) 1159 near the intersection with AA Highway (KY 9): 38.731° N, 84.101° W; the samples were collected from outcrop 40 of Algeo and Brett (Reference Algeo and Brett1999); LACMIP Locality 41960.
Fairview Formation, Johnsville West, Kentucky, west side of KY 9, outcrop 35A of Algeo and Brett (Reference Algeo and Brett1999), 38.758°N, 84.149°W; LACMIP Locality 41961.
Fairview Formation, 38.779°N, 84.209°W, Kentucky, Kentucky 9 AA Highway; Holst Creek Outcrop 29-B (Algeo and Brett, Reference Algeo and Brett1999); LACMIP Locality 41962.
Kope Formation, Kentucky, outcrop along KY 9 south of E. Alexandria Pike, 38.990°N, 84.397°W; LACMIP Locality 41963.
Kope Formation, Pavillion Parkway, Newport, Kentucky near I-471 Grand Avenue exit, 39.087°N, 84.477°W; LACMIP Locality 41964.
‘Arnheim’ Formation and others, Hamilton, Ohio, 39.409°N, 84.505°W; LACMIP Locality 41965.
Fairview Formation, near Campbell, Kentucky, KY 9 Highway, 38.921°N, 84.293°W; LACMIP Locality 41966.
‘Arnheim’ Formation, Ohio, I-275 at Kemper Road, Cincinnati, 3 m below Retrosirostra Schuchert and Cooper, Reference Schuchert and Cooper1931 layer, 39.295°N, 84.541°W; LACMIP Locality 41967.
Fairview Formation, Eden Ridge West, Kentucky, Outcrop 31A (Algeo and Brett, Reference Algeo and Brett1999), 38.767°N, 84.190°W; LACMIP Locality 41968.
Fairview Formation, Barker Road, Kentucky, Outcrop 17 (Algeo and Brett Reference Algeo and Brett1999), 38.859°N, 84.264°W; LACMIP Locality 41969.
New details of shell microstructure
Introduction and conventions
Nearly all of the fossils described herein are internal molds that began as internal coatings (Fig. 1). They reflect the inner shell surface, and thus provide a negative image of the original inner shell microstructure that faced the interior of the shell. The imprints in these fossils often reflect fundamental microstructural units, such as laths (blades), tablets, and prisms.
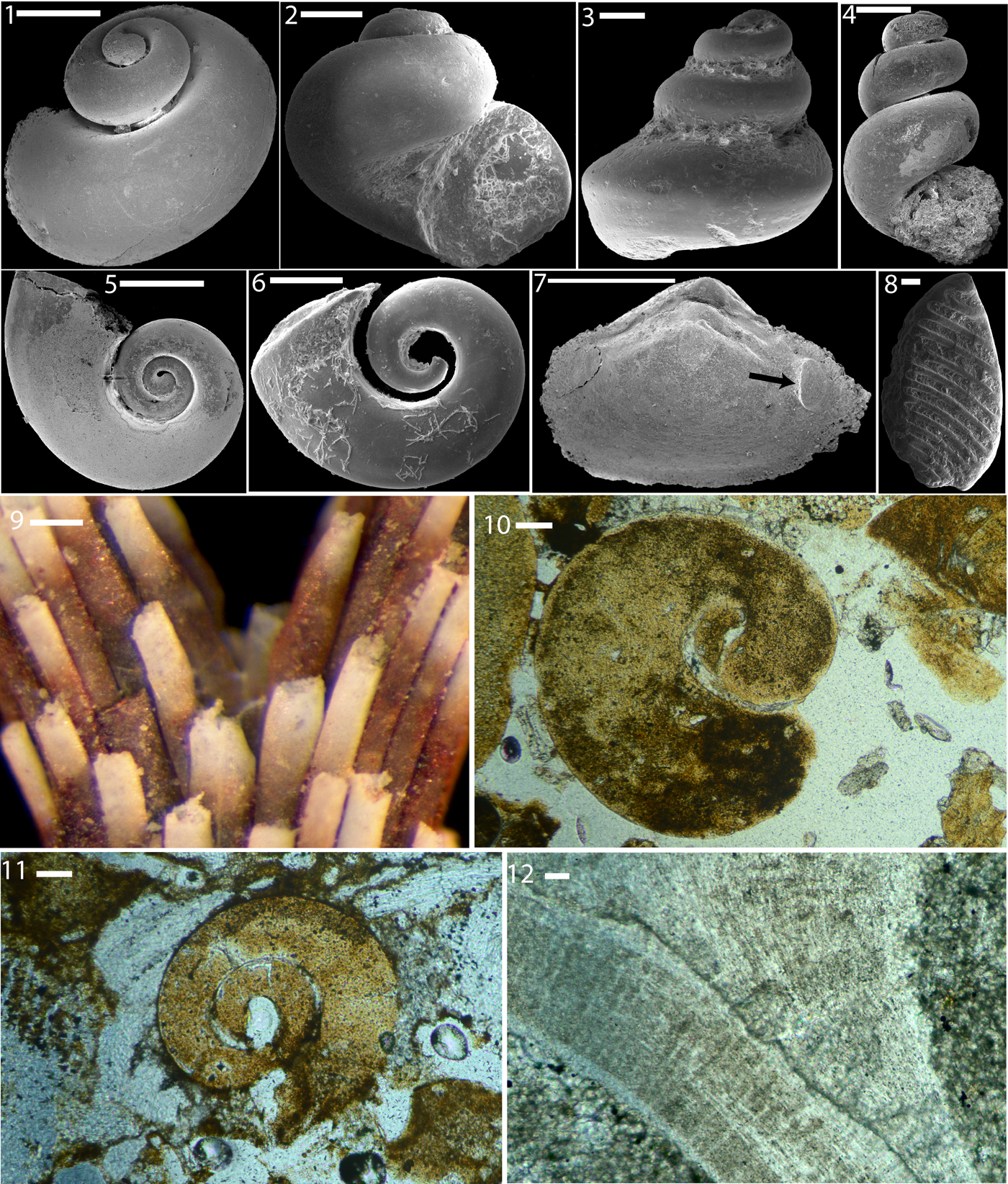
Figure 1. (1–11) Phosphatic steinkerns from the Upper Ordovician of Kentucky and Ohio, isolated (1–9) and in thin section (10, 11): (1–4, 10, 11) gastropods, (5, 6) cyrtonellid tergomyan, (7, 8) bivalves, and (9) bryozoan. (1) gastropod steinkern from the Kope Formation, AA Highway (KY 9) south of Alexandria Pike, LACMIP 14794 (LACMIP Locality 41957). (2) Gastropod steinkern, LACMIP 14795 (LACMIP Locality 41967). (3) Gastropod steinkern, LACMIP 14796 (LACMIP Locality 41967). (4) Gastropod steinkern, LACMIP 14797 (LACMIP Locality 41960). (5) Cyrtolites sp., LACMIP 14798 (LACMIP Locality 41960). (6) Cyrtolites sp., LACMIP 14799 (LACMIP Locality 41967). (7) Homilodonta sp., LACMIP 14800 (LACMIP Locality 41960). Arrow indicates muscle scar whose magnified views are shown in Figure 4.1–4.3. (8) Hinge-fill of Lyrodesma sp., LACMIP 14801 (LACMIP Locality 41958). (9) Bryozoan from the Kope Formation near Newport Kentucky, LACMIP 14802. (10) Thin section showing gastropod steinkern, LACMIP 14803 (LACMIP Locality 41968). (11) thin section showing gastropod steinkern, LACMIP 14804 (LACMIP Locality 41969). (12) Gastropod Cyclonema gracile, LACMIP 14805 (LACMIP Locality 41959). Thin section shows intersection of two whorls; upper whorl (left side) external surface is to the right. All scale bars are 200 μm.
Terms that indicate relative vertical position within the original shell (e.g., above, atop, below) are used with the up direction being from the inner shell surface (facing shell interior) toward the external (outer). Thus ‘smaller tablets below larger ones’ means there are smaller tablets on the very innermost shell surface, with larger ones closer to the external shell surface (i.e., following the sequence of secretion).
We utilize general terms for the layering within the shell, such as ‘inner shell layer’ or ‘outer layer’. We use these in place of the more specific terminology of layers defined in reference to the myostracum (e.g., m, m-1, m + 1) defined by MacClintock (Reference MacClintock1967) because in our specimens a complete vertical section of the shell microstructure is lacking, therefore it is impossible to know how many shell layers below the myostracum is the preserved inner shell layer. More importantly, the myostracum cuts diagonally through all other shell layers, so a system of layer identification based on the myostracum confuses the issue.
Guidelines for interpreting shell microstructure imprints in apatite are described in Runnegar (Reference Runnegar1985) and Vendrasco et al. (Reference Vendrasco, Rodríguez-Navarro, Checa, Devaere and Porter2015). A consideration of interfacial angles is important in the classification of the imprints wherever intersections of faces of the crystalline units (e.g., tablets and blades) are preserved. This method can distinguish, for example, between similar imprints produced by nacre and calcitic semi-nacre, two shell microstructures common in early mollusks and that have different structural properties. In rhombic calcitic semi-nacre, the calcite rhombs have expected angles of 78° and 102° (Taylor and Weedon, Reference Taylor and Weedon2000), whereas in nacre the typical expected angles are 64° (110/![]() $ {\bar 1} $10), 90° (100/010), 116° (110/1
$ {\bar 1} $10), 90° (100/010), 116° (110/1![]() $ {\bar 1} $0), and 122° (110/010) (Runnegar, Reference Runnegar1985). The two most common polygonal tablet shapes are diamond rhombuses showing 110,
$ {\bar 1} $0), and 122° (110/010) (Runnegar, Reference Runnegar1985). The two most common polygonal tablet shapes are diamond rhombuses showing 110, ![]() $ {\bar 1} $10,
$ {\bar 1} $10, ![]() $ {\bar 1} $
$ {\bar 1} $![]() $ {\bar 1} $0 and 1
$ {\bar 1} $0 and 1![]() $ {\bar 1} $0 faces, and hexagons showing all the rhombus faces plus added 010 and 0
$ {\bar 1} $0 faces, and hexagons showing all the rhombus faces plus added 010 and 0![]() $ {\bar 1} $0 faces (Taylor et al., Reference Taylor, Kennedy and Hall1969, figs. 6, 7; Wada, Reference Wada1972, fig. 15, types ɑ1, ɑ2, and ɑ5). For rhomboidal nacre tablets (ɑ1) the expected interfacial angles are 64° and 116° (equally common); for hexagonal tablets (ɑ2, ɑ5) the expected angles are ~116° and 122° (with 122° twice as common). For type ɑ4 (Wada, Reference Wada1972) rectangular tablets, only 90° angles are expected. For the rare octagonal tablets (e.g., types ɑ3 and ɑ6 of Wada, Reference Wada1972), 122° (110/010) and 148° (100/110) are expected at equal frequency. Electronically inverted images of nacre in modern mollusks (Fig. 2) are useful in comparing with shell microstructure imprints in the phosphatic steinkerns described herein.
$ {\bar 1} $0 faces (Taylor et al., Reference Taylor, Kennedy and Hall1969, figs. 6, 7; Wada, Reference Wada1972, fig. 15, types ɑ1, ɑ2, and ɑ5). For rhomboidal nacre tablets (ɑ1) the expected interfacial angles are 64° and 116° (equally common); for hexagonal tablets (ɑ2, ɑ5) the expected angles are ~116° and 122° (with 122° twice as common). For type ɑ4 (Wada, Reference Wada1972) rectangular tablets, only 90° angles are expected. For the rare octagonal tablets (e.g., types ɑ3 and ɑ6 of Wada, Reference Wada1972), 122° (110/010) and 148° (100/110) are expected at equal frequency. Electronically inverted images of nacre in modern mollusks (Fig. 2) are useful in comparing with shell microstructure imprints in the phosphatic steinkerns described herein.

Figure 2. Shell microstructure in modern mollusks, for comparison with fossil imprints. All images electronically inverted to allow direct comparison with the internal molds. (1) Aragonitic nacre-like tablets in the vetigastropod Ethminolia Iredale, Reference Iredale1924, LACMIP 14806; (2) foliated aragonite in the monoplacophoran Micropilina, LACMIP 14807; (3) nacre tablets in the bivalve Pteria hirundo, LACMIP 14808; (4) incipient nacre tablets in the bivalve Nucula, LACMIP 14809; (5) nacre tablets in the bivalve Pinctada martensii (Dunker, Reference Dunker and Küster1880), LACMIP 14810; (6, 7) nacre growth towers (6) and nacre tablets in the septum (7) of Nautilus pompilius Linnaeus, Reference Linnaeus1758, LACMIP 14811; (8) foliated calcite in the bivalve Anomia ephippium Linnaeus, Reference Linnaeus1758, LACMIP 14812. Scale bars are (1, 7) 10 μm; (2–6, 8) 3 μm.
Shell microstructure terms follow Carter (Reference Carter and Carter1990) and Carter et al. (Reference Carter, Harries, Malchus, Sartori, Anderson, Bieler, Bogan, Coan, Cope, Cragg, García-March, Hylleberg, Kelley, Kleemann, Kříž, McRoberts, Mikkelsen, Jojeta, Skelton, Tëmkin, Yancey and Zieritz2012). Higher-level molluscan taxonomy follows Carter et al. (Reference Carter, Altaba, Anderson, Araujo, Biakov, Bogan, Campbell, Campbell, Jin-hua, Cope, Delvene, Dijkstra, Zong-jie, Gardner, Gavrilova, Goncharova, Harries, Hartman, Hautmann, Hoeh, Hylleberg, Bao-yu, Johnston, Kirkendale, Kleemann, Koppka, Kříž, Machado, Malchus, Márquez-Aliaga, Masse, McRoberts, Middelfart, Mitchell, Nevesskaja, Özer, Pojeta, Polubotko, Pons, Popov, Sánchez, Sartori, Scott, Sey, Signorelli, Silantiev, Skelton, Steuber, Waterhouse, Wingard and Yancey2011) and Wagner (Reference Wagner2013).
Bivalves
Polygonal tablets merged into laminae were observed in internal molds of three species of bivalve in the assemblage: Homilodonta Cope, Reference Cope1997 (sub-class Protobranchia; order Nuculida), and Lyrodesma Conrad, Reference Conrad1841 (sub-class Autobranchia; sub-cohort Unioni), and a member of the Afghanodesmatida (sub-class Protobranchia) similar to Leiodysodonta Pojeta, Zhang, and Yang, Reference Pojeta, Zhang, Yang and Pojeta1986. The arrangement and morphologic varieties of the tablets vary among these bivalve taxa, but for each species the tablets tend to be more or less co-oriented within and between horizontal laminae. The ranges of size, shape, and interfacial angles are for each species consistent with nacre (see below). Tablet centers are offset in successive laminae, as is typical for bivalve nacre.
Some specimens of afghanodesmatid bivalves show good details of nacre, either regionally (e.g., in a comarginal band near the aperture in one specimen) or in patches at various places over the internal shell surface (Fig. 3). The nacre is preserved as overlapping laminae of merged and merging tablets. The entire outlines of tablets are preserved, indicating the nacreous laminae are horizontal. New tablets are not centered under overlying ones, but instead occur in various locations relative to the centers of slightly older tablets, typical of bivalve nacre. The extent of co-orientation of tablets within and between laminae varies somewhat from region to region on internal molds, but in many places the co-orientation is strong.
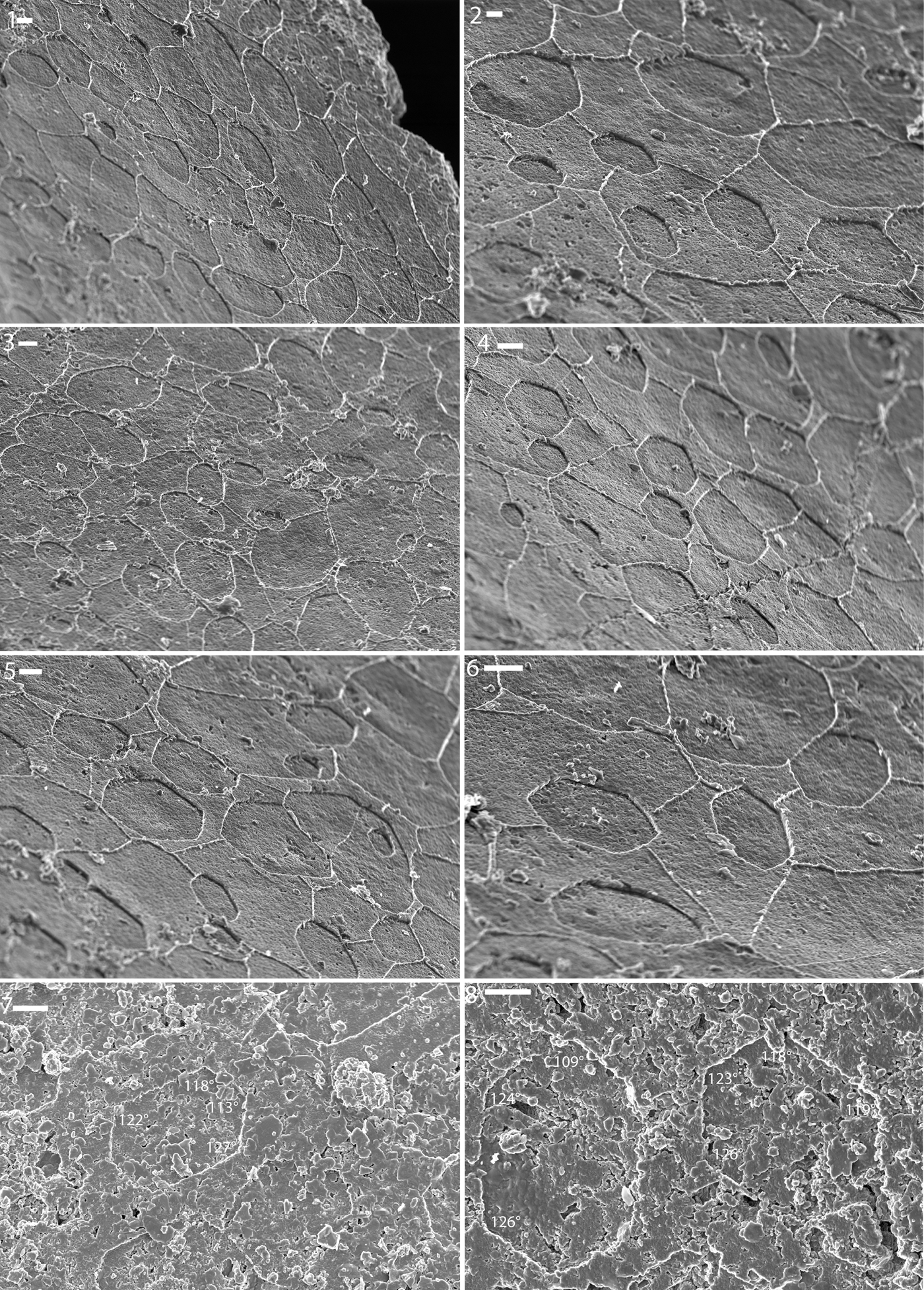
Figure 3. Shell microstructure imprints on steinkerns of Afghanodesmatid bivalves from the Ordovician Fairview Formation of Kentucky. (1–6) LACMIP 14813 (LACMIP Locality 41961); photos from near the aperture, visible in upper right of (1); (7, 8) LACMIP 14814 (LACMIP Locality 41962); near umbo. Numbers show measurements of well-preserved interfacial angles. Scale bars are (1, 3–8) 2 μm; (2) 1 μm.
Prior to merging with neighbors, tablets in the Afghanodesmatid bivalves are pseudo-hexagonal. Some tablets are roughly equidimensional and many are elongate. The elongate varieties are similar to those seen in a few modern mollusks, such as near the aperture of modern specimens of Nucula and in the septa of modern Nautilus (Fig. 2.7). Merged tablets show a variety of roughly equidimensional outlines, as in the nacre of modern mollusks. Isolated (not yet merged) tablets are typically on the order of a few micrometers in width (i.e., the shorter dimension in elongate tablets), whereas merged tablets are on the order of 5–10 μm in width. Interfacial angles of some of the best preserved tablets are 109.6°, 112.5°, 115.2°, 116.5°, 116.7°, 117.7°, 118.8°, 122.0°, 123.0°, 123.1°, 124.1°, 124.1°, 124.7°, 125.4°, 126°, 126.7°, 127.3°, 127.8°, and 128.7°, close to the expected angles 116° and 122°. Growing tablets are typically ~5–10 μm in length (measured along longer axis of tablet).
Specimens of Homilodonta show detailed preservation of nacre (Fig. 4). In the best-preserved specimens, imprints of tablets consistent with nacre can be seen over the entire inner surface of the shell. The tablets overlap, with smaller ones below larger ones on the inner shell surface (Fig. 4.6, 4.8). New tablets are co-oriented within and between laminae, and overlapping tablets are offset from each other such that new tablets initiated at boundaries between tablets in the overlying layer (Fig. 4.5). These observations are consistent with bivalve sheet nacre. In most cases, the entire outlines of tablets are preserved, indicating that the laminae are horizontal. In the muscle scars, closely spaced ends of laminae are preserved (Fig. 4.1, 4.2), indicating a slight inclination of the inner laminae in this region.

Figure 4. Shell microstructure imprints on phosphatic steinkerns of the bivalve Homilodonta sp. from the Ordovician Point Pleasant Formation, Kentucky (LACMIP Locality 41960). (1–5) LACMIP 14800: (1) region near the adductor muscle scar shown in the arrow in Figure 1.7; (2) magnified view of the same adductor muscle scar; (3) image from near same adductor muscle scar; (4) near umbo; (5) transitional zone from microstructure of the other adductor muscle scar (on left edge of image in Figure 1.7; without arrow) and rest of inner shell surface; (6–8) LACMIP 14815: (6) near middle/top (closer to umbo than aperture); (7) near umbo; (8) near aperture. Numbers show measurements of well-preserved interfacial angles. Scale bars are (1) 10 μm; (2) 1 μm; (3–8) 2 μm.
Tablets in Homilodonta range in shape from hexagonal to sub-circular to sub-oval. Tablets typically begin as polygonal and become more circular or oval as they begin to merge with neighbors. The hexagonal outline of isolated growing tablets and lack of distinct growth fronts of merged tablets are similar to what occurs in modern Nucula Lamarck, Reference Lamarck1799 (Fig. 2.4). Tablet imprints occur over a narrow size range from a few to nearly 10 μm in width/diameter, and they are ~400 nm in height, both of which are consistent with dimensions of nacre in modern mollusks. Interfacial angles of some of the better-preserved polygonal tablets are close to what is expected for nacre (112.9°, 115.0°, 115.3°, 120.2°, 124.1°, 124.8°, 126°, 126.4°, 126.6°, and 132.1°, compared with the expected 116° and 122°). Some large tablets appear to show spiral growth (Fig. 4.6), as is seen in the nacreous tablets of modern mollusks.
In and around the muscle scars is a laminar shell microstructure consisting of a broad stair-step texture. We interpret this as laminar aragonite because the ends of laminae are remarkably straight, as in the faces of nacre tablets and unlike foliated aragonite that should show the ends of blades. Also there is gradation between the stair-step pattern and nacre tablets along some borders of muscle scars (Fig. 4.5). The muscle scars of modern bivalves, reflecting the myostracum shell layer, typically display a prismatic shell microstructure. Nevertheless, a similar broad, shallow stair-step nacre-like shell microstructure can be seen in the muscle scars of modern abalone.
There are orientation patterns of apatite crystals within tablet imprints and exposed laminae of muscle scars, but these do not seem to reflect original tablet ultrastructure as described by Mutvei (Reference Mutvei1970, Reference Mutvei1978). There is a large variety of different orientations in the zones of co-oriented apatite crystals, in contrast to the 3–4 zones typical in modern nacre. Plus, adjacent regions do not show expected aragonite twin angles.
The fossil species we show here is characterized by an inner shell layer of nacre and muscle scars of a laminar aragonite texture. In Homilodonta the high occurrence of merging tablets over the entire internal surface indicates that active nacreous growth was occurring at a fast rate throughout the shell. Isolated tablets growing in a diffuse manner without active growth fronts of the nacreous laminae occur in modern bivalves such as Nucula (Fig. 2.4).
Fossils of Lyrodesma sp. occur as internal molds of segments of the ligament region of the shell (Dattilo et al., Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016). These hinge molds match the shape and size of spaces in the hinge of calcite-replaced shells of Lyrodesma. These molds reflect the shell microstructure expressed on the inner surface of the shell wherein the ligament had grown. Well-preserved tablets are preserved on the inner walls of the radiating hard hinge elements (Fig. 5.1–5.5). The tablets tend to be hexagonal, with angle matches close to expected typical angles of nacre, 106° and 122°: 113.0°, 113.1°, 115.4°, 118.4°, 120.7°, 132°. In one fragmentary specimen (Fig. 5.4), tablets have a more rounded, sub-polygonal shape, although rhomboidal tablets are also present in that individual.
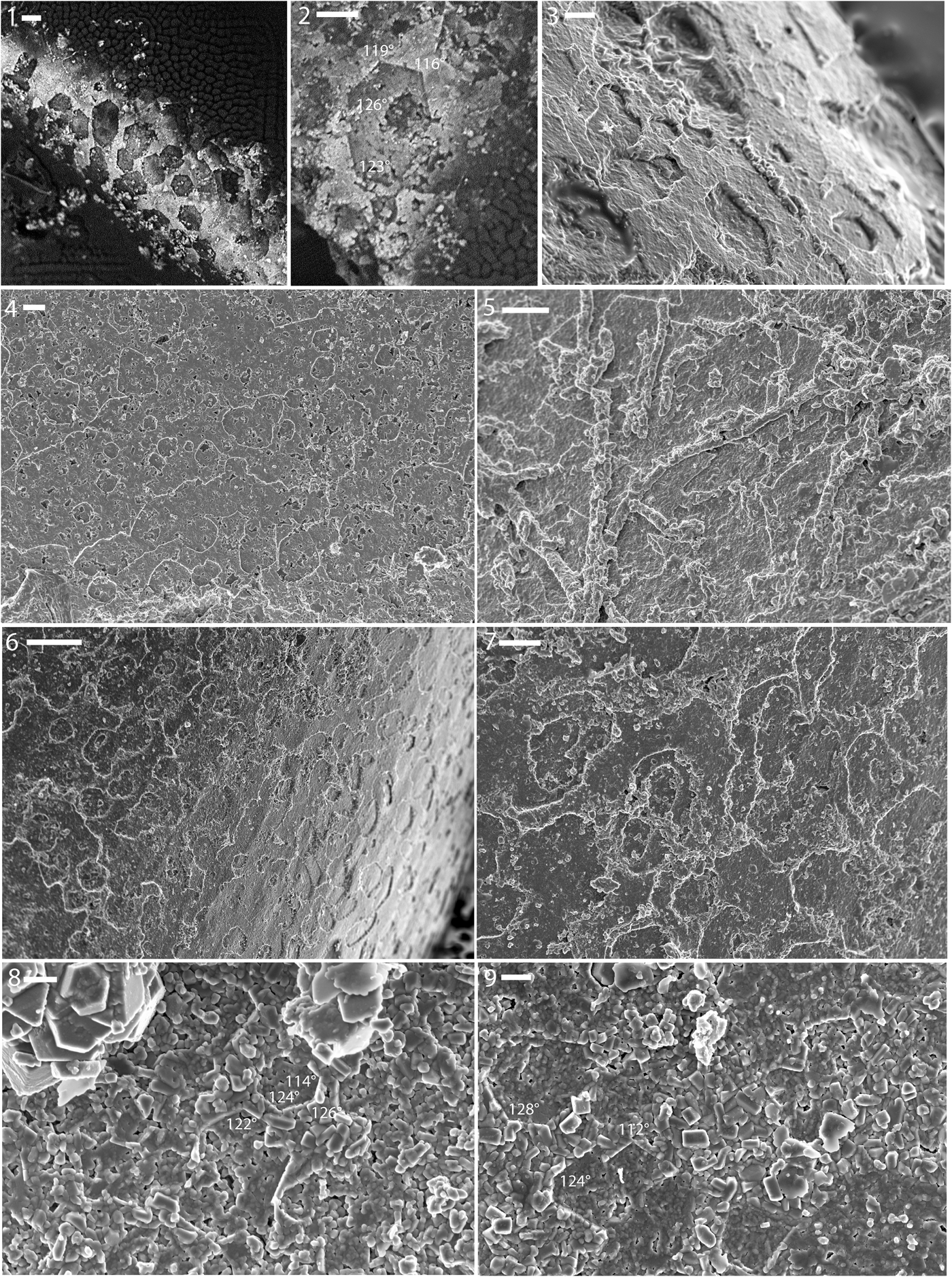
Figure 5. (1–5) Shell microstructure (nacre) imprints on internal molds of the hinge region of the bivalve Lyrodesma sp. from the Ordovician Fairview Formation of Kentucky (LACMIP Locality 41961). (1, 2) LACMIP 14816; (3) LACMIP 14817; (4) LACMIP 14818; (5) LACMIP 14819. (6–9) Shell microstructure (nacre and nacre-like) imprints on steinkerns of the bellerophontiform Cyrtolites sp. from the Ordovician Fairview (6, 7) and Point Pleasant (8, 9) formations. Numbers show measurements of well-preserved interfacial angles. (6, 7) LACMIP 14820 (LACMIP Locality 41961); (8, 9) LACMIP 14821 (LACMIP Locality 41960). Scale bars are (1, 6) 10 μm; (2, 7) 5 μm; (3) 2 μm; (4, 5) 3 μm; (8, 9) 1 μm.
Diamond-shaped tablets seen in other specimens of Lyrodesma are similar in outline to those of Pteria hirundo (Linnaeus, Reference Linnaeus1758) (Fig. 2.3) and Pinctada margaritifera (Linnaeus, Reference Linnaeus1758) (Taylor et al., Reference Taylor, Kennedy and Hall1969, fig. 10). The interfacial angles of rhomboidal tablets are close to what is expected for rhomboidal nacre (88°, 94.6°, 57.7°, and 73°, compared with the expected 90° and 64°), and those of hexagonal tablets are similar to that of hexagonal nacre (116°, 119°, 123°, and 126°, compared with the expected 116° and 122°).
Tergomyan monoplacophorans (cyrtonellids)
Tiny, symmetrically coiled cyrtonellid univalves that we tentatively identified as Cyrtolites Conrad, Reference Conrad1838 are relatively common in these beds. A few individuals preserve exceptional details of the inner shell microstructure of this species.
Imprints of subcircular to polygonal units were observed on the surface of internal molds of some individuals of Cyrtolites (Fig. 5.6–5.9). The units typically range from 5–10 μm in diameter and are isolated as well as partly merged into layers. The units show a high variation in outline (elongate-oval, elongate polygonal, sub-circular, diamond), even within the same individual. In many cases the impressions of smaller units are found within larger ones. The units are short, ~300 nm in height, and in some cases they show striking co-orientation. All these observations are consistent with the units being incipient nacre tablets, and the measured angles of the inferred tablets likewise are consistent with this interpretation. The best preserved angles are 109°, 112°, 115°, 117°, 117°, 118°, 118°, 120°, 121°, 121°, 122°, 122°, 124°, 124°, 127°, 128°, and 129°, which are close to the 116° and 122° expected for typical nacre.
One specimen showed highly inclined ends of presumably aragonitic tablets. Such semi-laminar, slightly inclined, aragonite tablet-rich types of shell microstructures are very common in the innermost shell layer of modern vetigastropods, typically occurring just below the nacreous layer (e.g., see Erben, Reference Erben1972, pl. 2).
Morphological gradation occurs among the symmetrically coiled fossils of the assemblage, and thus it is possible that they represent only one or a few species. The symmetrically coiled univalves in these phosphate-rich coquinas were considered to be Cyrtolites inornatum (Hall, Reference Hall1845) by Martin (Reference Martin1986). Hall (Reference Hall1845, p. 292) originally described this species as a member of the genus Microceras Hall, Reference Hall1845, and referred to it as one of the many tiny shells from what he referred to as the “decomposing marl slate of Cincinnati.” His description lines up with the fossils we observed, although external characters that diagnose Cyrtolites do not help in the classification of these internal molds. Wahlman (Reference Wahlman1992) classified Cyrtolites as a tergomyan monoplacophoran based on the presence of multiple sets of muscle scars in some species. The symmetrically coiled fossils from these shell beds were referred to as monoplacophorans by Dattilo et al. (Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016), but they did not classify them further.
Although nacre had been thought to be the characteristic inner shell layer of modern monoplacophorans, a closer analysis of modern representatives found that foliated aragonite is predominant in the group (Checa et al., Reference Checa, Ramírez-Rico, González-Segura and Sánchez-Navas2009a, b). In any case, the fossils described herein show that nacre had originated within at least one lineage of monoplacophorans at least by the Ordovician.
The nacre in the tergomyan monoplacophorans (Cyrtolites) of this assemblage show characteristics of both monoplacophoran and gastropod varieties. Overall, gastropods and cephalopods tend to show columnar nacre whereas monoplacophorans and bivalves show sheet nacre (Hedegaard, Reference Hedegaard1997). The tablets of nacre in the sheet form tend to be highly co-oriented, wherein some tablets have the b-axis pointing towards the local margin and other tablets are oriented with the b-axis ~120° and 240° to this direction, reflecting aragonitic twinning. In columnar nacre, the tablets tend to have less-horizontal co-orientation within any one sheet, although there are some exceptions (e.g., there is some degree of horizontal co-orientation near the aperture of Nautilus Linnaeus, Reference Linnaeus1758). The tablets of Cyrtolites are in some cases clearly stacked (Fig. 5.7), as in gastropods (Fig. 2.6), cephalopods (Fig. 2.7), and some bivalves near the margin.
Gastropods
The tiny gastropod fossils in these beds have been historically referred to as Cyclora, although most of them probably represent the juveniles of larger species (Dattilo et al., Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016), possibly in some cases Cyclonema Hall, Reference Hall1852. Identification to species is difficult with these internal molds, but there are high- and low-spired varieties that probably represent the apical region of multiple species (Fig. 1.1–1.4).
Some internal molds show isolated tablets of the same size (a few μm in width) and with the same angles (121°, 128°, 129°) as the common 122° of many forms of modern nacre (Fig. 6.9). Other specimens show an inclined laminar texture with straight or polygonal growth fronts, similar to what occurs in the innermost layer of modern gastropods (see description of textures in Cyrtolites above). In other instances a diffuse polygonal network is preserved in some regions of the internal mold, although it is unclear what this might represent. The clearest preservation of shell microstructure was observed in a specimen with a blunt top end (Fig. 6.4–6.10), possibly produced as the apex was sealed off in a manner similar to what was inferred by Dattilo et al. (Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016). At the blunt apical end of the specimen many sharply polygonal tablets typically ~5 μm in diameter can be observed (Fig. 6.5–6.10). These show some degree of co-orientation, and many of these tablet imprints directly overlie others in what must have been isolated stacks of tablets. Such stacks are reminiscent of the classic towered nacre of modern vetigastropods.
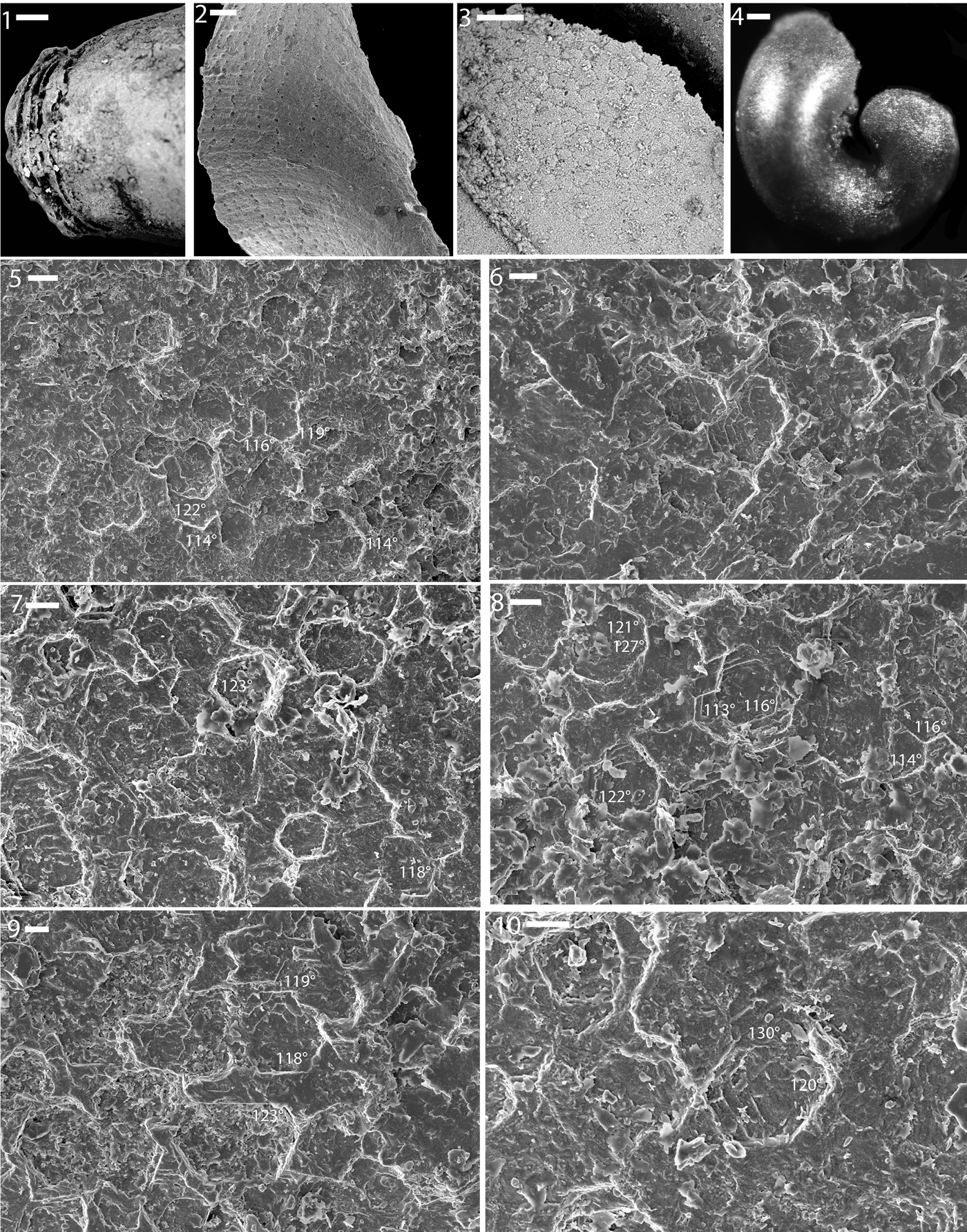
Figure 6. Shell microstructure (3, 5–10), septation (1), and phosphate cast of the umbilicus (2) on steinkerns of gastropods from the Ordovician Fairview (2) and Kope (1, 3–10) formations of Kentucky. (1) LACMIP 14822 (LACMIP Locality 41963), apical region showing septation; (2) LACMIP 14823 (LACMIP Locality 41961), phosphate fill of the umbilical region; (3) LACMIP 14824 (LACMIP Locality 41963), portion of an external mold where the overlying apical part of the steinkern is missing; (4–10) LACMIP 14825 (LACMIP Locality 41964); (4) entire specimen, showing that the apical region is blunt and appears incomplete; (5–10) closeup views of the apical region, showing nacre-like tablets; numbers show measurements of well-preserved interfacial angles. Scale bars are (1, 3) 10 μm; (2) 40 μm; (4) 100 μm; (5) 4 μm; (6–10) 2 μm.
Thompson (Reference Thompson1970) noted the common co-occurrence of the large platyceratid gastropod Cyclonema with the small steinkern gastropod fossils identified as Cyclora. Earlier, Ulrich (in Ulrich and Scofield, Reference Ulrich and Scofield1897) suggested that Cyclora was either the young or a dwarfed form of larger gastropods, such as Holopea Hall, Reference Hall1847 or Cyclonema, with which it occurs. Thompson (Reference Thompson1970) in particular noted the co-occurrence of Cyclora minuta Hall, Reference Hall1845 with Cyclonema bilix Conrad, Reference Conrad1842 and Cyclonema humerosum Ulrich and Scofield Reference Ulrich and Scofield1897, and a striking resemblance between the whorl profile in cross section of Cyclora minuta and the apical region of Cyclonema in the specimens that preserve it. Wagner (Reference Wagner2013) reassigned Cyclora minuta to Cyclonema, suggesting that Cyclora may represent the juvenile form of Cyclonema. Given the broad area over which these micro-teilsteinkerns were drawn from and their morphological variation (Fig. 1.1–1.4), these fossils may represent a number of different species, genera, families, or clades.
The shell of Cyclonema is remarkably well preserved, showing original color patterns and the whole shell in calcite with prismatic outer layer and laminar inner shell layer (Thompson, Reference Thompson1970). New specimens examined by us show the same pattern. Based on the quality of preservation of the shell, Thompson (Reference Thompson1970) inferred the entire shell was originally calcite, based on the fact that this is the only genus of gastropod from the Cincinnatian where the shell is typically preserved. If so, the laminar inner shell layer could not be nacre, which is exclusively aragonitic. Earlier, Knight et al. (Reference Knight, Cox, Keen, Batten, Yochelson, Robertson and Moore1960, p. I240) noted the laminar inner layer and inferred that the inner shell layer of Cyclonema was “seemingly nacreous and aragonitic.” Batten (Reference Batten1984) also made this claim about Cyclonema bilix, but inferred the laminar inner layer was unaltered aragonite, which is unlikely. Dattilo et al. (Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016) mentioned that the inner shell layer of Cyclonema consists of calcite recrystallized from aragonite, but evidence was not shown. Based on the exceptional images in Thompson (Reference Thompson1970), Batten (Reference Batten1984), and our own observations of Cyclonema gracile Ulrich and Scofield, Reference Ulrich and Scofield1897 from the Fairview Formation (Fig. 1.12), the fine laminae of the inner shell layer of Cyclonema is so often preserved in such detail as to represent original calcitic mineralogy, as inferred by Thompson (Reference Thompson1970).
If Cyclonema had a calcitic shell, this indicates that at least some of the gastropod micro-steinkerns from these shell beds are not from juvenile shells of Cyclonema. The shell microstructure of at least one of the teilsteinkerns previously classified as Cyclora is clearly aragonitic and represents something like nacre (Fig. 6.4–6.10).
Bryozoans
Apatite internal molds of stenolematan bryozoan zooecia are common in these beds (Figs. 1.9, 7.1–7.4), with Dattilo et al. (Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016) noting the common occurrence of trepostomes and, to a lesser extent, cryptostomes. Laminar and bladed types of calcitic shell microstructures in general characterize stenolamatans (Williams, Reference Williams and Carter1990). Distinct shell microstructures were observed in many phosphate molds of trepostome bryozoans. In isolated, broadly expanding zoecia with a sub-triangular cross section, pristine imprints of foliated calcite are preserved wherein adjacent blades of calcite are oriented with the long axis parallel to long axis of zoecia (Fig. 7.1; Vendrasco et al., Reference Vendrasco, Checa, Heimbrock and Baumann2013, figs. 8.2, 8.4). The ends of these blades vary from straight to angular, and those with angular edges show angles hovering around 100°. Foliated calcite in Novocrania has the c-axis in plane and elongate along it. This may also be the case in some bryozoans. In bivalves, by comparison, the c-axis is at a higher angle to the lath plane (45° with 104 calcite rhombohedra or 64° with 108 rhombohedra).
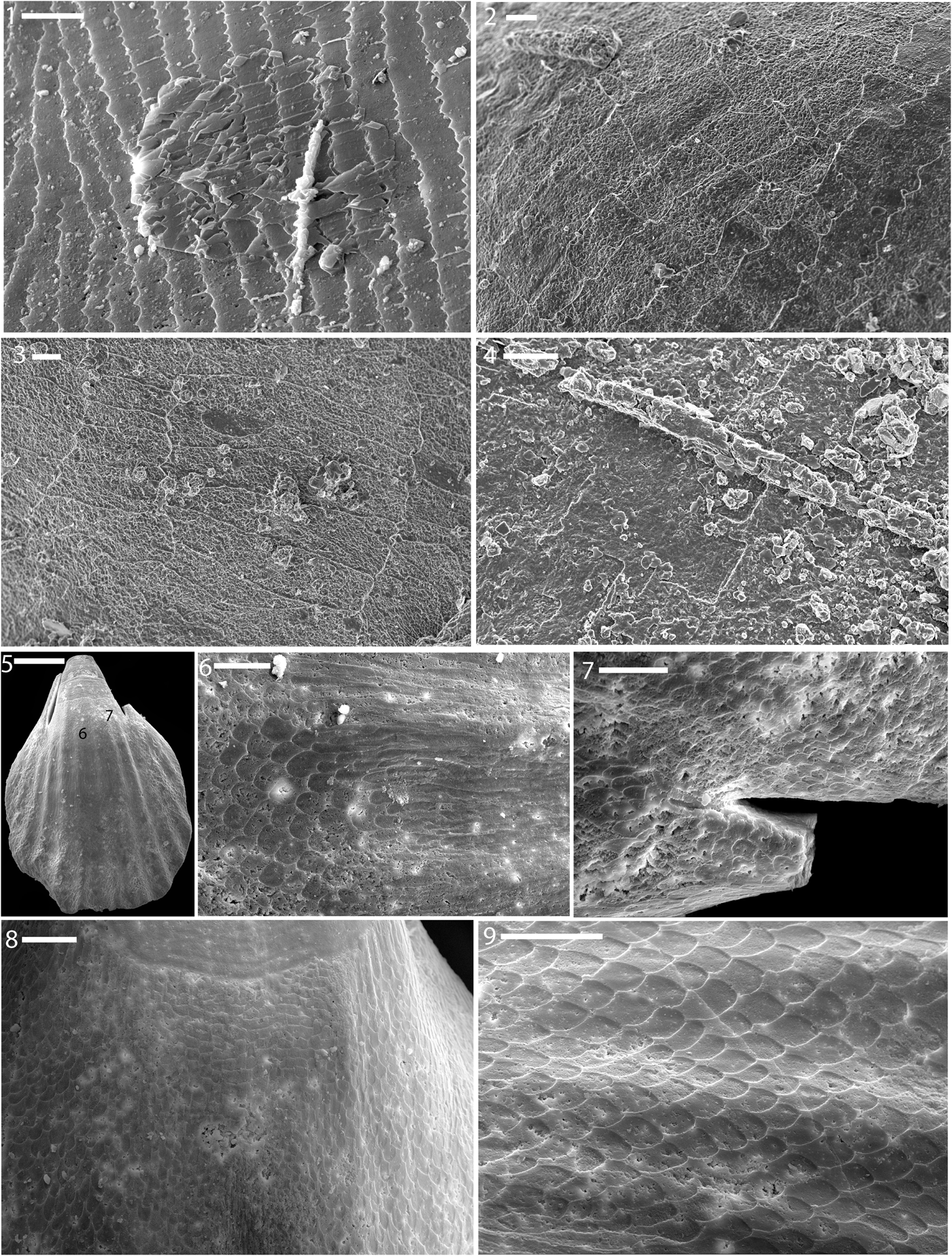
Figure 7. (1–4) Shell microstructure in phosphatic steinkerns of bryozoans from the Ordovician ‘Arnheim’ (1), Fairview (2, 3), and Kope (4) formations of Ohio (1) and Kentucky (2–4). (1) LACMIP 14449 (LACMIP Locality 41965); (2, 3) LACMIP 14826 (LACMIP Locality 41966); (4) LACMIP 14827 (LACMIP Locality 41963). (5–9) Shell microstructure in a phosphatic steinkern of the brachiopod Zygospira from the ‘Arnheim’ Formation of Ohio. (5–7) LACMIP 14828 (LACMIP Locality 41967); numbers in (5) show locations of photos (6, 9). (8, 9) LACMIP 14448 (LACMIP Locality 41967); (8) is from the a region similar to ‘6’ in the other specimen (5); (9) is from near the aperture. Scale bars are (1) 10 μm; (2–4) 4 μm; (5) 250 μm; (6–9) 50 μm.
The shell microstructures preserved in bryozoans, like those of brachiopods described below, are useful in that they provide a detailed record of the appearance of certain types of shell microstructure reflected on fine-grained internal molds. Unlike many early Paleozoic mollusks, the shell microstructures of these taxa are much better known, and therefore these images can serve to help in interpretation of problematic textures, such in those from Cambrian small skeletal fossils. In addition, shell microstructure seems to be under strong genetic control in Paleozoic stenolaemate bryozoans, and hence is of great use for classification (Boardman, Reference Boardman and Robison1983).
Brachiopods
Internal molds of the rhynchonellatan brachiopod Zygospira Hall, Reference Hall1862 show imprints of a remarkably well-organized calcitic fibrous prismatic shell microstructure (Fig. 7.5–7.9). The lateral regions of the inner shell layer show overlapping calcite blades, with only the tips preserved in most regions. These blade tips are slightly offset in subsequent layers, forming a scale-like pattern. In the middle-apical shell region, these calcite blades appear to have merged together to form a laminar type shell microstructure. Near the middle of the inner surface of the shell, elongate blades of calcite can be seen, and over the rest of the inner shell surface, the overlapping blade tip pattern predominates. Such a fibrous calcitic inner shell layer is characteristic of rhynchonellide brachiopods in general (Rowell and Grant, Reference Rowell, Grant, Boardman, Cheetham and Rowell1987). Other brachiopods from other beds of the Late Ordovician of the Cincinnati region show relatively unaltered calcitic bladed shell microstructures.
Comparison of nacre in the microfossil assemblages
A comparison of the fine structure of nacre in this assemblage of early mollusks can provide evidence for or against the hypothesis that nacre arose independently in mollusks, as advanced by Vendrasco et al. (Reference Vendrasco, Checa and Kouchinsky2011a). Some observations from this assemblage are consistent with the hypothesis, and others are not. First, aspects of crystal shape and arrangement vary among the mollusks in this assemblage. Differences include a strong trend towards tablet stacking in the gastropod and tergomyan versions versus more vertically offset arrangements in the bivalves. Nevertheless, there are also strong similarities among the nacre in these various forms. Most striking is the range in form of tablets from rounded to sharply polygonal, including some elongate tablets. In addition, nacre is clearly the dominant inner shell layer in this assemblage, occurring in representatives of the Bivalvia, Gastropoda, and Monoplacophora, in addition to its clear occurrence at the same time in the Cephalopoda.
These fossils also show good diversity of microstructures within the nacreous layer, particularly well demonstrated in specimens of Homilodonta, where tablets range from sub-circular to highly polygonal, from very well organized from layer to layer to more loosely organized, and from more to less of a sheet-like form. Such variation invites caution in over-splitting imprints of shell microstructure, especially where data are limited (e.g., for the gastropod microfossils in this assemblage).
Early evolution of mollusk shell microstructures
In contrast to the apparent commonality of nacre among Ordovician mollusks, its conspicuous absence or rarity from the Cambrian is notable. In contrast, existing shell microstructure data show that calcite was quite common in the inner shell layer of helcionellids and other Cambrian mollusks. It is possible that nacre did occur in the Cambrian, given the variability in the ultrastructure of nacre among modern bivalves and monoplacophorans and similar textures in some Cambrian mollusks (see below). At the very least there was a differential change among mollusks from the Cambrian to the Ordovician away from presumably aragonitic fibrous textures like lamello-fibrillar and foliated aragonite and calcitic textures like calcitic semi-nacre and foliated calcite towards a predominance of nacre.
Yochelcionella snorkorum Vendrasco et al., Reference Vendrasco, Porter, Kouchinsky, Li and Fernandez2010, middle Cambrian of Australia, known from phosphatic steinkerns, shows imprints similar to nacre. There is great spread in measured interfacial angles of Yochelcionella Runnegar and Pojeta, Reference Runnegar and Pojeta1974 (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a), although the viewing angle relative to the inner shell surface is in many cases highly tilted, and imprints of crystal faces are not always distinct. Measurements of well-preserved crystalline interfaces at a near-normal view are 58°, 66°, 70°, 90°, 109°, 112°, 113°, 115°, 119°, 126°. These are similar to the angles expected for typical nacre of 64° (110/![]() $ {\bar 1} $10), 90° (100/010), 116° (110/1
$ {\bar 1} $10), 90° (100/010), 116° (110/1![]() ${\bar 1}$0), and 122° (110/010). The tablet shapes range from polygonal (not rhomboidal like most of the helcionellids from the Cambrian) to rounded. This is a very nacre-like shell microstructure, except for the occurrence in Yochelcionella of regions of lamellae at slightly different dip angles, and occasional blade-like (not tablet) imprints. The overall pattern is similar to the mixture of foliated aragonite and nacre that is seen in some modern monoplacophorans, and the different lamellae dips also occur in the laminar inner shell layer of the modern monoplacophoran Veleropilina Starobogatov and Moskalev, Reference Starobogatov, Moskalev, Starobogatov, Golikov and Likarev1987 (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a). In Veleropilina, the stair-step configuration of laminae often have a tablet-like appearance (Checa et al., Reference Checa, Sánchez-Navas and Rodríguez-Navarro2009b). In any case, the shell microstructure in Yochelcionella is similar to what occurs in Veleropilina and may represent a precursor to nacre. For example the laminar inner shell microstructure in species of Yochelcionella from the middle Cambrian of Australia shows nacre-like tablets in stacks (Vendrasco et al., Reference Vendrasco, Porter, Kouchinsky, Li and Fernandez2010) that are similar to the mixed foliated-aragonite/nacre of Veleropilina (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a) and Micropilina Warén, Reference Warén1989 (see Cruz et al., Reference Cruz, Weissmüller and Farina2003).
${\bar 1}$0), and 122° (110/010). The tablet shapes range from polygonal (not rhomboidal like most of the helcionellids from the Cambrian) to rounded. This is a very nacre-like shell microstructure, except for the occurrence in Yochelcionella of regions of lamellae at slightly different dip angles, and occasional blade-like (not tablet) imprints. The overall pattern is similar to the mixture of foliated aragonite and nacre that is seen in some modern monoplacophorans, and the different lamellae dips also occur in the laminar inner shell layer of the modern monoplacophoran Veleropilina Starobogatov and Moskalev, Reference Starobogatov, Moskalev, Starobogatov, Golikov and Likarev1987 (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a). In Veleropilina, the stair-step configuration of laminae often have a tablet-like appearance (Checa et al., Reference Checa, Sánchez-Navas and Rodríguez-Navarro2009b). In any case, the shell microstructure in Yochelcionella is similar to what occurs in Veleropilina and may represent a precursor to nacre. For example the laminar inner shell microstructure in species of Yochelcionella from the middle Cambrian of Australia shows nacre-like tablets in stacks (Vendrasco et al., Reference Vendrasco, Porter, Kouchinsky, Li and Fernandez2010) that are similar to the mixed foliated-aragonite/nacre of Veleropilina (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a) and Micropilina Warén, Reference Warén1989 (see Cruz et al., Reference Cruz, Weissmüller and Farina2003).
Given that the earliest clam fossils also showed foliated aragonite (Vendrasco et al., Reference Vendrasco, Checa and Kouchinsky2011a), foliated aragonite was seen as a possible precursor to nacre. However, size may partly explain the pattern because it is the larger monoplacophoran Veleropilina that shows the transition from foliated aragonite to nacre at the innermost layer. What confounds the interpretation of patterns is that data are limited for the full range of Cambrian mollusks, and data that exist are concentrated about the innermost shell layer. Nacre may have occurred in the Cambrian. Some Cambrian fossils appear to show isolated nacre-like tablets preserved, and otherwise have similar morphologies (e.g., Yochelcionella). Nacre is predominant in fossil and modern shelled cephalopods and it likely represents the primitive inner shell layer in this group. Nacre was clearly present in Ordovician cephalopods (Vendrasco et al., Reference Vendrasco, Checa, Heimbrock and Baumann2013). Thus it is very likely that shell microstructures similar to nacre occurred during the Cambrian. Given the limits of the data, it seems best to compare across time intervals the proportions of taxa with certain types of shell microstructure.
It is surprising that there is no sign of crossed lamellar microstructure in this assemblage, because this is the dominant inner shell microstructure in modern mollusks and would be readily seen in phosphatic molds with this fidelity of preservation. The small sample size impedes conclusive statements about proportions in the Mollusca at the time, but it seems unlikely that crossed lamellar was primitive in the Mollusca. The only documented occurrence of crossed lamellar microstructure from the early Paleozoic is in Yuwenia Runnegar, Reference Runnegar1981 from the early Cambrian of Australia, where an endolith cast provides evidence of sets of fibers dipping at different vertical angles, which is diagnostic for crossed lamellar (Runnegar, Reference Runnegar1985). Sets of horizontal fibers at different angles are common in Cambrian mollusks, and in fact lamello-fibrillar, plywood-configuration shell microstructure has been noted as the inner shell layer of many Cambrian mollusks (Feng and Sun, Reference Feng and Sun2003), but these textures lack the vertical dips of first-order crossed lamellar.
Escalation
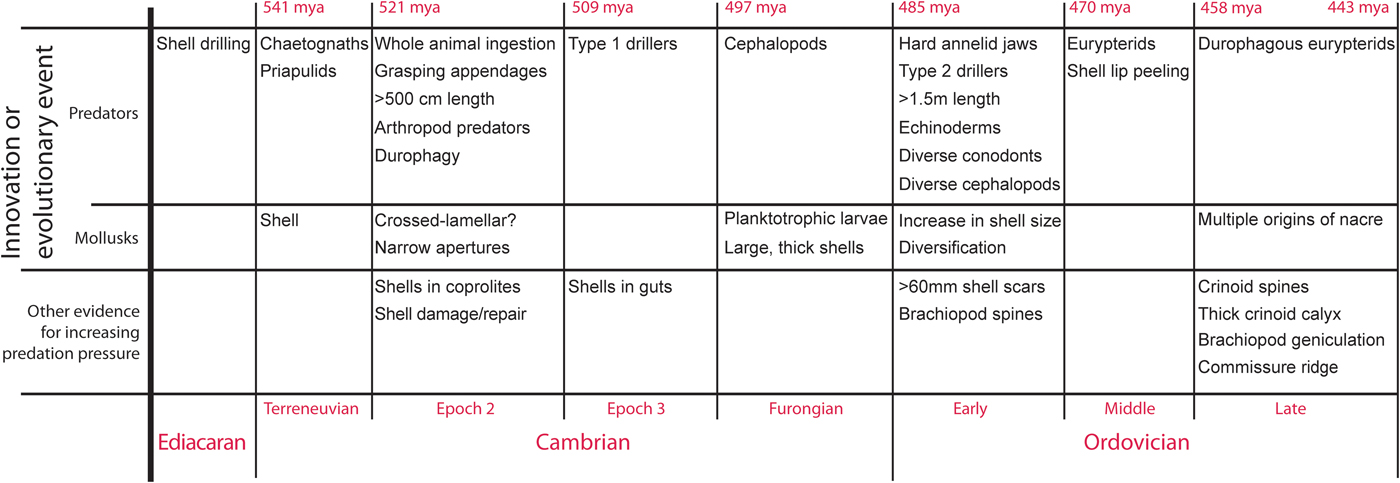
Prey defenses have reflected predator advances since the beginning of the Cambrian. By the Late Ordovician, shell-crushing predators were larger, more efficient, and higher in the water column (Fig. 8). Ordovician mollusks, like their Cambrian counterparts, were under attack from shell-crushing predators (Ebbestad and Peel, Reference Ebbestad and Peel1997; Ebbestad et al., Reference Ebbestad, Lindstrom and Peel2009). In response, through the Cambrian–Ordovician, many mollusk lineages increased in size (Runnegar and Pojeta, Reference Runnegar, Pojeta, Trueman and Clarke1985), the shell became much thicker, and ornamentation increased. In addition, nacre increased in dominance in mollusks through this interval. Nacre resists breakage better than any other natural material (Taylor and Layman, Reference Taylor and Layman1972; Currey, Reference Currey1977; Currey et al., Reference Currey, Zioupos, Davies and Casino2001), for example allowing the Nautilus shell to remain paper thin in spite of incredible pressures from the depths to which it descends.
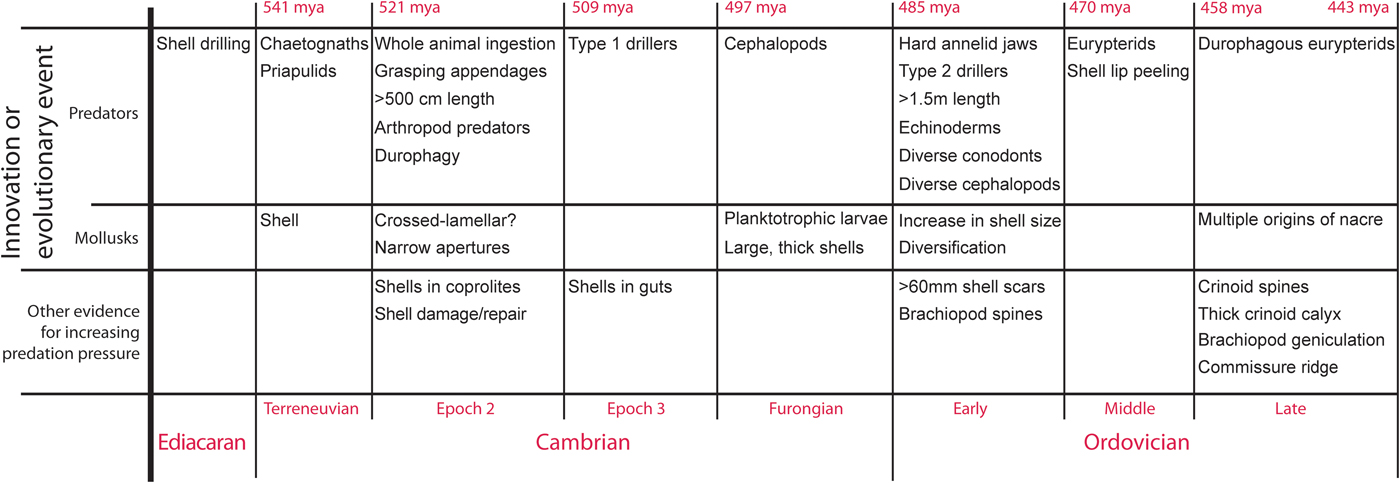
Figure 8. Details of the predator-prey arms race between mollusks and their predators through the Cambrian–Ordovician.
Fossils from the Cyclora beds reveal other molluscan responses to increasing predator efficiency, including internal thickening of the gastropod shell (Dattilo et al., Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016), sealing off near the apex, and a dominance in multiple lineages of the strongest shell microstructure known in nature, nacre. Two micro-steinkerns of gastropods provide details about the sealing off of the apex during growth. Figure 6.1 shows layering that might represent a type of apical occlusion described by Datillo et al. (Reference Dattilo, Freeman, Peters, Heimbrock, Deline, Martin, Kallmeyer, Reeder and Argast2016). The specimen in Figure 6.4–6.10 is unusual among the micro-steinkern gastropods we examined for having clear nacre tablets (out of more than 100 individuals examined via SEM) and a distinct seal near the apex.
Conclusions
Phosphatic steinkerns from the Late Ordovician of the Cincinnati Arch region provide excellent details on shell microstructures of mollusks and other invertebrates. Such small fossils from the taphonomically accumulated shell beds of the Ordovician of Ohio and Kentucky provide exceptional details on the first order microstructural units and reveal that nacre had become dominant among mollusks by that time, in step with increasing predation pressure associated with the Great Ordovician Biodiversification Event.
Acknowledgments
A. González Segura provided some assistance with FE-SEM, and S. Felton provided some samples; J. Carter provided identifications of bivalves from our samples. This project was funded by a Postdoctoral Fellowship (IIF 301668) from the Marie Skłodowska-Curie Actions to MJV with AGC from the European Commission, and grant CGL2013-48247-P from the Spanish Ministry of Science and Innovation to AGC and MJV. We are grateful to J. Carter and an anonymous reviewer who provided excellent feedback that dramatically improved the manuscript. Comments from the editors B. Hunda, M. Hautmann, and J. Kastigar also improved the manuscript.