Introduction
Large scale biogeographic patterns in deep-sea benthic fauna remain poorly understood (O'Hara et al., Reference O'Hara, Rowden and Bax2011; Watling et al., Reference Watling, Guinotte, Clark and Smith2013; Ingels et al., Reference Ingels, Amon, Bernardino, Bhadury, Bik, Clark, Dahlgren, Jones, McClain, Nunnally, Snelgrove, Tuhumwire and Yasuhara2021; Levin et al., Reference Levin, Auster, Clark, Hall-Spencer, Hopcroft, Ingels, Metaxas, Narayanaswamy, Tuhumwire and Yasuhara2021). One of the major unresolved questions is whether there is a distinct tropical fauna in the deep sea, or at least latitudinal zonation at low latitudes. Some studies showed clear zonation and the presence of a tropical fauna at bathyal depths (Zezina, Reference Zezina1997; O'Hara et al., Reference O'Hara, Rowden and Bax2011), whereas others did not (UNESCO, 2009; Watling et al., Reference Watling, Guinotte, Clark and Smith2013). There tends to be agreement that deeper, abyssal faunas are more homogeneous and lack clear latitudinal zonation (Zezina, Reference Zezina1997; UNESCO, 2009; O'Hara et al., Reference O'Hara, Rowden and Bax2011; Watling et al., Reference Watling, Guinotte, Clark and Smith2013). However, definition of the bathyal-abyssal boundary varies among studies (3500 m in UNESCO, 2009 and Watling et al., Reference Watling, Guinotte, Clark and Smith2013; 3000 m in Zezina, Reference Zezina1997; 2000 m in O'Hara et al., Reference O'Hara, Rowden and Bax2011). In addition, these studies exclusively analyze macrobenthos, megabenthos, and oceanographic parameters, and do not include any meiobenthic information.
A latitudinal diversity gradient is present in the deep sea, with higher diversity at lower latitudes (Culver and Buzas, Reference Culver and Buzas2000; Rex et al., Reference Rex, Stuart and Coyne2000; Yasuhara et al., Reference Yasuhara, Hunt, Cronin and Okahashi2009b, Reference Yasuhara, Hunt, van Dijken, Arrigo, Cronin and Wollenburg2012; Jöst et al., Reference Jöst, Yasuhara, Wei, Okahashi, Ostmann, Martínez Arbizu, Mamo, Svavarsson and Brix2019). Bottom temperature and particulate organic carbon flux, the major food source for deep-sea benthos, are commonly used to explain large-scale deep-sea diversity patterns in space and time (Rex and Etter, Reference Rex and Etter2010; Tittensor et al., Reference Tittensor, Rex, Stuart, McClain and Smith2011; Yasuhara et al., Reference Yasuhara, Okahashi, Cronin, Rasmussen and Hunt2014b, Reference Yasuhara, Huang, Hull, Rillo, Condamine, Tittensor, Kučera, Costello, Finnegan, O'Dea, Hong, Bonebrake, McKenzie, Doi, Wei, Kubota and Saupe2020; Yasuhara and Danovaro, Reference Yasuhara and Danovaro2016; Jöst et al., Reference Jöst, Yasuhara, Wei, Okahashi, Ostmann, Martínez Arbizu, Mamo, Svavarsson and Brix2019; Wei et al., Reference Wei, Chen, Wicksten and Rowe2020), but neither of them shows substantial change at the transition between the tropics and subtropics, nor do they show clear latitudinal trends in low–mid latitudes (O'Hara et al., Reference O'Hara, Rowden and Bax2011; Sweetman et al., Reference Sweetman, Thurber, Smith, Levin, Mora, Wei, Gooday, Jones, Rex, Ingels, Ruhl, Frieder, Danovaro, Würzberg, Baco, Grupe, Pasulka, Meyer, Dunlop, Henry and Roberts2017). Thus, to better understand high biodiversity in the deep sea, it is important to better understand the identity and affinities of tropical deep-sea faunas.
Ostracoda (Crustacea) is a major group of deep-sea meiobenthos (Yasuhara and Cronin, Reference Yasuhara and Cronin2008). They have reasonably good taxonomy, especially in the North Atlantic Ocean (Whatley and Coles, Reference Whatley and Coles1987; Yasuhara et al., Reference Yasuhara, Okahashi and Cronin2009c; Yasuhara and Okahashi, Reference Yasuhara and Okahashi2014, Reference Yasuhara and Okahashi2015), and their small size and calcitic shells mean that they are well preserved in sediments as fossils (Yasuhara et al., Reference Yasuhara, Tittensor, Hillebrand and Worm2017). Thus, they are useful to better understand deep-sea biogeography in the present as well as the past (Yasuhara et al., Reference Yasuhara, Hunt and Okahashi2019), and can give an insight into the above questions about the nature of the tropical deep-sea fauna from a meiofaunal perspective.
Here, we systematically describe Quaternary deep-sea ostracodes from the Ocean Drilling Program (ODP) Site 925, equatorial Atlantic Ocean, and show that this site has tropical ostracode faunal elements that are distinctive from higher latitude faunas, but in common with the tropical ostracodes in distant oceans. We suggest that this globally similar tropical deep-sea fauna may be a Tethyan legacy—the remainder of what was, during warmer climate intervals in the past, a more widely distributed fauna.
Materials and methods
Detailed information for the specimens used for the present study is shown in Appendix 1. All specimens are from the late Quaternary sediments of ODP Site 925 (Ceara Rise, western equatorial Atlantic; 4°12.2′N, 43°29.3′W; 3040 m water depth; Fig. 1), covering the last ca. 500 ka. Further details on samples, methods, chronology, paleoceanographical setting, and ostracode species diversity patterns are found in Yasuhara et al. (Reference Yasuhara, Hunt, Cronin and Okahashi2009b). Uncoated specimens were digitally imaged with a Philips XL-30 environmental scanning electron microscope (SEM) (at the Scanning Electron Microscope Lab, National Museum of Natural History, Smithsonian Institution) and a Hitachi S-3400N Variable Pressure SEM (at the Electron Microscope Unit, University of Hong Kong) in low-vacuum mode. High-resolution figures of ostracode SEM images (Figs. 2–13, 15–17) are available at Dryad (http://datadryad.org/; https://doi.org/10.5061/dryad.ns1rn8psq). For higher classification, we mainly referred to Whatley et al. (Reference Whatley, Siveter, Boomer and Benton1993), Horne et al. (Reference Horne, Cohen, Martens, Holmes and Chivas2002), and the World Ostracoda Database (Brandão and Karanovic, Reference Brandão and Karanovic2019).

Figure 1. Locality map indicating ODP Site 925 (4°12.2′N, 43°29.3′W). This map was generated with R package “marmap” by using the NOAA's ETOPO1 bathymetric data (Pante and Simon-Bouhet, Reference Pante and Simon-Bouhet2013).
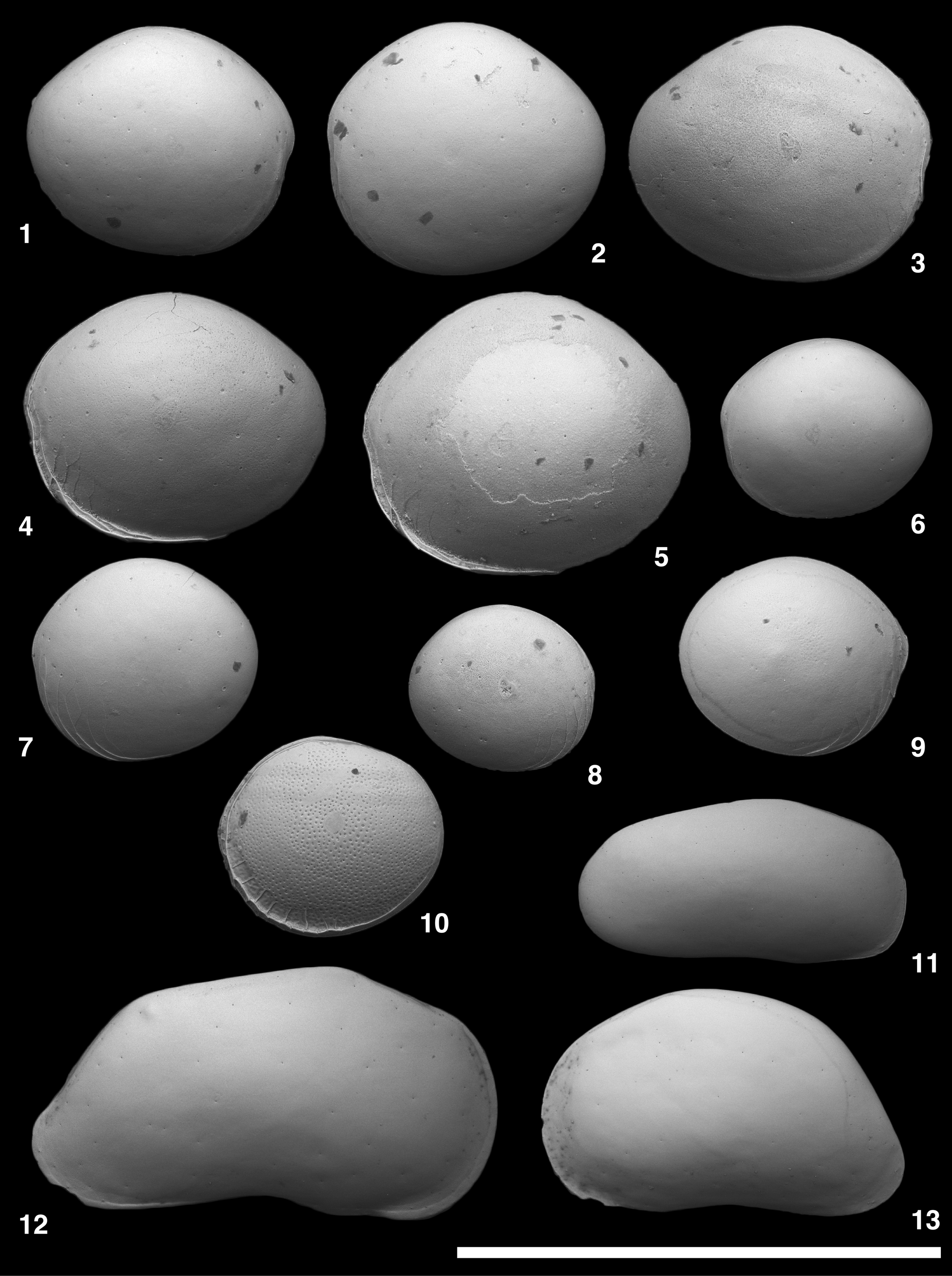
Figure 2. Scanning electron microscope images of Polycope, Bythocypris, and Zabythocypris species. (1–9) Polycope orbicularis s.l. Sars, Reference Sars1866; (1) USNM PAL 771616 (ODP925202), RV; (2) USNM PAL 771617 (ODP925203), LV; (3) USNM PAL 771618 (ODP925207), RV; (4) USNM PAL 771619 (ODP925208), LV; (5) USNM PAL 771620 (ODP925209), LV; (6) USNM PAL 771621 (ODP925211), LV; (7) USNM PAL 771622 (ODP925204), LV; (8) USNM PAL 771623 (ODP925205), RV; (9) USNM PAL 771624 (ODP925210), RV. (10) Polycope vasfiensis Sissingh, Reference Sissingh1972, USNM PAL 771625 (ODP925206), LV. (11) Bythocypris weddellensis Brandão, Reference Brandão2008, USNM PAL 771626 (ODP925013), juvenile? RV. (12, 13) Zabythocypris ancipita Maddocks, Reference Maddocks1969; (12) USNM PAL 771627 (ODP925011), adult? RV; (13) USNM PAL 771628 (ODP925012), juvenile LV. All lateral views. Scale bar = 1 mm.
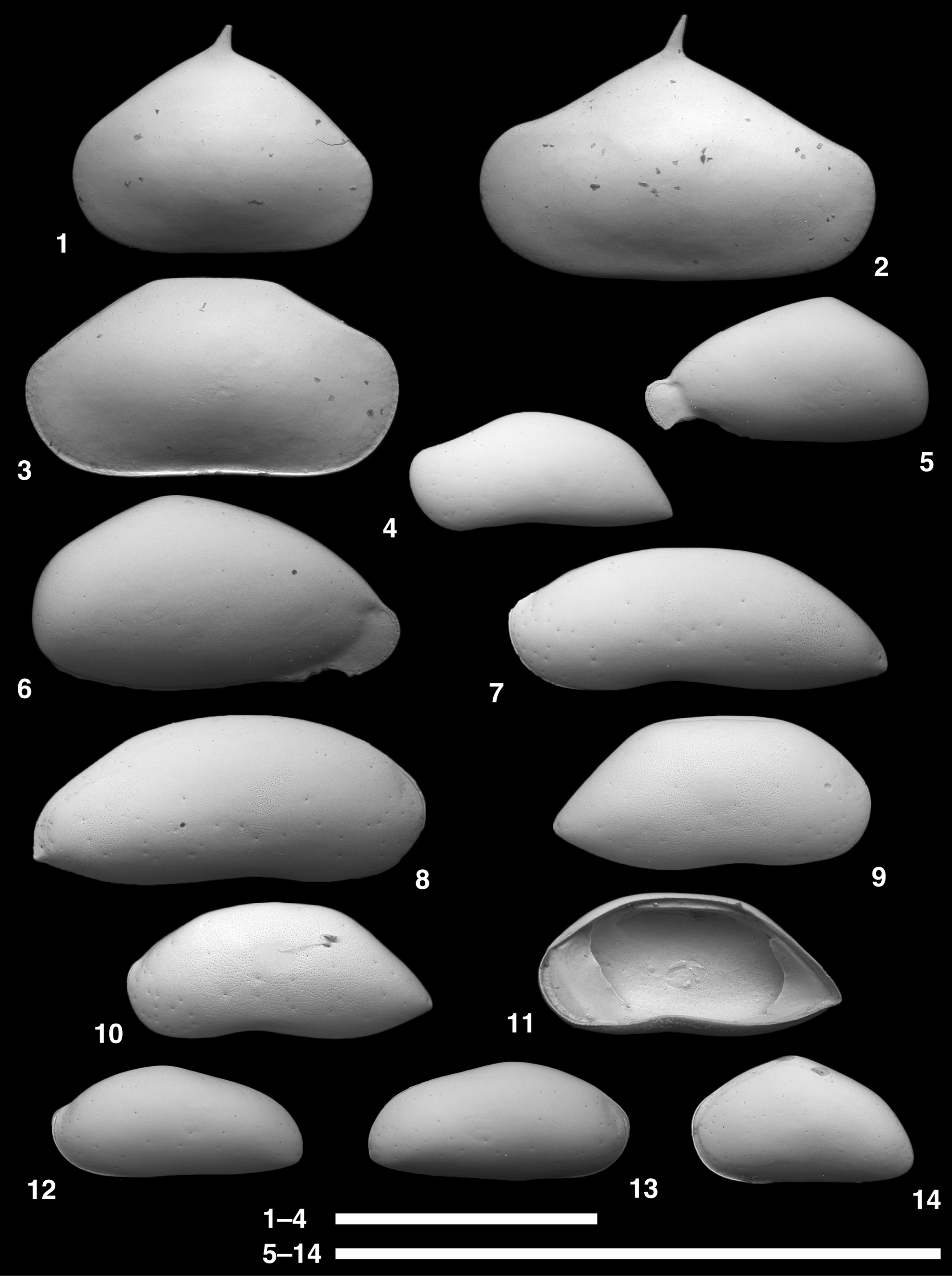
Figure 3. Scanning electron microscope images of Zabythocypris, Macrocypris, Aratrocypris, Argilloecia, and Propontocypris species. (1–3) Zabythocypris heterodoxa (Chapman, Reference Chapman1910); (1) USNM PAL 771629 (ODP925257), A-1 juvenile LV; (2) USNM PAL 771630 (ODP925258), adult LV; (3) USNM PAL 771631 (ODP925259), adult RV. (4) Macrocypris miranda s.l. Maddocks, Reference Maddocks1990, USNM PAL 771632 (ODP925212), adult? LV. (5, 6) Aratrocypris sp. 1; (5) USNM PAL 771633 (ODP925003), juvenile? LV; (6) USNM PAL 771634 (ODP925002), juvenile? RV. (7, 8) Argilloecia acuminata Müller, Reference Müller1894; (7) USNM PAL 771635 (ODP925004), adult LV; (8) USNM PAL 771636 (ODP925005), adult? RV. (9–11) Argilloecia labri Yasuhara and Okahashi, Reference Yasuhara and Okahashi2015; (9) USNM PAL 771637 (ODP925006), adult? RV; (10) USNM PAL 771638 (ODP925007), adult? LV; (11) USNM PAL 771639 (ODP925159), adult RV. (12, 13) Argilloecia sp. 1; (12) USNM PAL 771640 (ODP925008), adult? LV; (13) USNM PAL 771641 (ODP925009), adult? RV. (14) Propontocypris trigonella s.l. (Sars, Reference Sars1866), USNM PAL 771642 (ODP925218), juvenile LV. All lateral views, except (11), internal view. Scale bars = 1 mm.
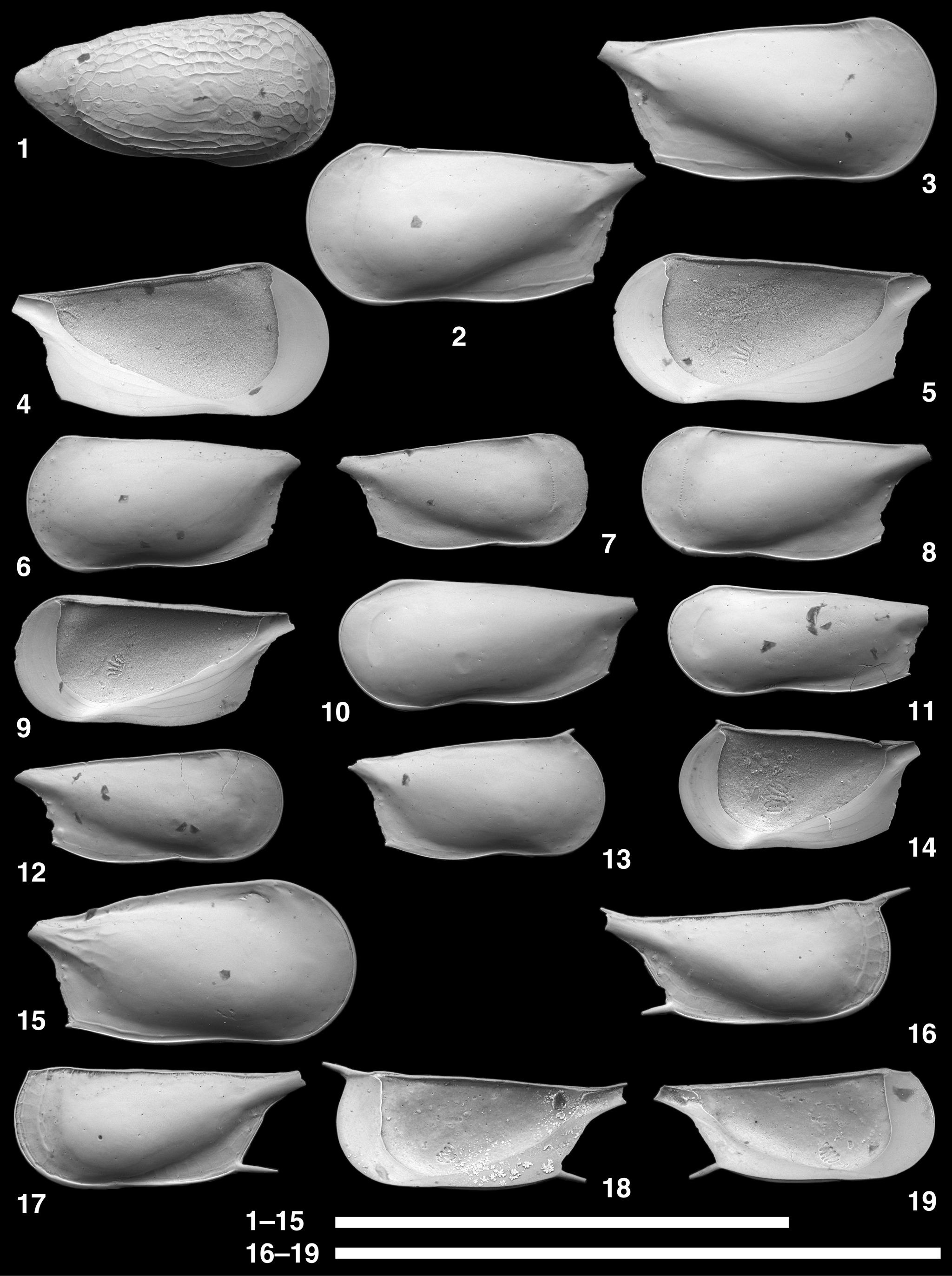
Figure 4. Scanning electron microscope images of Pseudocythere species. (1) Pseudocythere fuegiensis Brady, Reference Brady1880, USNM PAL 771643 (ODP925223), adult RV. (2–14) Pseudocythere caudata Sars, Reference Sars1866; (2) USNM PAL 771644 (ODP925224), adult LV; (3) USNM PAL 771645 (ODP925225), adult RV; (4) USNM PAL 771646 (ODP925233), adult LV; (5) USNM PAL 771647 (ODP925234), adult RV; (6) USNM PAL 771648 (ODP925227), adult LV; (7) USNM PAL 771649 (ODP925232), adult RV; (8) USNM PAL 771650 (ODP925235), adult LV; (9) USNM PAL 771651 (ODP925236), adult RV; (10) USNM PAL 771652 (ODP925238), adult LV; (11) USNM PAL 771653 (ODP925239), adult LV; (12) USNM PAL 771654 (ODP925241), adult RV; (13) USNM PAL 771655 (ODP925237), adult RV; (14) USNM PAL 771656 (ODP925240), adult RV. (15) Pseudocythere sp. 1, USNM PAL 771657 (ODP925226), adult RV. (16–19) Pseudocythere spinae n. sp.; (16) USNM PAL 771658 (ODP925229), holotype, adult RV; (17) USNM PAL 771659 (ODP925228), paratype, adult LV; (18) USNM PAL 771660 (ODP925231), paratype, adult RV; (19) USNM PAL 771661 (ODP925230), paratype, adult LV. (1–3, 6–8, 10–13, 15–17) lateral views; (4, 5, 9, 14, 18, 19) internal views. Scale bars = 1 mm.
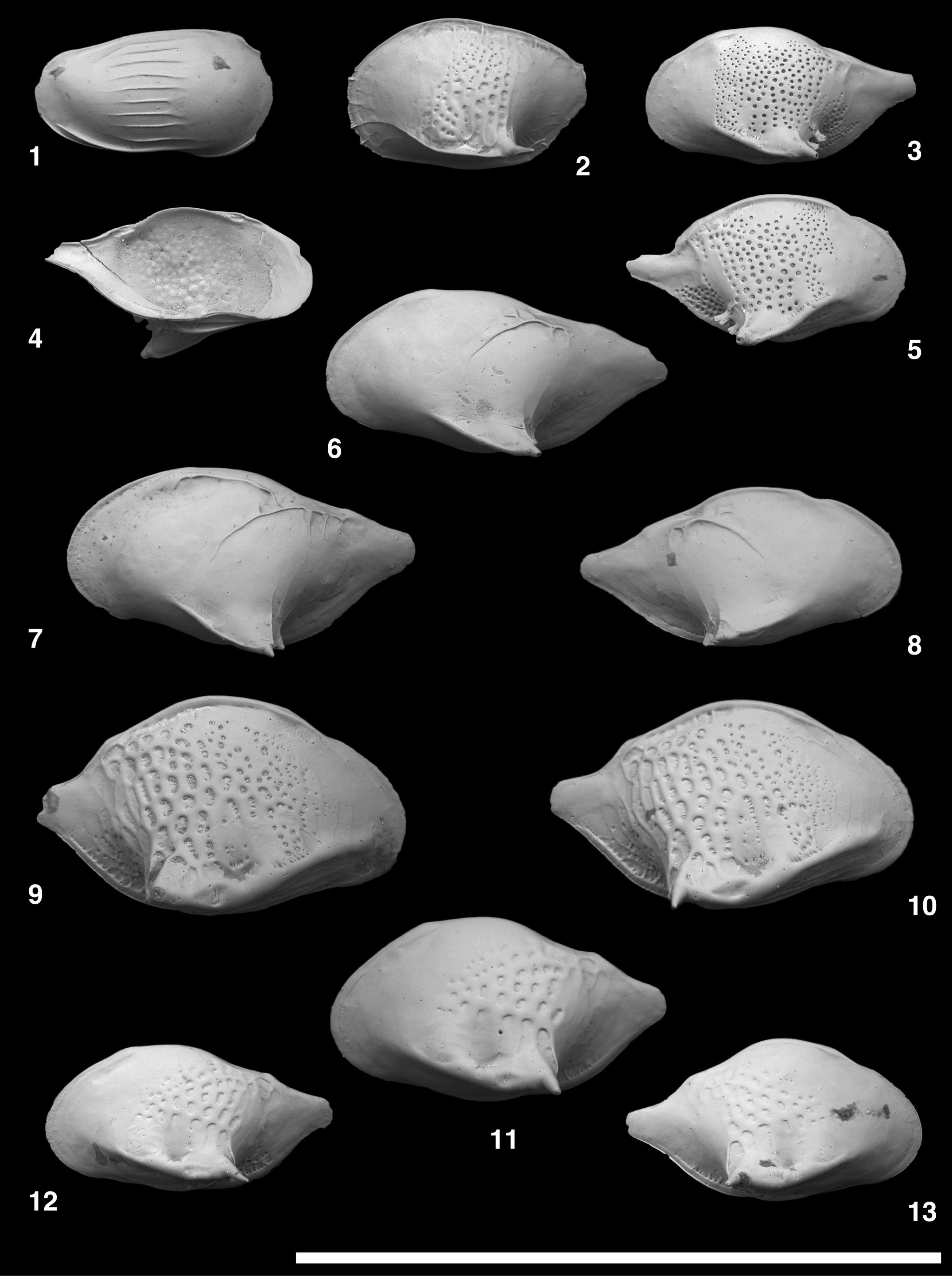
Figure 5. Scanning electron microscope images of Ruggieriella, Aversovalva, and Cytheropteron species. (1) Ruggieriella mcmanusi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c, USNM PAL 771662 (ODP925244), adult RV. (2) Aversovalva atlantica Whatley and Coles, Reference Whatley and Coles1987, USNM PAL 771663 (ODP925010), juvenile LV. (3–5) Cytheropteron carolinae Whatley and Coles, Reference Whatley and Coles1987; (3) USNM PAL 771664 (ODP925014), adult LV; (4) USNM PAL 771665 (ODP925015), adult LV; (5) USNM PAL 771666 (ODP925016), adult RV. (6–8) Cytheropteron omega Aiello, Barra, and Bonaduce, Reference Aiello, Barra and Bonaduce1996; (6) USNM PAL 771667 (ODP925018), adult LV; (7) USNM PAL 771668 (ODP925019), adult LV; (8) USNM PAL 771669 (ODP925030), adult RV. (9, 10) Cytheropteron porterae Whatley and Coles, Reference Whatley and Coles1987; (9) USNM PAL 771670 (ODP925020), juvenile? RV; (10) USNM PAL 771671 (ODP925029), adult RV. (11–13) Cytheropteron demenocali Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (11) USNM PAL 771672 (ODP925021), adult LV; (12) USNM PAL 771673 (ODP925242), adult LV; (13) USNM PAL 771674 (ODP925243), adult? RV. All lateral views, except (4), internal view. Scale bar = 1 mm.

Figure 6. Scanning electron microscope images of Cytheropteron species. (1–4) Cytheropteron cf. C. lineoporosa Whatley and Coles, Reference Whatley and Coles1987; (1) USNM PAL 771675 (ODP925023), adult LV; (2) USNM PAL 771676 (ODP925024), adult RV; (3) USNM PAL 771677 (ODP925025), adult LV; (4) USNM PAL 771678 (ODP925026), adult RV. (5–7) Cytheropteron lineoporosa Whatley and Coles, Reference Whatley and Coles1987; (5) USNM PAL 771679 (ODP925027), adult RV; (6) USNM PAL 771680 (ODP925028), adult LV; (7) USNM PAL 771681 (ODP925017), adult RV. (8) Cytheropteron sp. 1, USNM PAL 771682 (ODP925022), adult RV. All lateral views, except (3, 4), internal views. Scale bar = 1 mm.
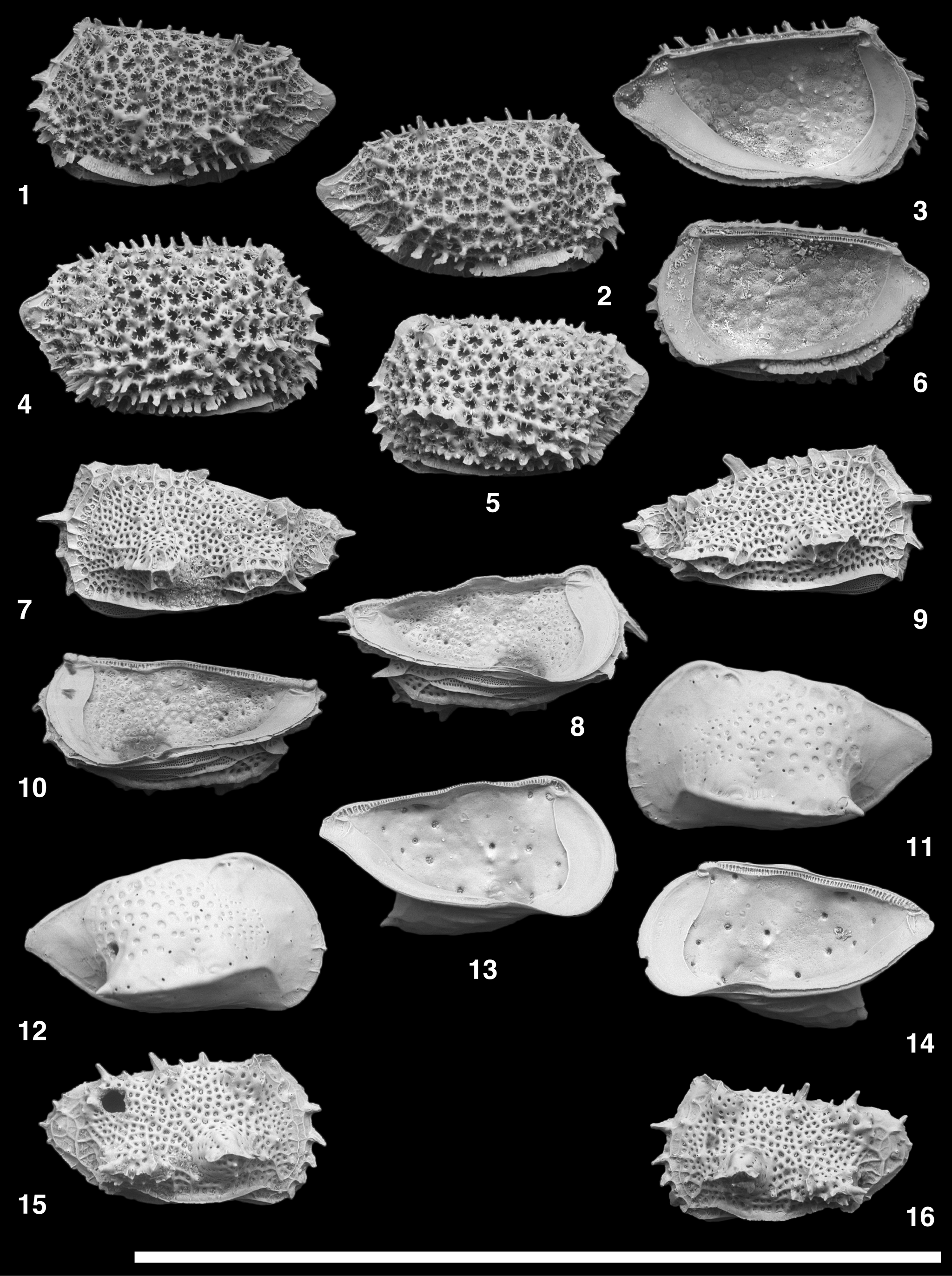
Figure 7. Scanning electron microscope images of Eucytherura and Hemiparacytheridea species. (1–3) Eucytherura spinicorona Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (1) USNM PAL 771683 (ODP925033), adult? LV; (2) USNM PAL 771684 (ODP925034), adult? RV; (3) USNM PAL 771685 (ODP925035), adult? LV. (4–6) Eucytherura calabra (Colalongo and Pasini, Reference Colalongo and Pasini1980); (4) USNM PAL 771686 (ODP925036), adult RV; (5) USNM PAL 771687 (ODP925037), adult LV; (6) USNM PAL 771688 (ODP925038), adult RV. (7–10) Eucytherura downingae (Coles and Whatley, Reference Coles and Whatley1989); (7) USNM PAL 771689 (ODP925039), adult LV; (8) USNM PAL 771690 (ODP925042), adult LV; (9) USNM PAL 771691 (ODP925040), adult RV; (10) USNM PAL 771692 (ODP925041), adult RV. (11–14) Hemiparacytheridea zarikiani n. sp.; (11) USNM PAL 771693 (ODP925044), holotype, adult LV; (12) USNM PAL 771694 (ODP925048), paratype, adult RV; (13) USNM PAL 771695 (ODP925049), paratype, adult LV; (14) USNM PAL 771696 (ODP925050), paratype, adult RV. (15, 16) Eucytherura multituberculata Ayress et al., Reference Ayress, Whatley, Downing and Millson1995; (15) USNM PAL 771697 (ODP925046), adult RV; (16) USNM PAL 771698 (ODP925043), adult LV. (1, 2, 4, 5, 7, 9, 11, 12, 15, 16) lateral views; (3, 6, 8, 10, 13, 14) internal views. Scale bar = 1 mm.
Figure 8. Scanning electron microscope images of Pedicythere species. (1, 2) Pedicythere atroposopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c, USNM PAL 771699 (ODP925198), adult LV. (3–16) Pedicythere kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (3, 4) USNM PAL 771700 (ODP925183), adult LV; (5, 6) USNM PAL 771701 (ODP925187), adult LV; (7, 8) USNM PAL 771702 (ODP925188), adult LV; (9, 10) USNM PAL 771703 (ODP925192), adult RV; (11, 12) USNM PAL 771704 (ODP925193), adult RV; (13, 14) USNM PAL 771705 (ODP925196), adult LV; (15, 16) USNM PAL 771706 (ODP925200), adult LV. (17–20) Pedicythere cf. P. kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (17, 18) USNM PAL 771707 (ODP925191), adult LV; (19) USNM PAL 771708 (ODP925185), adult LV; (20) USNM PAL 771709 (ODP925184), adult LV. (1–6, 9, 10, 13–20) lateral views; (7, 8, 11, 12) internal views; (2, 4, 6, 7, 10, 11, 14, 16, 18) oblique views. Scale bar = 1 mm.

Figure 9. Scanning electron microscope images of Pedicythere species. (1–4) Pedicythere cf. P. kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (1, 2) USNM PAL 771710 (ODP925194), adult LV; (3, 4) USNM PAL 771711 (ODP925201), adult RV. (5–8) Pedicythere canis n. sp.; (5, 6) USNM PAL 771712 (ODP925199), holotype, adult RV; (7, 8) USNM PAL 771713 (ODP925195), paratype, adult LV. (9–11) Pedicythere sp. 1; (9, 10) USNM PAL 771714 (ODP925189), adult LV; (11) USNM PAL 771715 (ODP925190), adult LV. (12, 13) Pedicythere lachesisopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c, USNM PAL 771716 (ODP925186), adult LV. (14, 15) Pedicythere sp. 2, USNM PAL 771717 (ODP925197), adult RV. (3–10, 12–15) lateral views; (1, 2, 11) internal views; (1, 4, 6, 8, 10, 11, 13, 15) oblique views. Scale bar = 1 mm.

Figure 10. Scanning electron microscope images of Rimacytheropteron, Semicytherura, Eucythere, and Xylocythere species. (1) Rimacytheropteron longipunctatum (Breman, Reference Breman1976), USNM PAL 771718 (ODP925245), adult RV. (2–5) Semicytherura pulchra (Coles and Whatley, Reference Coles and Whatley1989); (2, 3) USNM PAL 771719 (ODP925045), adult LV; (4, 5) USNM PAL 771720 (ODP925047), adult LV. (6–9) Semicytherura coeca Ciampo, Reference Ciampo1986; (6, 7) USNM PAL 771721 (ODP925255), adult LV; (8, 9) USNM PAL 771722 (ODP925256), adult RV. (10, 11) Eucythere pubera Bonaduce, Ciampo, and Masoli, Reference Bonaduce, Ciampo and Masoli1976; (10) USNM PAL 771723 (ODP925031), juvenile? LV; (11) USNM PAL 771724 (ODP925032), juvenile RV. (12–15) Xylocythere denticulata n. sp.; (12) USNM PAL 771725 (ODP925251), holotype, adult LV; (13) USNM PAL 771726 (ODP925252), paratype, adult LV; (14) USNM PAL 771727 (ODP925253), paratype, adult RV; (15) USNM PAL 771728 (ODP925254), paratype, adult LV. (1, 2, 4, 7, 8, 10–14) lateral views; (3, 5, 6, 9, 15) inner views. Scale bars = 1 mm.
Figure 11. Scanning electron microscope images of Chejudocythere and Paracytherois species. (1, 2) Chejudocythere subtriangulata Hao in Ruan and Hao, Reference Ruan, Hao, Rong and Shu1988, USNM PAL 771729 (ODP925260), adult LV. (3–20) Paracytherois bondi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (3) USNM PAL 771730 (ODP925151), adult LV; (4) USNM PAL 771731 (ODP925152), adult RV; (5, 6) USNM PAL 771732 (ODP925154), adult RV; (7) USNM PAL 771733 (ODP925153), adult LV; (8) USNM PAL 771734 (ODP925155), adult LV; (9, 10) USNM PAL 771735 (ODP925156), adult RV; (11) USNM PAL 771736 (ODP925157), adult LV; (12) USNM PAL 771737 (ODP925158), adult RV; (13, 14) USNM PAL 771738 (ODP925164), adult LV; (15) USNM PAL 771739 (ODP925165), adult RV; (16) USNM PAL 771740 (ODP925166), adult LV; (17) USNM PAL 771741 (ODP925167), adult RV; (18) USNM PAL 771742 (ODP925168), adult LV; (19, 20) USNM PAL 771743 (ODP925169), adult RV. (1–7, 11–17) lateral views; (8–10, 18–20) internal views; (2) closeup of a sieve-type pore; (5, 14) closeup of fine striations; (9, 19) closeup of subcentral muscle scars. Scale bars of closeup views (2, 5, 9, 14, 19) = 10 μm; scale bars for the other images (1, 3, 4, 6–8, 10–13, 15–18, 20) = 0.5 mm.

Figure 12. Scanning electron microscope images of Paracytherois species. (1–3) Paracytherois bondi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (1) USNM PAL 771744 (ODP925171), adult RV; (2) USNM PAL 771745 (ODP925172), adult LV; (3) USNM PAL 771746 (ODP925173), adult RV. (4–12) Paracytherois obtusa n. sp.; (4) USNM PAL 771747 (ODP925170), paratype, adult LV; (5, 6) USNM PAL 771748 (ODP925174), holotype, adult LV; (7) USNM PAL 771749 (ODP925175), paratype, adult RV; (8) USNM PAL 771750 (ODP925176), paratype, adult RV; (9) USNM PAL 771751 (ODP925177), paratype, adult LV; (10, 11) USNM PAL 771752 (ODP925178), paratype, adult RV; (12) USNM PAL 771753 (ODP925179), paratype, adult LV. (13–16) Paracytherois productum (Brady and Norman, Reference Brady and Norman1889); (13) USNM PAL 771754 (ODP925180), adult LV; (14) USNM PAL 771755 (ODP925181), adult RV; (15, 16) USNM PAL 771756 (ODP925182), adult LV. (1–8, 12–14) lateral views; (9–11, 15, 16) internal views; (6) closeup of fine striations; (11, 16) closeup of subcentral muscle scars. Scale bars of closeup views (6, 11, 16) = 10 μm; scale bars for the other images (1–5, 7–10, 12–15) = 1 mm.

Figure 13. Scanning electron microscope images of Poseidonamicus species. (1–6) Poseidonamicus sculptus n. sp.; (1) USNM PAL 771757 (ODP925213), holotype, adult female LV; (2) USNM PAL 771758 (ODP925215), paratype, adult male RV; (3, 4) USNM PAL 771759 (ODP925216), paratype, adult female LV; (5, 6) USNM PAL 771760 (ODP925217), paratype, adult female RV. (7) Poseidonamicus cf. P. sculptus n. sp., USNM PAL 771761 (ODP925214), adult male LV. (8, 9) Poseidonamicus pintoi Benson, Reference Benson1972 from Albatross Station 2763, South Atlantic Ocean (24.28°S, 42.8°W); (8) USNM PAL 527091 (SI55-01), adult female LV; (9) USNM PAL 771762 (SI55-19), adult male RV. (1, 2, 4, 6–9) lateral views; (3, 5) internal views. Scale bar = 1 mm.

Figure 14. Length-to-height ratio for adults of Poseidonamicus pintoi and Poseidonamicus sculptus n. sp. Boxplots are for Poseidonamicus pintoi from its type locality (white) and a large sample of Poseidonamicus sculptus n. sp. from Chain Core 82-24 (gray) from the North Atlantic Ocean (41.7°N, 32.9°W). Males and females are shown separately. Sexes were identified using the procedure of Hunt et al. (Reference Hunt, Martins, Puckett, Lockwood, Swaddle, Hall and Stedman2017); numbers inside boxes indicate sample sizes. Black dots indicate measurements from the four figured individuals from ODP 925 (Fig. 13.1, 13.2, 13.4, and 13.6).

Figure 15. Scanning electron microscope images of Pterygocythere, Xestoleberis, and Abyssocythere species. (1–4) Pterygocythere nobilis (Jellinek, Swanson, and Mazzini, Reference Jellinek, Swanson and Mazzini2006); (1) USNM PAL 771763 (ODP925219), adult RV; (2) USNM PAL 771764 (ODP925220), adult RV; (3) USNM PAL 771765 (ODP925221), adult LV; (4) USNM PAL 771766 (ODP925222), A-1 juvenile? LV. (5–9) Xestoleberis oppoae Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c; (5) USNM PAL 771767 (ODP925246), juvenile? RV; (6) USNM PAL 771768 (ODP925248), adult RV; (7) USNM PAL 771769 (ODP925247), adult LV; (8) USNM PAL 771770 (ODP925249), adult LV; (9) USNM PAL 771771 (ODP925250), adult RV. (10) Abyssocythere atlantica Benson, Reference Benson1971, USNM PAL 771772 (ODP925001), juvenile RV. (2–7, 10) lateral views; (1, 8, 9) internal views. Scale bars = 1 mm.

Figure 16. Scanning electron microscope images of Henryhowella asperrima (Reuss, Reference Reuss1850). (1) USNM PAL 771773 (ODP925051), adult LV; (2) USNM PAL 771774 (ODP925052), adult RV; (3) USNM PAL 771775 (ODP925053), adult LV; (4) USNM PAL 771776 (ODP925054), adult RV; (5) USNM PAL 771777 (ODP925055), adult LV; (6) USNM PAL 771778 (ODP925056), adult LV; (7) USNM PAL 771779 (ODP925058), adult LV; (8) USNM PAL 771780 (ODP925057), adult RV. (1–5, 7) lateral views; (6, 8) internal views. Scale bar = 1 mm.

Figure 17. Scanning electron microscope images of Legitimocythere acanthoderma (Brady, Reference Brady1880). (1) USNM PAL 771781 (ODP925150), adult LV; (2) USNM PAL 771782 (ODP925160), adult LV; (3) USNM PAL 771783 (ODP925161), adult RV; (4) USNM PAL 771784 (ODP925162), adult LV; (5) USNM PAL 771785 (ODP925163), adult RV. (1–3) lateral views; (4, 5) internal views. Scale bar = 1 mm.
Repository and institutional abbreviation
Figured specimens are deposited in the National Museum of Natural History (Washington DC, catalogue numbers USNM PAL 527091 and 771616–771785). MY's personal catalogue numbers are also shown.
Systematic paleontology
Abbreviations
LV, left valve; RV, right valve; L, length; H, height.
Class Ostracoda Latreille, Reference Latreille1802
Subclass Myodocopa Sars, Reference Sars1866
Order Halocyprida Dana, Reference Dana1853
Suborder Cladocopina Sars, Reference Sars1866
Superfamily Polycopoidea Sars, Reference Sars1866
Family Polycopidae Sars, Reference Sars1866
Genus Polycope Sars, Reference Sars1866
Type species
Polycope orbicularis Sars, Reference Sars1866.
Polycope orbicularis s.l. Sars, Reference Sars1866
Figure 2.1–2.9
- Reference Zhao and Zheng1996
Polycope sp. 4 Zhao and Zheng, pl. 4, fig. 12.
- Reference Yasuhara, Okahashi and Cronin2009c
Polycope cf. orbicularis Sars; Yasuhara et al., p. 881, pl. 1, fig. 5.
- Reference Yasuhara, Okahashi and Cronin2009c
Polycope orbicularis s.l. Sars; Yasuhara et al., p. 881.
- Reference Alvarez Zarikian2009
Polycope orbicularis Sars; Alvarez Zarikian, p. 3, pl. P1, fig. 7.
- Reference Yasuhara and Okahashi2015
Polycope orbicularis s.l.; Yasuhara and Okahashi, p. 25, fig. 2F, G.
Remarks
See Yasuhara et al. (Reference Yasuhara, Okahashi and Cronin2009c) for details of this species and its ‘sensu lato’ status.
Polycope vasfiensis Sissingh, Reference Sissingh1972
Figure 2.10
- Reference Sissingh1972
Polycope vasfiensis Sissingh; p. 68, pl. 1, fig. 6.
- Reference Bonaduce, Ciampo and Masoli1976
Polycope vasfiensis; Bonaduce et al., p. 18, text-fig. 6, pl. 1, figs. 6–8.
- Reference Ruan, Hao, Rong and Shu1988
Polycope vasfiensis; Ruan and Hao, p. 393, pl. 74, figs. 2–5.
- ?Reference Zhao and Zheng1996
Polycope sp. 3 Zhao and Zheng, pl. 4, fig. 11.
- Reference Aiello, Barra and Bonaduce2000
Polycope vasfiensis; Aiello et al., p. 85, pl. 1, fig. 1.
- Reference Yasuhara, Okahashi and Cronin2009c
Polycope vasfiensis; Yasuhara et al., p. 882, pl. 1, figs. 1, 2.
- Reference Yasuhara and Okahashi2015
Polycope vasfiensis; Yasuhara and Okahashi, p. 25, fig. 2H, I.
Holotype
LV (Utrecht Micropaleontological Collection, Netherlands; catalog number not shown) from Vasfi Formation, Rhodos, Aegean Sea, Pleistocene.
Remarks
This species is widely known from the Mediterranean, North Atlantic, and North Pacific.
Subclass Podocopa Sars, Reference Sars1866
Order Podocopida Sars, Reference Sars1866
Suborder Bairdiocopina Gründel, Reference Gründel1967
Superfamily Bairdioidea Sars, Reference Sars1866
Family Bythocyprididae Maddocks, Reference Maddocks1969
Genus Bythocypris Brady, Reference Brady1880
Type species
Bythocypris reniformis Brady, Reference Brady1880.
Bythocypris weddellensis Brandão, Reference Brandão2008
Figure 2.11
- Reference Brandão2008
Bythocypris weddellensis Brandão, p. 428, figs. 40A–N, 41, 42.
Holotype
ZMH K-41325, Weddell Sea, Southern Ocean, living.
Remarks
Bythocypris weddellensis is similar to Bythocypris tenera Breman, Reference Breman1975, but the latter is more slender.
Genus Zabythocypris Maddocks, Reference Maddocks1969
Type species
?Bythocypris heterodoxa Chapman, Reference Chapman1910.
Zabythocypris ancipita Maddocks, Reference Maddocks1969
Figure 2.12, 2.13
- Reference Maddocks1969
Zabythocypris ancipita Maddocks, p. 108, fig. 59.
- ?Reference Schornikov1980
Zabythocypris ancipita; Schornikov, p. 188.
- ?Reference Whatley and Coles1987
Bythocypris sp. 1 Whatley and Coles, pl. 1, fig. 3.
- ?Reference Whatley, Ayress, Hanai, Ikeya and Ishizaki1988
Zabythocypris ancipita; Whatley and Ayress, p. 747.
Holotype
LV (National Museum of Natural History, Washington DC, USA), Mozambique Channel, western Indian Ocean, Recent.
Remarks
Our specimens are almost identical to the asymmetrical dimorph of Zabythocypris ancipita Maddocks, Reference Maddocks1969. Schornikov (Reference Schornikov1980) and Whatley and Ayress (Reference Whatley, Ayress, Hanai, Ikeya and Ishizaki1988) reported this species, but without any drawings or microphotographic images.
Zabythocypris heterodoxa (Chapman, Reference Chapman1910)
Figure 3.1–3.3
- Reference Chapman1910
?Bythocypris heterodoxa Chapman, p. 429, pl. 56, fig. 20a, b.
- Reference Maddocks1969
Zabythocypris heterodoxa (Chapman); Maddocks, p. 102, figs. 56a–c, 57, 58.
- Reference Schornikov1980
Zabythocypris heterodoxa; Schornikov, p. 188.
Holotype
Not designated. The original description shows a left valve from the western Pacific Ocean, Recent.
Remarks
Several species very similar to Zabythocypris heterodoxa (Chapman, Reference Chapman1910) are known (Athersuch and Gooday, Reference Athersuch and Gooday1979; Schornikov, Reference Schornikov1980). Our specimens are most similar to the specimens shown as Zabythocypris heterodoxa in Maddocks (Reference Maddocks1969) in lateral outline and in the nearly vertical angle of dorsal spine.
Suborder Cypridocopina Jones, Reference Jones1901
Superfamily Macrocypridoidea Müller, Reference Müller1912
Family Macrocyprididae Müller, Reference Müller1912
Genus Macrocypris Brady, Reference Brady1868b
Type species
Cythere minna Baird, Reference Baird1850.
Macrocypris miranda s.l. Maddocks, Reference Maddocks1990
Figure 3.4
- Reference Whatley and Coles1987
Macrocypris sp. cf. M. minna (Baird); Whatley and Coles, pl. 1, fig. 7.
- Reference Maddocks1990
Macrocypris miranda Maddocks, p. 46, figs. 2.5, 2.6, 3.5, 3.6, 18.3, 22.17, 24.2, 25.11, 25.12, 29.13, 32.11, 38.3, 46.7, 46.8, 48.10–48.13, 49.10–49.13, 56.17, 56.36, 57.1, 57.31, 58.1, 59.12, 59.29, 59.34, 60.3, 63.13, 63.22, 64.2, 65.1, 80.2, pls. 4.7–4.12, 5.7–5.12, 58.1, 58.2, 58.9–58.15, 59.1, 59.2, 59.11, 64.5–64.8, 78.8–78.10, 82.13, 82.14, 83.5, 83.6, 85.10–85.12, 98.3–98.7, 110.2, 110.3.
- Reference Didié and Bauch2000
Macrocypris sp. Didié and Bauch, pl. 4, fig. 16.
Holotype
USNM 240194 (National Museum of Natural History, Washington DC, USA), southeastern Atlantic Ocean, Holocene.
Remarks
We tentatively assign our juvenile specimen in this species in a broad sense.
Superfamily Pontocypridoidea Müller, Reference Müller1894
Family Pontocyprididae Müller, Reference Müller1894
Genus Aratrocypris Whatley et al., Reference Whatley, Ayress, Downing, Harlow and Kesler1985
Type species
Aratrocypris rectoporrecta Whatley et al., Reference Whatley, Ayress, Downing, Harlow and Kesler1985.
Aratrocypris sp. 1
Figure 3.5, 3.6
- Reference Whatley, Ayress, Downing, Harlow and Kesler1985
Aratrocypris sp. Whatley et al., p. 72, pl. 2, figs. ?14, 16, ?17 (non fig. 15).
- Reference Whatley and Coles1987
Aratrocypris sp. cf. A. rectoporrecta Whatley and Coles, pl. 1, fig. 10.
- ?Reference Whatley, Witte and Coles1989
Aratrocypris gigantea Whatley, Witte, and Coles, p. 212, pl. 1, figs. 11, 12, pl. 2, figs. 1–3, 6.
- Reference Zhao and Zheng1996
Aratrocypris sp. Zhao and Zheng, pl. 1, fig. 15.
Remarks
Our specimens are identical to the specimen shown as Aratrocypris sp. in Whatley et al. (Reference Whatley, Ayress, Downing, Harlow and Kesler1985, pl. 2, fig. 16) from the Pleistocene southwestern Pacific. Although Aratrocypris sp. of Whatley et al. (Reference Whatley, Ayress, Downing, Harlow and Kesler1985) was described later as Aratrocypris maddocksae Whatley, Witte, and Coles, Reference Whatley, Witte and Coles1989, this Pleistocene southwestern Pacific specimen differs from the type specimens from the Paleogene North Atlantic Ocean in outline (the former has a narrower and more rounded posterior margin). Our specimens are also very similar to Aratrocypris gigantea Whatley et al., Reference Whatley, Witte and Coles1989 (type locality and horizon: tropical North Atlantic, Recent) and their outlines are identical each other. The only differences are: (1) our specimens are much smaller, and (2) the plough-like anteroventral structure is denticulate in Aratrocypris gigantea. It is likely that our specimens (as well as the Pleistocene southwestern Pacific specimen of Aratrocypris sp.) are juveniles of Aratrocypris gigantea. Given this considerable uncertainty, however, we prefer to call our specimens Aratrocypris sp. 1.
Genus Argilloecia Sars, Reference Sars1866
Type species
Argilloecia cylindrica Sars, Reference Sars1866.
Argilloecia acuminata Müller, Reference Müller1894
Figure 3.7, 3.8
- Reference Müller1894
Argilloecia acuminata Müller, p. 261, pl. 12, figs. 1, 2, 12–22.
- Reference Breman1975
Argilloecia acuminata; Breman, p. 82, pl. 2, fig. 21, pl. 6, fig. 69.
- Reference Whatley and Coles1987
Argilloecia sp. 5 Whatley and Coles, p. 87, pl. 1, figs. 19, 20.
- Reference Guernet and Fourcade1988
Cardobairdia gr. asymmetrica (van den Bold); Guernet and Fourcade, p. 144, pl. 3, fig. 10.
- Reference Ruan, Hao, Rong and Shu1988
Argilloecia acuminata; Ruan and Hao, p. 239, pl. 36, figs. 23–26.
- Reference Ruan, Hao, Rong and Shu1988
Argilloecia conoidea Sars; Ruan and Hao, p. 239, pl. 37, fig. 4.
- Reference Wang, Zhang, Zhao, Min, Bian, Zheng, Cheng and Chen1988
Argilloecia conoidea; Wang et al., p. 231, fig. 5.70, pl. 36, figs. 11–13.
- Reference Malz and Jellinek1994
Argilloecia (Robustoargilloecia) acuminata Müller; Malz and Jellinek, p. 24, pl. 5, figs. 27, 28.
- Reference Aiello and Szczechura2004
Argilloecia acuminata; Aiello and Szczechura, p. 16, pl. 1, fig. 2.
- Reference Hou and Gou2007
Argilloecia acuminata; Hou and Gou, p. 546, pl. 108, figs. 22, 23.
- Reference Hou and Gou2007
Argilloecia conoidea; Hou and Gou, p. 547, pl. 165, fig. 21, pl. 225, figs. 10, 11.
- Reference Yasuhara, Okahashi and Cronin2009c
Argilloecia acuminata; Yasuhara et al., p. 886, pl. 3, figs. 1, 2, 4, 5.
- Reference Alvarez Zarikian2009
Argilloecia sp. 2 Alvarez Zarikian, p. 7, pl. P8, fig. 4 (part; non fig. 3).
- Reference Yasuhara and Okahashi2014
Argilloecia acuminata; Yasuhara and Okahashi, p. 774, fig. 2.5.
- Reference Yasuhara, Grimm, Brandão, Jöst, Okahashi, Iwatani, Ostman and Martínez Arbizu2014a
Argilloecia acuminata; Yasuhara et al., p. 347, fig. 3.1, 3.2.
- Reference Yasuhara and Okahashi2015
Argilloecia acuminata; Yasuhara and Okahashi, p. 28, fig. 4A–D.
- Reference Bergue, Coimbra, Pivel, Petró and Mizusakia2017
Argilloecia acuminata; Bergue et al., p. 506, pl. 4, figs. 15, 16.
Holotype
Not designated. The type locality is Bay of Naples, Italy, Recent.
Remarks
This species is widely known from the Mediterranean Sea and the Atlantic and Pacific oceans.
Argilloecia labri Yasuhara and Okahashi, Reference Yasuhara and Okahashi2015
Figure 3.9–3.11
- Reference Whatley and Coles1987
Argilloecia sp. 4 Whatley and Coles, p. 86, pl. 1, figs. 17, 18.
- Reference Didié and Bauch2000
Argilloecia sp. 2 Didié and Bauch, p. 116, pl. 3, figs. 3, 4.
- Reference Yasuhara and Okahashi2015
Argilloecia labri Yasuhara and Okahashi, p. 28, fig. 4G–J.
Holotype
Adult LV, USNM PAL 603651 (National Museum of Natural History, Washington DC, USA), eastern North Atlantic Ocean, Quaternary.
Remarks
This species has been reported from the North Atlantic Ocean.
Argilloecia sp. 1
Figure 3.12, 3.13
Remarks
This distinctly shaped Argilloecia species is probably an undescribed species, but we are not sure whether our specimens are adults or juveniles. Thus, we prefer to keep this species in open nomenclature until definitively adult specimens are recovered.
Genus Propontocypris Sylvester-Bradley, Reference Sylvester-Bradley1947
Type species
Pontocypris trigonella Sars, Reference Sars1866.
Propontocypris trigonella s.l. (Sars, Reference Sars1866)
Figure 3.14
- Reference Sars1866
Potocypris trigonella (sic.) Sars, p. 16.
- Reference Sars1923
Pontocypris trigonella Sars; Sars, p. 48, pl. 20, figs. 1–16.
- Reference Athersuch and Whittaker1982
Propontocypris trigonella (Sars); Athersuch and Whittaker, p. 67, text-figs. 1–4, pls. 9.64, 9.66.
- Reference Whatley and Coles1987
Pontocypris sp. cf. P. trigonella Sars; Whatley and Coles, pl. 1, fig. 25.
- Reference Didié and Bauch2000
Propontocypris trigonella; Didié and Bauch, pl. 3, fig. 17.
- Reference Alvarez Zarikian2009
Propontocypris trigonella; Alvarez Zarikian, p. 7, pl. P8, fig. 10.
Holotype
Not designated. The type locality is off Norway, Norwegian Sea, Recent.
Remarks
Specimens resembling this juvenile have been identified as Propontocypris trigonella or its affinity in the North Atlantic Ocean (Whatley and Coles, Reference Whatley and Coles1987; Didié and Bauch, Reference Didié and Bauch2000; Alvarez Zarikian, Reference Alvarez Zarikian2009). But it is not clear if they are conspecific. We tentatively identify this juvenile specimen as Propontocypris trigonella (Sars, Reference Sars1866) in a broad sense.
Suborder Cytherocopina Gründel, Reference Gründel1967
Superfamily Cytheroidea Baird, Reference Baird1850
Family Bythocytheridae Sars, Reference Sars1866
Genus Pseudocythere Sars, Reference Sars1866
Type species
Pseudocythere caudata Sars, Reference Sars1866.
Pseudocythere caudata Sars, Reference Sars1866
Figure 4.2–4.14
- Reference Sars1866
Pseudocythere caudata Sars, p. 88.
- Reference Sars1926
Pseudocythere caudata; Sars, p. 239, pl. 109, fig. 2a–k.
- Reference Moore1961
Pseudocythere caudata; Moore, p. Q268, fig. 195.5.
- Reference Neale1967
Pseudocythere caudata; Neale, fig. 5e–i, pl. 1, fig. e, f.
- Reference Neale1967
Pseudocythere cf. P. caudata Sars; Neale, p. 14, fig. 5a–d, pl. 1, figs. a, b.
- Reference Bonaduce, Ciampo and Masoli1976
Pseudocythere caudata; Bonaduce et al., p. 119, pl. 14, figs. 9, 10.
- Reference Joy and Clark1977
Pseudocythere caudata; Joy and Clark, p. 137, pl. 1, figs. 1–3.
- Reference Bonaduce, Masoli, Pugliese and McKenzie1980
Pseudocythere caudata mediterranea Bonaduce et al., p. 136, pl. 1, fig. 1, pl. 2, figs. 1, 2, 6.
- Reference Horne1986
Pseudocythere caudata; Horne, p. 119, figs. 1m, 2c.
- Reference Athersuch, Horne and Whittaker1989
Pseudocythere caudata; Athersuch et al., p. 255, fig. 108.
- Reference Malz and Jellinek1994
Pseudocythere cf. caudata; Malz and Jellinek, figs. 3, 4, 6.
- Reference Whatley, Eynon and Moguilevsky1996a
Pseudocythere caudata; Whatley et al., pl. 1, figs. 10, 12.
- Reference Coles, Ainsworth, Whatley and Jones1996
Pseudocythere gr. caudata Sars; Coles et al., p. 150, pl. 2, figs. 3, 4.
- Reference Freiwald and Mostafawi1998
Pseudocythere caudata; Freiwald and Mostafawi, pl. 60, fig. 5.
- Reference Whatley, Eynon and Moguilevsky1998a
Pseudocythere caudata; Whatley et al., pl. 1, figs. 8, 9.
- Reference Didié and Bauch2001
Pseudocythere caudata; Didié and Bauch, pl. 1, fig. 20 (as erratum for Didié and Bauch, Reference Didié and Bauch2000).
- Reference Stepanova, Taldenkova and Bauch2003
Pseudocythere caudata; Stepanova et al., pl. 1, fig. 4.
- Reference Guernet2005
Pseudocythere (Dopseucythere) caudata Sars; Guernet, p. 108.
- Reference Alvarez Zarikian2009
Pseudocythere caudata; Alvarez Zarikian, p. 3, pl. P2, fig. 4.
- Reference Yasuhara, Okahashi and Cronin2009c
Pseudocythere caudata; Yasuhara et al., p. 892, pl. 4, figs. 7–12.
- Reference Yasuhara and Okahashi2014
Pseudocythere caudata; Yasuhara and Okahashi, p. 774, fig. 2.9, 2.10.
- Reference Yasuhara, Grimm, Brandão, Jöst, Okahashi, Iwatani, Ostman and Martínez Arbizu2014a
Pseudocythere caudata; Yasuhara et al., p. 348, fig. 5.1, 5.2.
- Reference Yasuhara, Stepanova, Okahashi, Cronin and Brouwers2014c
Pseudocythere caudata; Yasuhara et al., p. 412, pl. 6, figs. 1–12.
- Reference Yasuhara and Okahashi2015
Pseudocythere caudata; Yasuhara and Okahashi, p. 31, fig. 5F, G.
- Reference Alvarez Zarikian2016
Pseudocythere caudata; Alvarez Zarikian, p. 98, pl. 1, fig. 8.
Holotype
Not designated. The type locality is off Norway, Norwegian Sea, Recent.
Remarks
This species has considerable intraspecific variation. See Yasuhara et al. (Reference Yasuhara, Stepanova, Okahashi, Cronin and Brouwers2014c) for detailed discussion.
Pseudocythere fuegiensis Brady, Reference Brady1880
Figure 4.1
- Reference Brady1880
Pseudocythere fuegiensis Brady, p. 145, pl. 1, fig. 7a–d.
- Reference Puri and Hulings1976
Pseudocythere fuegiensis; Puri and Hulings, p. 309, pl. 1, figs. 9, 10.
Holotype
Adult RV, BM 81.5.54 (Natural History Museum, London, UK), southeastern Pacific, Recent.
Remarks
Pseudocythere fuegiensis Brady, Reference Brady1880 is very similar to ?Pseudocythere sp.1 of Didié and Bauch (Reference Didié and Bauch2000), but has more upturned caudal process and better developed primary reticulation (referred to as ‘striae’ in previous papers). This species is also very similar to ?Pseudocythere sp.2 (Didié and Bauch, Reference Didié and Bauch2000; Alvarez Zarikian, Reference Alvarez Zarikian2009) in outline, but surface reticulation patterns differ.
Pseudocythere spinae new species
Figure 4.16–4.19
Holotype
Adult RV, USNM PAL 771658 (ODP925229) (Fig. 4.16) from the Ceara Rise, western equatorial Atlantic, ODP Site 925D, 1/5/137–139 (ca. 238 ka).
Paratypes
Adult LV, USNM PAL 771659 (ODP925228) (Fig. 4.17); adult RV, USNM PAL 771660 (ODP925231) (Fig. 4.18); adult LV, USNM PAL 771661(ODP925230) (Fig. 4.19).
Diagnosis
A slender species of Pseudocythere with well-developed spine(s) and a slightly concave dorsal margin.
Description
Carapace moderately calcified, small in size, height similar throughout because of parallel dorsal and ventral margins. Outline subrectangular in lateral view; anterior margin rounded in ventral half; caudal process well developed, prominent, upturned, bearing a long spine ventrally; dorsal margin straight in LV and slightly concave in RV; ventral margin slightly rounded, but almost straight. Anterodorsal corner angular and bearing a long spine in RV; posterodorsal corner absent. Lateral surface almost smooth, but with pore conuli scattered, very weak reticulation in anterior margin, and a fine, long ridge along dorsal margin. Inner lamella broad. Hingement adont. Frontal scar subrectangular or I-shaped; adductor muscle scars consisting of vertical row of five elongate scars.
Etymology
From Latin spinae (noun, genitive singular) = spine.
Dimensions
USNM PAL 771658 (ODP925229) (holotype), L = 514 μm, H = 194 μm; USNM PAL 771659 (ODP925228) (paratype), L = 483 μm, H= 199 μm.
Remarks
Pseudocythere spinae n. sp. is similar to Pseudocythere hastata Bonaduce et al., Reference Bonaduce, Masoli, Pugliese and McKenzie1980 in having a long spine at the anterodorsal corner, but distinguished by having a posteroventral spine and straighter dorsal and ventral margins.
Pseudocythere sp. 1
Figure 4.15
Remarks
This species is similar to Pseudoloxoconcha? sp. of Malz and Jellinek (Reference Malz and Jellinek1994), but it is distinguished by weaker development of carinae on the lateral surface (restricted to the posterior one-fifth of the carapace in this species, but broadly developed in posterior half in Pseudoloxoconcha? sp.) and the presence of a flat area along dorsal margin.
Genus Ruggieriella Colalongo and Pasini, Reference Colalongo and Pasini1980
Type species
Ruggieriella decemcostata Colalongo and Pasini, Reference Colalongo and Pasini1980.
Ruggieriella mcmanusi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 5.1
- Reference Yasuhara, Okahashi and Cronin2009c
Ruggieriella mcmanusi Yasuhara, Okahashi, and Cronin, p. 892, pl. 4, figs. 1–5.
Holotype
Adult RV, USNM PAL 537137 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This is the second report of this species, which originally was described from the Carolina Slope, northwestern Atlantic Ocean.
Family Cytheruridae Müller, Reference Müller1894
Genus Aversovalva Hornibrook, Reference Hornibrook1952
Type species
Cytheropteron (Aversovalva) aureum Hornibrook, Reference Hornibrook1952.
Aversovalva atlantica Whatley and Coles, Reference Whatley and Coles1987
Figure 5.2
- Reference Cronin1983
Cytheropteron sp. B Cronin, pl. 8, fig. H.
- Reference Whatley and Coles1987
Aversovalva atlantica Whatley and Coles, p. 68, pl. 3, figs. 7–9.
- Reference Whatley, Ayress, Hanai, Ikeya and Ishizaki1988
Aversovalva sp. 1 Whatley and Ayress, pl. 2, fig. 4a, b.
- Reference Zhao2005
Aversovalva atlantica; Zhao, pl. 2, figs. 9, 10.
Holotype
RV, OS 12550 (Natural History Museum, London, UK), North Atlantic Ocean, Pleistocene.
Remarks
This species has been reported both from the Atlantic and Pacific oceans.
Genus Cytheropteron Sars, Reference Sars1866
Type species.—Cythere latissima Norman, Reference Norman1865 (designated by Brady and Norman, Reference Brady and Norman1889; see Horne and Whittaker, Reference Horne and Whittaker1988, for details and lectotype).
Cytheropteron carolinae Whatley and Coles, Reference Whatley and Coles1987
Figure 5.3–5.5
- Reference Whatley and Coles1987
Cytheropteron carolinae Whatley and Coles, p. 60, pl. 2, figs. 6, 7, 9.
- Reference Whatley, Eynon and Moguilevsky1996a
Cytheropteron carolinae; Whatley et al., pl. 1, figs. 13, 14.
- Reference Whatley, Eynon and Moguilevsky1998a
Cytheropteron carolinae; Whatley et al., pl. 1, figs. 13, 14.
- non Reference Cronin1996
Cytheropteron carolinae; Cronin, fig. 6a.
- non Reference Didié and Bauch2000
Cytheropteron sp. cf. C. carolinae Whatley and Coles; Didié and Bauch, pl. 2, fig. 23.
- non Reference Alvarez Zarikian2009
Cytheropteron carolinae; Alvarez Zarikian, p. 4, pl. P4, fig. 7.
- Reference Yasuhara, Okahashi and Cronin2009c
Cytheropteron carolinae; Yasuhara et al., p. 900, pl. 7, figs. 8, 9.
- Reference Yasuhara, Grimm, Brandão, Jöst, Okahashi, Iwatani, Ostman and Martínez Arbizu2014a
Cytheropteron carolinae s.l. Whatley and Coles; Yasuhara et al., p. 349, fig. 6.3, 6.4.
- Reference Yasuhara, Stepanova, Okahashi, Cronin and Brouwers2014c
Cytheropteron carolinae; Yasuhara et al., p. 418, pl. 5, figs. 8, 9, pl. 10, figs. 4, 5.
- Reference Jöst, Yasuhara, Okahashi, Brix, Martínez Arbizu and Ostmann2018
Cytheropteron carolinae; Jöst et al., p. 769, fig. 2.7–2.10.
- Reference Bergue, Brandão and Anjos-Zerfass2019
Cytheropteron carolinae; Bergue et al., p. 1502, fig. 3J.
Holotype
RV, OS 12526 (Natural History Museum, London, UK), North Atlantic Ocean, Pleistocene.
Remarks
This species has been widely reported from the Atlantic and Arctic oceans.
Cytheropteron omega Aiello, Barra, and Bonaduce, Reference Aiello, Barra and Bonaduce1996
Figure 5.6–5.8
- Reference Whatley and Coles1987
Cytheropteron syntomoalatum Whatley and Coles, pl. 2, fig. 27 (non pl. 2, figs. 25, 26, 28, 29).
- Reference Aiello, Barra and Bonaduce1996
Cytheropteron omega Aiello, Barra, and Bonaduce, p. 170, pl. 2, figs. 7–9.
- Reference Yasuhara and Okahashi2015
Cytheropteron omega; Yasuhara and Okahashi, p. 35, fig. 8C–F.
Holotype
LV, B.O.C. 2151 (Paleontological Department, the University “Federico II” of Napes, Italy), Monte San Nicole Section, Italy, Pliocene.
Remarks
Our specimens are slightly more slender compared to the type specimens, but are otherwise identical.
Cytheropteron porterae Whatley and Coles, Reference Whatley and Coles1987
Figure 5.9, 5.10
- Reference Whatley and Coles1987
Cytheropteron porterae Whatley and Coles, p. 64, pl. 2, figs. 21–23.
- Reference Didié and Bauch2000
Cytheropteron porterae; Didié and Bauch, p. 110, pl. 2, figs. 19–21.
- Reference Alvarez Zarikian2009
Cytheropteron porterae; Alvarez Zarikian, p. 4, pl. P4, figs. 5, 6.
Holotype
RV, OS 12536 (Natural History Museum, London, UK), North Atlantic Ocean, Pliocene.
Remarks
Reliable records of this species (showing specimens with thick alae) are so far known only from the North and equatorial Atlantic Ocean.
Cytheropteron demenocali Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 5.11–5.13
- Reference Boomer1999
Cytheropteron sp. A Boomer, pl. 3, figs. 18, 19.
- ?Reference Zhao, Whatley and Zhou2000
Cytheropteron sp. F Zhao, Whatley, and Zhou, p. 278, pl. 4, fig. 24.
- Reference Yasuhara, Okahashi and Cronin2009c
Cytheropteron demenocali Yasuhara, Okahashi, and Cronin, p. 900, pl. 9, figs. 1–10.
- Reference Yasuhara and Okahashi2014
Cytheropteron demenocali; Yasuhara and Okahashi, p. 776, fig. 3.3, 3.4.
- Reference Yasuhara and Okahashi2015
Cytheropteron demenocali; Yasuhara and Okahashi, p. 36, fig. 9C, D.
- Reference Alvarez Zarikian2015
Cytheropteron sp. D Alvarez Zarikian, pl. 3, figs. 7, 8.
- Reference Alvarez Zarikian2016
Cytheropteron sp. C Alvarez Zarikian, p. 103, pl. 1, fig. 4.
- Reference Jöst, Yasuhara, Okahashi, Brix, Martínez Arbizu and Ostmann2018
Cytheropteron demenocali; Jöst et al., p. 769, fig. 2.17–2.26.
- Reference Jöst, Yasuhara, Wei, Okahashi, Ostmann, Martínez Arbizu, Mamo, Svavarsson and Brix2019
Cytheropteron demenocali; Yasuhara et al., p. 94, fig. 2E–H.
Holotype
Adult RV, USNM PAL 536984 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This species has been reported widely from the Atlantic, Arctic, and Pacific oceans.
Cytheropteron lineoporosa Whatley and Coles, Reference Whatley and Coles1987
Figure 6.5–6.7
- Reference Whatley and Coles1987
Cytheropteron lineoporosa Whatley and Coles, p. 62, pl. 2, figs. 11–14.
- Reference Didié and Bauch2000
Cytheropteron lineoporosa; Didié and Bauch, p. 110, pl. 2, fig. 14.
- Reference Alvarez Zarikian2009
Cytheropteron lineoporosa; Alvarez Zarikian, p. 4, pl. P3, figs. 3, 5.
- Reference Jöst, Yasuhara, Okahashi, Brix, Martínez Arbizu and Ostmann2018
Cytheropteron lineoporosa; Jöst et al., p. 770, fig. 3.10.
- non Reference Bergue, Brandão and Anjos-Zerfass2019
Cytheropteron lineoporosa; Bergue et al., p. 1505, fig. 3L.
Holotype
RV, OS 12530 (Natural History Museum, London, UK), North Atlantic Ocean, Pleistocene.
Remarks
The specimens of Figure 6.5, 6.6 have a weak carina close to the posterodorsal corner, which is absent in Figure 6.7 and in the type specimens (Whatley and Coles, Reference Whatley and Coles1987).
Cytheropteron cf. C. lineoporosa Whatley and Coles, Reference Whatley and Coles1987
Figure 6.1–6.4
Remarks
This species is similar to Cytheropteron lineoporosa Whatley and Coles, Reference Whatley and Coles1987, but has a carina and punctation in the posterodorsal area close to posterodorsal corner and lacks punctation in the posteroventral area just behind the ala.
Cytheropteron sp. 1
Figure 6.8
Remarks
We found only one specimen of this species and thus keep this species in open nomenclature, awaiting recovery of additional specimens.
Genus Eucytherura Müller, Reference Müller1894
Type species
Cythere complexa Brady, Reference Brady1867 (designated by Alexander, Reference Alexander1936).
Eucytherura spinicorona Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 7.1–7.3
- Reference Whatley and Coles1987
Eucytherura calabra (Colalongo and Pasini); Whatley and Coles, p. 91, pl. 3, figs. 14–16.
- ?Reference Coles, Ainsworth, Whatley and Jones1996
Eucytherura calabra; Coles et al., p. 137, pl. 3, fig. 18.
- Reference Didié and Bauch2001
Eucytherura calabra; Didié and Bauch (as erratum for Didié and Bauch, Reference Didié and Bauch2000), p. 104, pl. 1, figs. 9, 10.
- Reference Yasuhara, Okahashi and Cronin2009c
Eucytherura spinicorona Yasuhara, Okahashi, and Cronin, p. 912, pl. 12, figs. 2–7.
Holotype
Adult female RV, USNM PAL 537046 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This species has been recorded from the North Atlantic Ocean.
Eucytherura calabra (Colalongo and Pasini, Reference Colalongo and Pasini1980)
Figure 7.4–7.6
- Reference Colalongo and Pasini1980
Typhloeucytherura calabra Colalongo and Pasini, p. 122, pl. 20, figs. 1–8, pl. 21, figs. 1, 2.
- Reference Cronin1983
Typhloeucytherura sp. Cronin, pl. 6, fig. C.
- Reference Nohara1987
Eucytherura shinzatoensis Nohara, p. 88, pl. 7, fig. 2a–c.
- Reference Whatley and Coles1987
Eucytherura calabra (Colalongo and Pasini); Whatley and Coles, pl. 3, figs. 14–16.
- Reference Ruan, Hao, Rong and Shu1988
Eucytherura sp. 1; Ruan and Hao, p. 291, pl. 49, fig. 18.
- Reference Ruan, Hao, Rong and Shu1988
Eucytherura spinosa Ruan in Ruan and Hao, Reference Ruan, Hao, Rong and Shu1988, p. 289, pl. 49, figs. 15–17.
- Reference Whatley, Ayress, Hanai, Ikeya and Ishizaki1988
Eucytherura calabra; Whatley and Ayress, pl. 1, fig. 9a, b.
- Reference Ayress, Whatley, Downing and Millson1995
Eucytherura calabra; Ayress et al., p. 211, fig. 3A–D.
- Reference Ayress1996
Eucytherura calabra; Ayress, p. 22, pl. 3, figs. 9, 10.
- Reference Coles, Ainsworth, Whatley and Jones1996
Eucytherura calabra; Coles et al., p. 136, pl. 3, fig. 18.
- Reference Zhao and Zheng1996
Eucytherura calabra; Zhao and Zheng, p. 72, pl. 2, fig. 36.
- Reference Didié and Bauch2001
Eucytherura calabra; Didié and Bauch (as erratum of Didié and Bauch, Reference Didié and Bauch2000), p. 103, pl. 1, figs. 9, 10.
- Reference Yasuhara and Okahashi2015
Eucytherura calabra; Yasuhara and Okahashi, p. 38, fig. 10F–H.
- ?Reference Alvarez Zarikian2015
Eucytherura calabra; Alvarez Zarikian, pl. 4, figs. 4, 5.
- ?Reference Alvarez Zarikian2015
Eucytherura sp. B Alvarez Zarikian, pl. 4, fig. 3.
Holotype
Female RV, LO.195/a (Istituto di Paleontologia Universita di Bologna, Italy), Vrica coastal section, Italy, Pleistocene.
Remarks
This species has been widely reported from the Mediterranean Sea and the Atlantic and Pacific oceans.
Eucytherura downingae (Coles and Whatley, Reference Coles and Whatley1989)
Figure 7.7–7.10
- Reference Whatley and Coles1987
Eucytherura sp. 3 Whatley and Coles, pl. 3, fig. 19.
- Reference Ruan, Hao, Rong and Shu1988
Gen. et sp. 3 Ruan and Hao, p. 389, pl. 45, fig. 22.
- Reference Ruan and Hao1989
Gen. 1 et sp. Ruan, p. 131, pl. 24, figs. 15, 16.
- Reference Coles and Whatley1989
Parahemingwayella downingae Coles and Whatley, p. 91, pl. 2, figs. 14–16.
- Reference Whatley and Coles1991
Parahemingwayella downingae; Whatley and Coles, p. 132.
- Reference Ayress, Whatley, Downing and Millson1995
Eucytherura downingae (Coles and Whatley); Ayress et al., p. 212, fig. 4E.
- ?Reference Zhao and Zheng1996
Parahemingwayella downingae; Zhao and Zheng, pl. 3, fig. 38.
- ?Reference Boomer1999
Parahemingwayella downingae; Boomer, pl. 3, fig.13.
- Reference Hou and Gou2007
Parahemingwayella downingae; Hou and Gou, p. 327, pl. 152, figs. 3–6.
Holotype
LV, 13186 (Natural History Museum, London, UK), North Atlantic Ocean, Oligocene.
Remarks
This species is known both from the Atlantic and Pacific oceans with a long stratigraphic range from Eocene to Quaternary.
Eucytherura multituberculata Ayress, Whatley, Downing, and Millson, Reference Ayress, Whatley, Downing and Millson1995
Figure 7.15, 7.16
- Reference Cronin1983
?Tuberculocythere sp. Cronin, pl. 6, fig. A.
- Reference Whatley and Coles1987
Eucytherura sp. 2 Whatley and Coles, pl. 3, fig. 18.
- Reference Ayress, Whatley, Downing and Millson1995
Eucytherura multituberculata Ayress, Whatley, Downing, and Millson, p. 213, fig. 5A–E.
- Reference Yasuhara, Okahashi and Cronin2009c
Eucytherura sp. 3 Yasuhara, Okahashi, and Cronin, p. 914, pl. 12, fig. 13.
- Reference Yasuhara and Okahashi2015
Eucytherura multituberculata; Yasuhara and Okahashi, p. 38, fig. 10I, J.
- Reference Alvarez Zarikian2015
Eucytherura multituberculata; Alvarez Zarikian, pl. 4, fig. 13.
Holotype
Adult LV, OS 14071 (Natural History Museum, London, UK), southwestern Pacific, Pliocene.
Remarks
This species has been reported from the North Atlantic and southwestern Pacific oceans.
Genus Hemiparacytheridea Herrig, Reference Herrig1963
Type species
Hemiparacytheridea occulta Herrig, Reference Herrig1963.
Hemiparacytheridea zarikiani new species
Figure 7.11–7.14
- Reference Alvarez Zarikian2015
Eucytherura sp. A Alvarez Zarikian, pl. 4, figs. 7, 8.
Holotype
Adult LV, USNM PAL 771693 (ODP925044) (Fig. 7.11) from the Ceara Rise, western equatorial Atlantic, ODP Site 925C, 1/1/6–8 (ca. 2 ka).
Paratypes
Adult RV, USNM PAL 771694 (ODP925048) (Fig. 7.12); adult LV, USNM PAL 771695 (ODP925049) (Fig. 7.13); adult RV, USNM PAL 771696 (ODP925050) (Fig. 7.14).
Diagnosis
A moderately calcified Hemiparacytheridea species with a subtriangular outline, well-developed caudal process, weakly punctate carapace, and thin ventrolateral ridge while lacking large tubercles.
Description
Carapace moderately calcified, small, highest at anterior cardinal angle. Outline subtriangular in lateral view; anterior margin rounded, weakly rimmed; caudal process well developed and subtriangular, pointed at mid-height; dorsal and ventral margins slightly sinuous. Anterodorsal corner moderately angular in LV, weakly angular in RV; posterodorsal corner almost straight and slightly convex. Lateral surface ornamented with weak punctation in the central part and smooth in the posterior and anterior margins; sizes of puncta increase posteriorly; ventrolateral lateral ridge weakly curved, bearing a spine at its posterior end; normal pores scattered. Internal features as for genus. Hingement typical of genus, lacking posterior terminal tooth in RV.
Etymology
In honor of Carlos A. Alvarez Zarikian, Texas A&M University, for his work on Cenozoic deep-sea ostracodes. He first recognized this species.
Dimensions
USNM PAL 771693 (ODP925044) (holotype), L = 379 μm, H = 209 μm; USNM PAL 771694 (ODP925048) (paratype), L = 375 μm, H= 199 μm.
Remarks
Hemiparacytheridea zarikiani n. sp. is similar to Hemiparacytheridea vanharteni Ayress et al., Reference Ayress, Whatley, Downing and Millson1995 in having a subtriangular outline and relatively smooth lateral surface, but distinguished by having punctation and lacking any large tubercle on its lateral surface.
Genus Pedicythere Eagar, Reference Eagar1965
Type species
Pedicythere tessae Eagar, Reference Eagar1965.
Remarks
Terminology for this genus follows that of Schornikov (Reference Schornikov2005).
Pedicythere atroposopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 8.1, 8.2
- ?Reference Guernet and Bellier2000
Pedicythere sp. B Guernet and Bellier, p. 270, pl. 5, fig. 3.
- Reference Yasuhara, Okahashi and Cronin2009c
Pedicythere atroposopetasi Yasuhara, Okahashi, and Cronin, p. 914, pl. 15, figs. 1–13.
- Reference Yasuhara and Okahashi2015
Pedicythere atroposopetasi; Yasuhara and Okahashi, p. 39, figs. 11F–I, 12A–D.
Holotype
Adult LV, USNM PAL 537011 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This species is known both from the northwestern and northeastern Atlantic Ocean.
Pedicythere canis new species
Figure 9.5–9.8
Holotype
Adult RV, USNM PAL 771712 (ODP925199) (Fig. 9.5, 9.6) from the Ceara Rise, western equatorial Atlantic, ODP Site 925D, 1/4/137–139 (ca. 197 ka).
Diagnosis
A small, weakly calcified Pedicythere species with a denticulate dorsal margin, a hand-shaped process at the anterior cardinal angle (of RV), feather-like posteroventral and ventrolateral processes, and an ala with a very well-developed anterior carina.
Description
Carapace thin, small, highest at anterior cardinal angle. Outline subtriangular in lateral view; anterior margin rounded, bearing five spines; caudal process prominent and upturned, bearing a feather-like posteroventral process; dorsal margin denticulate, straight in RV, slightly convex in LV. Alae extending below ventral margin, bearing very well-developed anterior carina; three fine carinae running on and along ala. Anterodorsal corner bearing a hand-shaped process at the anterior cardinal angle; posterodorsal corner absent. Lateral surface smooth, with normal pores scattered. Internal features as for genus.
Etymology
From Latin canis (noun, genitive singular), meaning ‘dog,’ referring to the lateral view that looks like a dog face. Hand-shaped process at the anterior cardinal angle, caudal process, and posteroventral and ventrolateral processes and ala as ear, nose, and beard of a dog, respectively.
Dimensions
USNM PAL 771712 (ODP925199) (holotype), L = 496 μm, H = 210 μm; USNM PAL 771713 (ODP925195) (paratype), L = 477 μm, H= 221 μm.
Remarks
Pedicythere canis n. sp. is distinguished from other Pedicythere species (e.g., Schornikov, Reference Schornikov2005; Yasuhara et al., Reference Yasuhara, Okahashi and Cronin2009c) by having a hand-shaped process at the anterior cardinal angle, a denticulate dorsal margin, and three fine carinae running on and along the ala. Note that the hand-shaped process of anterior cardinal angle seems to be broken in one of our specimens (Fig. 9.7, 9.8).
Pedicythere kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 8.3–8.16
- Reference Guernet and Bellier2000
Pedicythere sp. A Guernet and Bellier, p. 270, pl. 5, fig. 2 (non fig. 1).
- Reference Yasuhara, Okahashi and Cronin2009c
Pedicythere kennettopetasi Yasuhara, Okahashi, and Cronin, p. 916, pl. 16, figs. 1–10.
- Reference Yasuhara and Okahashi2015
Pedicythere kennettopetasi; Yasuhara and Okahashi, p. 39, fig. 13E, F.
Holotype
Adult RV, USNM PAL 537023 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This species is known both from the northwestern and northeastern Atlantic Ocean.
Pedicythere cf. P. kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figures 8.17–8.20, 9.1–9.4
Remarks
This species is very similar to Pedicythere kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c, but the blade-like carina at the anterior edge of the ala is less developed in this species.
Pedicythere lachesisopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 9.12, 9.13
- Reference Cronin1983
Pedicythere sp. A Cronin, p. 110, pl. 4H.
- Reference Bergue and Coimbra2008
Pedicythere sp. Bergue and Coimbra, p. 130, pl. 6, fig. 13.
- Reference Yasuhara, Okahashi and Cronin2009c
Pedicythere lachesisopetasi Yasuhara, Okahashi, and Cronin, p. 918, pl. 16, figs. 11–21.
- Reference Yasuhara and Okahashi2015
Pedicythere lachesisopetasi; Yasuhara and Okahashi, p. 40, figs. 12E–J, 13A–D.
Holotype
Adult LV, USNM PAL 537025 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
Like Pedicythere atroposopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c and Pedicythere kennettopetasi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c, this species is also known both from the northwestern and northeastern Atlantic Ocean.
Pedicythere sp. 1
Figure 9.9–9.11
Remarks
This rare species is kept in open nomenclature in this study.
Pedicythere sp. 2
Figure 9.14, 9.15
Remarks
This rare species is kept in open nomenclature in this study.
Genus Rimacytheropteron Whatley and Coles, Reference Whatley and Coles1987
Type species
Monoceratina longipunctata Breman, Reference Breman1976.
Rimacytheropteron longipunctatum (Breman, Reference Breman1976)
Figure 10.1
- Reference Breman1976
Monoceratina longipunctata Breman, p. 15, pl. 1, figs. 4a, b, pl. 2, figs. 4c–i.
- Reference Bonaduce, Ciampo and Masoli1976
‘Pedicythere’ tessellata Bonaduce, Ciampo, and Masoli, p. 88, pl. 36, figs. 12–15.
- Reference Whatley and Coles1987
Rimacytheropteron longipunctata (Breman); Whatley and Coles, p. 70, pl. 3, figs. 12, 13.
- Reference Zhao and Zheng1996
Rimacytheropteron longipunctata; Zhao and Zheng, pl. 4, fig. 29.
- Reference Aiello, Barra and Bonaduce2000
Rimacytheropteron longipunctatum; Aiello et al., p. 97, pl. 3, fig. 11.
- Reference Didié and Bauch2000
Rimacytheropteron longipunctata; Didié and Bauch, p. 115, pl. 4, fig. 26.
- Reference Aiello and Szczechura2004
Rimacytheropteron longipunctatum; Aiello and Szczechura, p. 56, pl. 14, figs. 7, 8.
- Reference Bergue, Costa, Dwyer and Moura2006
Rimacytheropteron longipunctatum; Bergue et al., p. 207, fig. 7M.
- Reference Bergue and Coimbra2008
Rimacytheropteron longipunctatum; Bergue and Coimbra, p. 133, pl. 7, fig. 12.
- Reference Alvarez Zarikian2009
Rimacytheropteron longipunctatum; Alvarez Zarikian, p. 4, pl. P3, fig. 8.
- Reference Yasuhara, Okahashi and Cronin2009c
Rimacytheropteron longipunctatum; Yasuhara et al., p. 918, pl. 14, figs. 1–5.
- Reference Alvarez Zarikian2015
Rimacytheropteron longipunctatum; Alvarez Zarikian, pl. 3, fig. 13.
Holotype
Adult LV, EB-NS-118-1 (Paleontological Department, Instituut voor Aardwetenschappen, Vrije Universiteit, Netherlands), Adriatic Sea, Holocene.
Remarks
This species is widely known from the Mediterranean Sea and Atlantic and Pacific oceans.
Genus Semicytherura Wagner, Reference Wagner1957
Type species
Cythere nigrescens Baird, Reference Baird1838.
Remarks
Following Ayress and Correge (Reference Ayress and Correge1992), we consider Mayburya Coles and Whatley as a junior synonym of Semicytherura Wagner, Reference Wagner1957. Internal views of well-preserved specimens of the type species of Mayburya, i.e., Semicytherura pulchra (Coles and Whatley, Reference Coles and Whatley1989) (Fig. 10.3, 10.5) clearly show typical characters of Semicytherura including very broad inner lamella.
Semicytherura pulchra (Coles and Whatley, Reference Coles and Whatley1989)
Figure 10.2–10.5
- Reference Ciampo1986
Trinacriacythere cornuta Ciampo, p. 104, pl. 15, figs. 1–4, pl. 18, fig. 6.
- Reference Whatley, Ayress, Hanai, Ikeya and Ishizaki1988
Rostrocythere? sp. Whatley and Ayress, pl. 1, figs. 2, 3.
- Reference Coles and Whatley1989
Mayburya pulchra Coles and Whatley, p. 87, pl. 1, figs. 5–7.
- Reference Ayress and Correge1992
Semicytherura pulchra (Coles and Whatley); Ayress and Correge, p. 57, pl. 19.
- Reference Zhao and Zheng1996
Mayburya pulchra; Zhao and Zheng, pl. 3, fig. 18.
- non Reference Whatley, Moguilevsky, Ramos and Coxill1998b
Semicytherura pulchra; Whatley et al., p. 124, pl. 3, fig. 21.
- Reference Boomer1999
Semicytherura cf. S. pulchra (Coles and Whatley); Boomer, pl. 3, figs. 12, 15.
- Reference Didié and Bauch2000
Semicytherura pulchra; Didié and Bauch, p. 111, pl. 4, fig. 9.
- Reference Alvarez Zarikian2015
Semicytherura pulchra; Alvarez Zarikian, p. 138, pl. 4, fig. 15.
Holotype
RV, 13168 (Natural History Museum, London, UK), North Atlantic, Oligocene.
Remarks
The name Semicytherura cornuta (Ciampo, Reference Ciampo1986) is a junior homonym of Semicytherura cornuta (Brady, Reference Brady1868a), and thus cannot be used for this species (Ayress and Correge, Reference Ayress and Correge1992).
Semicytherura coeca Ciampo, Reference Ciampo1986
Figure 10.6–10.9
- Reference Ciampo1980
Semicytherura sp. 3 Ciampo, pl. 2, fig. 5.
- Reference Ciampo1986
Semicytherura coeca Ciampo, p. 95, pl. 7, fig. 7.
- ?Reference Ruan, Hao, Rong and Shu1988
Semicytherura prona Ruan in Ruan and Hao, Reference Ruan, Hao, Rong and Shu1988, p. 304, pl. 53, figs. 21–24.
- Reference Ayress1995
Semicytherura coeca; Ayress, p. 901.
- Reference Ayress1996
Semicytherura coeca; Ayress, p. 25, pl. 4, fig. 9.
- Reference Coles, Ainsworth, Whatley and Jones1996
Semicytherura coeca; Coles et al., p. 151, pl. 2, figs. 7, 8.
Holotype
LV, COC no. 520 (Dipartamento della Scienze della Terra, Universita di Napoli, Italy), Santa Agata Fossili, Italy, Miocene.
Remarks
Semicytherura prona Ruan in Ruan and Hao, Reference Ruan, Hao, Rong and Shu1988 is almost identical to Semicytherura coeca Ciampo, Reference Ciampo1986, except for the presence of a spine on the posterior end of the ventrolateral ridge that the type specimen of Semicytherura coeca and our specimens do not have. We are not sure if Semicytherura coeca and Semicytherura prona are conspecific.
Genus Xylocythere Maddocks and Steineck, Reference Maddocks and Steineck1987
Type species
Xylocythere turnerae Maddocks and Steineck, Reference Maddocks and Steineck1987.
Xylocythere denticulata new species
Figure 10.12–10.15
- ?Reference Steineck, Maddocks, Turner, Coles, Whatley, Whatley and Maybury1990
Xylocythere sp. 5 Steineck et al., pl. 1, fig. 6, pl. 2, fig. 5.
Holotype
Adult LV, USNM PAL 771725 (ODP925251) (Fig. 10.12) from the Ceara Rise, western equatorial Atlantic, ODP Site 925C, 1/3/27–29 (ca. 78 ka).
Paratypes
Adult RV, USNM PAL 771726 (ODP925252) (Fig. 10.13); adult LV, USNM PAL 771727 (ODP925253) (Fig. 10.14); adult RV, USNM PAL 771728 (ODP925254) (Fig. 10.15).
Diagnosis
A species of Xylocythere ornamented with denticulation.
Description
Carapace well calcified, medium in size, highest at anterior cardinal angle. Outline subrectangular-oval in lateral view; anterior margin evenly rounded in ventral half, bearing short spines but straighter and smoother in dorsal half; posterior margin upturned; dorsal margin weakly sinuous and ventral margin almost straight. Anterodorsal and posterodorsal corners angular. Lateral surface ornamented with well-developed primary and secondary reticulation; denticulation on muri; a posteroventoral spine; pore conuli scattered on muri; and a fine ridge running along ventral margin. Anterior marginal rim and sulcus present. Inner lamella broad. Hingement merodont type. Frontal scar divided; adductor muscle scars consisting of vertical row of four elongate scars.
Etymology
From Latin denticulata (adjective, nominative singular, gender feminine), referring to denticulate muri.
Dimensions
USNM PAL 771725 (ODP925251) (holotype), L = 642 μm, H = 350 μm; USNM PAL 771727 (ODP925253) (paratype), L = 655 μm, H = 328 μm.
Remarks
Xylocythere denticulata n. sp. is distinguished from other Xylocythere species by having well-developed denticulation on the lateral surface. This species is similar to Xylocythere sp. of Yasuhara et al. (Reference Yasuhara, Okahashi and Cronin2009c), but is distinguished by its much stronger primary reticulation and denticulation on the lateral surface. Xylocythere is known from wood-fall and chemosynthetic environments (Maddocks and Steineck, Reference Maddocks and Steineck1987; Steineck et al., Reference Steineck, Maddocks, Turner, Coles, Whatley, Whatley and Maybury1990; Tanaka et al., Reference Tanaka, Lelièvre and Yasuhara2019). It is uncertain whether Xylocythere denticulata n. sp. is a Xylocythere species adapted to the normal soft-sediment environment or if the presence of this species suggests a wood-fall environment nearby, especially given low abundance of this species in the studied site.
Family Eucytheridae Puri, Reference Puri1954
Genus Eucythere Brady, Reference Brady1868a
Type species
Cythere declivis Norman, Reference Norman1867 (designated by Brady and Norman,Reference Brady and Norman1889; see Horne and Whittaker, Reference Horne and Whittaker1985, for details and lectotype).
Eucythere pubera Bonaduce, Ciampo, and Masoli, Reference Bonaduce, Ciampo and Masoli1976
Figure 10.10, 10.11
- Reference Bonaduce, Ciampo and Masoli1976
Eucythere pubera Bonaduce, Ciampo, and Masoli, p. 64, text-fig. 28, pl. 37, figs. 1–8.
- Reference Whatley and Downing1983
Eucythere (Eucythere) parapubera Whatley and Downing, p. 366, pl. 3, figs. 19–21.
- Reference Whatley and Coles1987
Eucythere pubera; Whatley and Coles, p. 93, pl. 4, fig. 15.
- Reference Whatley, Ayress, Hanai, Ikeya and Ishizaki1988
Eucythere parapubera Whatley and Downing; Whatley and Ayress, p. 740, pl. 1, fig. 4a, b.
- Reference Wang, Zhang, Zhao, Min, Bian, Zheng, Cheng and Chen1988
Eucythere serrata Zhao in Wang et al., p. 238, fig. 5.75, pl. 39, figs. 11–16.
- Reference McKenzie, Reyment and Reyment1993
Pseudeucythere parapubera (Whatley and Downing); McKenzie et al., p. 88, pl. 2, figs. 23, 24.
- ?Reference Ayress1995
Eucythere cf. parapubera Whatley and Downing; Ayress, fig. 5.11.
- Reference Aiello, Barra and Bonaduce2000
Eucythere pubera; Aiello et al., p. 97, pl. 3, fig. 12.
- Reference Didié and Bauch2000
Eucythere pubera; Didié and Bauch, p. 116, pl. 3, fig. 23.
- Reference Zhao2005
Eucythere pubera; Zhao, p. 41, pl. 3, fig. 8.
- Reference Hou and Gou2007
Eucythere serrata; Hou and Gou, p. 252, pl. 94, figs. 5–7.
- Reference Alvarez Zarikian2009
Eucythere pubera; Alvarez Zarikian, p. 4, pl. P6, fig. 3.
- Reference Yasuhara and Okahashi2014
Eucythere pubera; Yasuhara and Okahashi, p. 780, fig. 6.1.
Holotype
LV, no. 233 (Zoological Station of Naples, Italy), Adriatic Sea, Recent.
Remarks
This species is widely known from the Mediterranean Sea and the Atlantic and Pacific oceans.
Family Krithidae Mandelstam in Bubikyan, Reference Bubikyan1958
Genus Krithe Brady, Crosskey, and Robertson, Reference Brady, Crosskey and Robertson1874
Type species
Ilyobates praetexta Sars, Reference Sars1866.
Remarks
Krithe is the dominant genus in this core, including Krithe trinidadensis van den Bold, Reference van den Bold1958; Krithe minima Coles, Whatley, and Moguilevsky, Reference Coles, Whatley and Moguilevsky1994; Krithe lamellata Coles, Whatley, and Moguilevsky, Reference Coles, Whatley and Moguilevsky1994; Krithe reversa van den Bold, Reference van den Bold1958; and other species. We follow the taxonomic scheme of Coles et al. (Reference Coles, Whatley and Moguilevsky1994), but the detailed taxonomy of this genus will be discussed elsewhere.
Family Paracytheridae Puri, Reference Puri1974
Genus Chejudocythere Ishizaki, Reference Ishizaki1981
Type species
Chejudocythere higashikawai Ishizaki, Reference Ishizaki1981.
Chejudocythere subtriangulata Hao in Ruan and Hao, Reference Ruan, Hao, Rong and Shu1988
Figure 11.1, 11.2
- Reference Ruan, Hao, Rong and Shu1988
Chejudocythere subtriangulata Hao in Ruan and Hao, p. 251, pl. 39, figs. 20–23.
Holotype
LV, 40330 (repository unknown), Okinawa Trough, northwestern Pacific, Quaternary.
Remarks
This species originally was described from the northwestern Pacific Ocean.
Family Paradoxostomatidae Brady and Norman, Reference Brady and Norman1889
Genus Paracytherois Müller, Reference Müller1894
Type species
Paracytherois striata Müller, Reference Müller1894 (designated by Howe, Reference Howe1955; he considered this species a junior synonym of Paradoxostoma flexuosum [Brady, Reference Brady1868b] [sic: correctly, Bythocythere? flexuosa Brady, Reference Brady1867]; see Ellis and Messina Catalogue at www.micropress.org/em).
Paracytherois bondi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figures 11.3–11.20, 12.1–12.3
- Reference Yasuhara, Okahashi and Cronin2009c
Paracytherois bondi Yasuhara, Okahashi, and Cronin, p. 924, pl. 19, figs. 5–10, 15 (?12).
- Reference Yasuhara and Okahashi2015
Paracytherois bondi; Yasuhara and Okahashi, p. 44, fig. 15B, C.
Holotype
Adult RV, USNM PAL 537066 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This species may be conspecific with Paracytherois striata Mueller, Reference Müller1894, but detailed comparison is difficult because only a sketch is available for Paracytherois striata. Therefore, we prefer to call this species Paracytherois bondi Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c, at least for now.
Paracytherois obtusa new species
Figure 12.4–12.12
Holotype
Adult female? LV, USNM PAL 771748 (ODP925174) (Fig. 12.5, 12.6) from the Ceara Rise, western equatorial Atlantic, ODP Site 925C, 1/1/6–8 (ca. 2 ka).
Paratypes
Adult male? LV, USNM PAL 771747 (ODP925170) (Fig. 12.4); adult female? RV, USNM PAL 771749 (ODP925175) (Fig. 12.7); adult male? RV, USNM PAL 771750 (ODP925176) (Fig. 12.8); adult female? LV, USNM PAL 771751 (ODP925177) (Fig. 12.9); adult female? RV, USNM PAL 771752 (ODP925178) (Fig. 12.10, 12.11); adult male? LV, USNM PAL 771753 (ODP925179) (Fig. 12.12).
Diagnosis
A small, weakly calcified Paracytherois species, elongate in lateral view; posterior margin obtuse and rounded; lateral surface covered with horizontal striations.
Description
Carapace weakly calcified, small, highest at posterior cardinal angle. Outline elongate in lateral view; anterior margin acutely rounded and pointed at mid height; posterior margin obtuse and rounded; dorsal margin slightly arched; ventral margin concave. Anterodorsal and posterodorsal corners rounded. Lateral surface covered with very fine, horizontal striations. Internal features as for genus. Adductor muscle scars consisting of vertical row of four scars; the dorsal scar is small and rounded, with the others elongated.
Etymology
From Latin obtusa (adjective, nominative singular, gender feminine), referring to obtuse and rounded posterior margin.
Dimensions
USNM PAL 771748 (ODP925174) (holotype), L = 580 μm, H = 240 μm; USNM PAL 771749 (ODP925175) (paratype), L = 574 μm, H = 248 μm.
Remarks
Paracytherois obtusa n. sp. is distinguished from other Paracytherois species by having a non-acuminate posterior margin.
Paracytherois productum (Brady and Norman, Reference Brady and Norman1889)
Figure 12.13–12.16
- Reference Brady and Norman1889
Paradoxostoma productum Brady and Norman, p. 236, pl. 21, figs. 9, 10.
- Reference Corrége1993
?Paracytherois sp. 1 Corrége, pl. 1, fig. 5.
- Reference Didié and Bauch2000
Paracytherois sp. Didié and Bauch, p. 115, pl. 4, fig. 14.
- Reference Yasuhara, Okahashi and Cronin2009c
Paracytherois productum (Brady and Norman); Yasuhara et al., p. 924, pl. 19, figs. 1–4 (?11).
- Reference Jöst, Yasuhara, Okahashi, Ostmann, Martínez Arbizu and Brix2017
Paracytherois bondi (Brady and Norman) [sic.]; Jöst et al., fig. 3.23.
Holotype
Unknown. Type locality is the Bergen Fjord, Norway, Recent.
Remarks
Our specimens are identical to the original sketch of the lateral view of Paracytherois productum (Brady and Norman, Reference Brady and Norman1889).
Family Thaerocytheridae Hazel, Reference Hazel1967
Genus Poseidonamicus Benson, Reference Benson1972
Type species
Poseidonamicus major Benson, Reference Benson1972.
Poseidonamicus sculptus new species
Figure 13.1–13.6
- Reference Whatley and Coles1987
Poseidonamicus sp. cf. P. major Benson; Whatley and Coles, pl. 6, fig. 11.
- Reference Whatley and Coles1987
Poseidonamicus sp. cf. P. pintoi Benson; Whatley and Coles, pl. 6, fig. 12.
- Reference Malz1990
Poseidonamicus pintoi Benson; Malz, pl. 4, figs. 3, 4, pl. 6, fig. 9.
- Reference Hunt2007
Poseidonamicus pintoi; Hunt, fig. 12.2, 12.3.
- Reference Hunt and Yasuhara2010
Poseidonamicus pintoi; Hunt and Yasuhara, fig. 1.
- Reference Yasuhara, Hunt, Okahashi and Brandão2015
Poseidonamicus pintoi; Yasuhara et al., p. 165, fig. 94R.
Holotype
Adult LV, USNM PAL 771757 (ODP925213) (Fig. 13.1) from the Ceara Rise, western equatorial Atlantic, ODP Site 925B, 3/3/7–9 (ca. 491 ka).
Paratypes
Adult RV, USNM PAL 771758 (ODP925215) (Fig. 13.2); adult LV, USNM PAL 771759 (ODP925216) (Fig. 13.3, 13.4); adult RV, USNM PAL 771760 (ODP925217) (Fig. 13.5, 13.6).
Diagnosis
Well-calcified species of Poseidonamicus with a dorsal ridge that is developed only in the posterior half and whose dorsal edge is straight or shifts upward towards the posterior; coarsely reticulate with alternating robust and quite reduced vertical muri in the region immediately posterior and ventral to the adductor muscle scars.
Description
Carapace well calcified, medium-large in size, highest at anterior cardinal angle. Outline subrectangular in lateral view. Anterior margin evenly rounded with ~14 denticles; posterior margin slightly pointed, with a maximum extent at about mid-height, bearing several denticles and 1–2 longer spines; well-developed marginal rim on anterior, ventral, and posterior edges. Anterodorsal corner moderately well developed; posterodorsal corner less developed, especially in RV. Lateral surface coarsely reticulate, divided into an anterior field with low and even reticulation and distinctly rounded fossae, and a posterior field with larger, polygonal fossae. Vertical muri generally more strongly developed than horizontal muri in the posterior field, although the vertical muri posterior and ventral to the muscle scars (separating the A and B fossae of Hunt, Reference Hunt2007) are quite reduced, sometimes nearly absent. Prominent ventral ridge that curves gently, terminating in a posterior spine; dorsal ridge mostly straight or shifting upward towards the posterior, well developed only in the posterior half. Inner lamella moderately broad, with a well-developed selvage in RV. Hingement holamphidont, with a stepped anterior tooth and subtly trilobate posterior tooth in RV; medial bar is smooth. Muscle scars typical for the genus: a vertical row of four adductors, the middle two more elongate than the top and bottom; two oval frontal scars, with the ventral one larger than the dorsal.
Etymology
From Latin sculptus (adjective, singular, masculine), carved, referring to the well-defined ridges of the reticulum.
Dimensions
USNM PAL 771757 (ODP925213) (holotype), L = 1073 μm, H = 626 μm; USNM PAL 771758 (ODP925215) (paratype), L = 1088 μm, H= 569 μm.
Remarks
Poseidonamicus sculptus n. sp. is the most commonly encountered species of the genus in the deep North Atlantic Ocean from the Pliocene to Recent. When figured, it usually has been identified as, or compared to, Poseidonamicus pintoi Benson, Reference Benson1972. The two species are similar in that their reticulum generally emphasizes vertical elements, and they both share a dorsal ridge that is well developed only in the posterior. There are, however, also clear differences between these two species. Poseidonamicus pintoi is somewhat smaller, less elongate (Fig. 14), and it has a more even reticulum and markedly narrower anterior marginal rims than Poseidonamicus sculptus n. sp. These features are clear in the figured specimens (Fig. 13.8, 13.9), which are from Benson's type sample for Poseidonamicus pintoi (Albatross station 2763, South Atlantic Ocean, 24.28°S, 42.8°W).
Specimens that we consider to be true Poseidonamicus pintoi have been reported from only a small area off the coast of southeast Brazil in the Campos and Santos basins, from the Pleistocene to Recent (Benson, Reference Benson1972; Bergue and Coimbra, Reference Bergue and Coimbra2008; Bergue et al., Reference Bergue, Coimbra, Pivel, Petró and Mizusakia2017). These occurrences are from samples that range between 1100–1300 m in water depth, considerably shallower than depths for all the published occurrences for Poseidonamicus sculptus n. sp. in the synonymy list above (>2400 m). Some reports of Poseidonamicus pintoi we consider to be neither that species, nor Poseidonamicus scultpus n. sp. (e.g., Whatley et al., Reference Whatley, Moguilevsky, Ramos and Coxill1998b; Zhao, Reference Zhao2005).
Poseidonamicus scultpus n. sp. is also similar in shape to Poseidonamicus riograndensis Benson in Benson and Peypouquet, Reference Benson and Peypouquet1983, and has a similar arrangement of emphasized muri posterior to the central scars, but that species is smaller, and its dorsal ridge is evenly developed and curves downward in the posterior. See also the remarks under Poseidonamicus cf. P. sculptus n. sp.
Poseidonamicus cf. P. sculptus new species
Figure 13.7
- Reference Whatley and Coles1987
Poseidonamicus sp. cf. P. pintoi Benson; Whatley and Coles, pl. 6, fig. 13.
- ?Reference Guernet and Fourcade1988
Poseidonamicus pintoi Benson; Guernet and Fourcade, p. 144, pl. 3, fig. 1.
- Reference Hunt2007
Poseidonamicus species 4 Hunt, fig. 10.2.
Remarks
Hunt (Reference Hunt2007) recorded this species from the Pliocene through Recent of the North Atlantic Ocean. It often co-occurs with, but at a lower abundance than, Poseidonamicus sculptus n. sp., with which it is easily confused because of its quite similar size and shape. This species differs from Poseidonamicus sculptus n. sp. in its lower and more even reticulum, in being more prone to secondary reticulation, especially in the anterior field, and in that it bears a large sieve pore in the area antero-dorsal to the muscle scars that is absent in Poseidonamicus sculptus n. sp. (see Hunt, Reference Hunt2007, character 8).
Family Trachyleberididae Sylvester-Bradley, Reference Sylvester-Bradley1948
Genus Abyssocythere Benson, Reference Benson1971
Type species
Abyssocythere casca Benson, Reference Benson1971.
Abyssocythere atlantica Benson, Reference Benson1971
Figure 15.10
- Reference Benson1971
Abyssocythere atlantica Benson, p. 13, fig. 10, pl. 3, fig. 1.
- Reference Whatley and Coles1987
Abyssocythere atlantica; Whatley and Coles, pl. 6, fig. 10.
- ?Reference Steineck, Dehler, Hoose, McCalla, Hanai, Ikeya and Ishizaki1988
Abyssocythere trinidadensis (van den Bold); Steineck et al., pl. 1, fig. 11.
- ?Reference Gründel1989
Abyssocythere atlantica; Gründel, fig. 15.
- Reference Guernet and Moullade1994
Abyssocythere atlantica; Guernet and Moullade, p. 263, pl. 2, fig. 8 (?6).
- Reference Yasuhara, Hunt, Okahashi and Brandão2015
Abyssocythere atlantica; Yasuhara et al., p. 29, figs. 7E–L, 8F–K.
Holotype
LV, 170280 (National Museum of Natural History, Washington DC, USA), equatorial-south Atlantic, Pleistocene.
Remarks
This species is widely known from the deep North and South Atlantic oceans.
Genus Henryhowella Puri, Reference Puri1957
Type species.—Cythere evax Ulrich and Bassler, Reference Ulrich and Bassler1904.
Henryhowella asperrima (Reuss, Reference Reuss1850)
Figure 16
- Reference Reuss1850
Cypridina asperrima Reuss, p. 74, pl. 10, fig. 5a, b.
- Reference Costa1853
Cypridina hirta Costa, p. 174, pl. 15, fig. 2a, c.
- Reference Müller1894
Cythereis sarsii Müller, p. 370, pl. 8, fig. 8.
- Reference Tressler1941
Cythereis dunelmensis Norman; Tressler, p. 100, pl. 19, fig. 21.
- Reference van den Bold1960
Henryhowella asperrima (Reuss); van den Bold, p. 169, pl. 4, fig. 10, pl. 8, fig. 2.
- Reference Oertli1961
Henryhowella ruggierii Oertli, p. 28, pl. 4, figs. 39–45.
- Reference Laughton, Berggren, Benson, Davies, Franz, Musich, Perch-Nielsen, Ruffman, van Hinte and Whitmarsh1972
Henryhowella (generic assignment only); Laughton et al., pl. 11, fig. 4.
- Reference Berggren, Benson, Haq, Riedel, Sanfilippo, Schrader and Tjalsma1976
Henryhowella asperrima; Berggren et al., pl. 6, fig. 4.
- Reference Bonaduce, Ciampo and Masoli1976
Henryhowella sarsi (Müller); Bonaduce et al., p. 52, pl. 31, figs. 1–7.
- Reference Benson1977
Henryhowella asperrima? (Reuss); Benson, pl. 2, fig. 2.
- Reference Joy and Clark1977
Echinocythereis dasyderma (Brady); Joy and Clark, p. 142, pl. 2, figs. 14–17.
- Reference Benson1978
Henryhowella asperrima; Benson, pl. 1, fig. 3.
- Reference Rosenfeld and Bein1978
Henryhowella asperrima; Rosenfeld and Bein, p. 18, pl. 1, fig. 23.
- Reference Ducasse and Peypouquet1979
Henryhowella asperrima; Ducasse and Peypouquet, pl. 3, fig. 1.
- Reference Steineck1981
Henryhowella ex. gr. H. asperrima (Reuss); Steineck, p. 346, pl. 2, fig. 1.
- Reference Uffenorde1981
Henryhowella asperrima s.l. (Reuss); Uffenorde, p. 148, pl. 2, figs. 14, 15, 17–19.
- ?Reference Cronin1983
Henryhowella asperrima; Cronin, pl. 4, fig. F.
- Reference Benson and Peypouquet1983
Henryhowella asperrima?; Benson and Peypouquet, pl. 2, figs. 1, 3.
- Reference Malz and Jellinek1984
Henryhowella asperrima; Malz and Jellinek, pl. 5, figs. 38, 39.
- Reference Cronin and Compton-Gooding1987
Henryhowella sp. Cronin and Compton-Gooding, pl. 1, figs. 5, 6, pl. 2, fig. 1.
- Reference Whatley and Coles1987
Henryhowella asperrima; Whatley and Coles, pl. 5, figs. 9–11.
- Reference Guernet and Fourcade1988
Henryhowella cf. evax (Ulrich and Bassler); Guernet and Fourcade, pl. 3, figs. 18–20.
- Reference Dingle, Lord and Boomer1990
Henryhowella melobesioides (Brady); Dingle et al., p. 311, figs. 42E, F, 43A–F, 44A–D, 47A (non fig. 42C, D).
- Reference Malz1990
Henryhowella asperrima; Malz, fig. 6.8.
- Reference Kempf and Nink1993
Henryhowella asperrima; Kempf and Nink, p. 95, figs. 1–30.
- Reference Guernet and Moullade1994
Henryhowella cf. asperrima (Reuss); Guernet and Moullade, p. 268, pl. 3, figs. 8–11, 14.
- Reference Cronin1996
Henryhowella asperrima; Cronin, fig. 7a.
- Reference Coles, Ainsworth, Whatley and Jones1996
Henryhowella gr. asperrima (Brady) [sic]; Coles et al., pl. 6, figs. 2, 3.
- non Reference Whatley, Staunton, Kaesler and Moguilevsky1996b
Henryhowella asperrima; Whatley et al., p. 67, pl. 3, fig. 8.
- Reference Guernet1998
Henryhowella melobesioides; Guernet, pl. 2, figs. 4–6.
- Reference Whatley, Eynon and Moguilevsky1998a
Henryhowella dasyderma (Brady); Whatley et al., pl. 3, figs. 20, 21.
- non Reference Whatley, Moguilevsky, Ramos and Coxill1998b
Henryhowella asperrima; Whatley et al., p. 129, pl. 4, figs. 22, 23.
- non Reference Boomer1999
Henryhowella cf. H. asperrima (Reuss); Boomer, p. 145, pl. 2, figs. 1, 2, 4.
- Reference Bonaduce, Barra and Aiello1999
Henryhowella asperrima; Bonaduce et al., p. 60, pl. 1, figs. 1, 2.
- Reference Bonaduce, Barra and Aiello1999
Henryhowella? asperrima (Reuss); Bonaduce et al., p. 61, pl. 1, figs. 3, 4.
- Reference Bonaduce, Barra and Aiello1999
Henryhowella sarsii sarsii (Müller); Bonaduce et al., p. 64, pl. 2, figs. 1–10, pl. 3, fig. 12, pl. 4, figs. 9, 10, pl. 5, figs. 1, 2, 6–8, 11.
- Reference Bonaduce, Barra and Aiello1999
Henryhowella sarsii profunda Bonaduce, Barra, and Aiello, p. 68, pl. 1, figs. 5–12, pl. 4, figs. 1–8.
- Reference Guernet and Bellier2000
Henryhowella sp. Guernet and Bellier, p. 267, pl. 4, figs. 12, 15.
- Reference Didié and Bauch2001
Henryhowella sp. cf. H. dasyderma (Brady); Didié and Bauch, pl. 1, figs. 1, 2.
- Reference Barra and Bonaduce2001
Henryhowella sarsii profunda; Barra and Bonaduce, p. 64, pl. 4, fig. 8.
- Reference Dall'Antonia and Bossio2001
Henryhowella asperrima; Dall'Antonia and Bossio, p. 418, pl. 5, figs. 3–7.
- Reference Aiello and Szczechura2004
Henryhowella asperrima; Aiello and Szczechura, p. 26, pl. 4, figs. 12–14.
- Reference Mazzini2005
Henryhowella asperrima; Mazzini, p. 50, figs. 26A–I, 27B.
- Reference Mazzini2005
Fallacihowella sp. B Mazzini, p. 57, fig. 32A–Q.
- Reference Alvarez Zarikian2009
Henryhowella dasyderma; Alvarez Zarikian, p. 6, pl. P9, figs. 6–8.
- Reference Yasuhara, Okahashi and Cronin2009c
Henryhowella cf. asperrima; Yasuhara et al., p. 926, pl. 20, fig. 7, pl. 21, figs. 1–4.
- Reference Bergue and Govindan2010
Henryhowella asperrima; Bergue and Govindan, p. 751, fig. 3.14.
- Reference Bergue and Govindan2010
Henryhowella sp. 1 Bergue and Govindan, p. 752, fig. 3.15.
- Reference Nachite and Bekkali2010
Henryhowella asperrima; Nachite and Bekkali, pl. 2, fig. 10.
- Reference Pirkenseer and Berger2011
Henryhowella asperrima; Pirkenseer and Berger, p. 54, pl. 7, figs. 6a–c, 7a–c, pl. 8, figs. 1a–c, 2a–c, 3a–c.
- Reference Hajek-Tadesse and Prtoljan2011
Henryhowella asperrima; Hajek-Tadesse and Prtoljan, fig. 3.4.
- Reference Seko, Pipík and Doláková2012
Henryhowella asperrima; Seko et al., fig. 8O.
- Reference Russo, Pugliese and Serventi2012
Henryhowella asperrima; Russo et al., pl. 1, fig 5.
- Reference Sciuto2014
Henryhowella ex H. hirta (Costa) group; Sciuto, p. 6, pl. 1, fig. H.
- Reference Sciuto2014
Henryhowella ex H. profunda Bonaduce et al. group; Sciuto, p. 8, pl. 1, fig. I.
- Reference Yasuhara and Okahashi2014
Henryhowella asperrima; Yasuhara and Okahashi, p. 782, fig. 8.4.
- Reference Yasuhara, Grimm, Brandão, Jöst, Okahashi, Iwatani, Ostman and Martínez Arbizu2014a
Henryhowella asperrima; Yasuhara et al., p. 354, fig. 7.7, 7.8.
- Reference Yasuhara, Stepanova, Okahashi, Cronin and Brouwers2014c
Henryhowella asperrima; Yasuhara et al., p. 432, pl. 16, figs. 3–10.
- Reference Alvarez Zarikian2015
Henryhowella asperrima; Alvarez Zarikian, pl. 10, figs. 4–6.
- Reference DeNinno, Cronin, Rodriguez-Lazaro and Brenner2015
Henryhowella asperrima; DeNinno et al., p. 91, pl. 3, figs. 4, 5.
- Reference Yasuhara and Okahashi2015
Henryhowella asperrima; Yasuhara and Okahashi, p. 46, fig. 16E–K.
- Reference Yasuhara, Hunt, Okahashi and Brandão2015
Henryhowella asperrima; Yasuhara et al., p. 82, figs. 40Q–U, 41G–K, 42A–O, 43A–H.
- Reference Gemery, Cronin, Briggs, Brouwers, Schornikov, Stepanova, Wood and Yasuhara2017
Henryhowella asperrima; Gemery et al., p. 62, fig. 20.2, 20.3.
- Reference Bergue, Coimbra, Pivel, Petró and Mizusakia2017
Henryhowella asperrima; Bergue et al., p. 501, pl. 2, fig. 18, pl. 3, fig. 1.
- Reference Bergue, Brandão and Anjos-Zerfass2019
Henryhowella asperrima; Bergue et al., p. 1508, fig. 4J, K.
Holotype
Not designated.
Remarks
See Yasuhara et al. (Reference Yasuhara, Hunt, Okahashi and Brandão2015) for detailed discussion of this species, along with SEM images of topotype specimens.
Genus Legitimocythere Coles and Whatley, Reference Coles and Whatley1989
Type species.—Cythere acanthoderma Brady, Reference Brady1880.
Legitimocythere acanthoderma (Brady, Reference Brady1880)
Figure 17
- Reference Brady1880
Cythere acanthoderma Brady, p. 104, pl. 18, fig. 5a–e.
- Reference Tressler1941
Cythereis ericea (Brady); Tressler, p. 101, pl. 19, fig. 23.
- Reference Puri and Hulings1976
Cythere acanthoderma; Puri and Hulings, 267, pl. 11, figs. 16–18.
- ?Reference Ducasse and Peypouquet1979
Thalassocythere acanthoderma (Brady); Ducasse and Peypouquet, pl. 3, fig. 4.
- non Reference Benson, DelGrosso and Steineck1983
“Thalassocythere” acanthoderma (Brady); Benson et al., pl. 2, fig. 9.
- ?Reference Cronin and Compton-Gooding1987
“Thalassocythere” sp. Cronin and Compton-Gooding, pl. 2, fig. 3.
- Reference Malz1987
“Thalassocythere” acanthoderma; Malz, fig. 2b.
- Reference Whatley and Coles1987
“Thalassocythere” acanthoderma; Whatley and Coles, pl. 6, figs. 1, 2.
- non Reference Coles and Whatley1989
Legitimocythere acanthoderma (Brady); Coles and Whatley, p. 100, pl. 4, fig. 9.
- Reference Dingle and Lord1990
Legitimocythere acanthoderma; Dingle and Lord, fig. 2.11.
- Reference Malz1990
“Thalassocythere” acanthoderma; Malz, fig. 8.5–8.7.
- Reference Didié and Bauch2000
Thalassocythere acanthoderma; Didié and Bauch, pl. 3, figs. 15, 16.
- Reference Jellinek and Swanson2003
Legitimocythere acanthoderma; Jellinek and Swanson, p. 33.
- Reference Jellinek and Swanson2003
Legitimocythere sp. A Jellinek and Swanson, p. 37, pl. 27, figs. 1, 2.
- Reference Jellinek and Swanson2003
Legitimocythere sp. B Jellinek and Swanson, p. 37, pl. 27, figs. 3, 4.
- Reference Ayress, De Deckker and Coles2004
Legitimocythere acanthoderma; Ayress et al., p. 36, pl. 1, figs. 6, 7.
- Reference Mazzini2005
Legitimocythere acanthoderma; Mazzini, p. 42, figs. 22A–L, 23A–F.
- Reference Mazzini2005
Legitimocythere geniculata Mazzini; p. 44, fig. 24A–M.
- Reference Alvarez Zarikian2009
Legitimocythere acanthoderma; Alvarez Zarikian, p. 6, pl. P1, figs. 4, 5 [sic: this should be pl. 1, figs. 4, 6].
- Reference Yasuhara, Cronin, Hunt and Hodell2009a
Legitimocythere acanthoderma; Yasuhara et al., p. 922, figs. 5.5, 11.1–11.6.
- Reference Yasuhara, Okahashi and Cronin2009c
Legitimocythere sp. Yasuhara, Okahashi, and Cronin, p. 927, pl. 21, fig. 5, pl. 22, figs. 7, 8.
- Reference Brandão2013
Legitimocythere acanthoderma; Brandão, p. 16, pls. 1, 2.
- Reference Yasuhara, Grimm, Brandão, Jöst, Okahashi, Iwatani, Ostman and Martínez Arbizu2014a
Legitimocythere acanthoderma; Yasuhara et al., p. 354, fig. 8.4.
- Reference Yasuhara, Hunt, Okahashi and Brandão2015
Legitimocythere acanthoderma s.l. (Brady); Yasuhara et al., p. 133, figs. 63R, S, 65A–J, 73N–X, 74A–C, Q, 75E–N, 76, 77A–I.
Holotype
Lectotype, juvenile LV, NHM 80.38.48.A.1 (Natural History Museum, London, UK), Southern Ocean, Recent.
Remarks
See Yasuhara et al. (Reference Yasuhara, Hunt, Okahashi and Brandão2015) and Brandão (Reference Brandão2013) for detailed discussion of this species.
Genus Pterygocythere Hill, Reference Hill1954
Type species
Cypridina alata Bosquet, Reference Bosquet1847.
Pterygocythere nobilis (Jellinek, Swanson, and Mazzini, Reference Jellinek, Swanson and Mazzini2006)
Figure 15.1–15.4
- Reference Tressler1941
Cytheropteron mucronalatum Brady; Tressler, p. 102, pl. 19, fig. 25.
- Reference Benson, DelGrosso and Steineck1983
Brachycythere mucronalatum (Brady); Benson et al., pl. 1, figs. 6, 7.
- Reference Whatley and Coles1987
Bosquetina mucronalatum (Brady); Whatley and Coles, pl. 5, figs. 1, 2.
- Reference Jellinek, Swanson and Mazzini2006
Pseudobosquetina nobilis Jellinek, Swanson, and Mazzini, p. 41, figs. 6–8.
- Reference Yasuhara, Hunt, Okahashi and Brandão2015
Pseudobosquetina nobilis; Yasuhara et al., p. 156, figs. 87K–N, 88G–S.
Holotype
Female carapace, SMF Xe 21746 (Senckenberg Research Institute and Natural History Museum Frankfurt, Germany), South Atlantic, living.
Remarks
We consider Pseudobosquetina a junior synonym of Pterygocythere. See Ayress et al. (Reference Ayress, De Deckker and Coles2004) and Yasuhara et al. (Reference Yasuhara, Hunt, Okahashi and Brandão2015) for detailed discussion.
Family Xestoleberididae Sars, Reference Sars1928
Genus Xestoleberis Sars, Reference Sars1866
Type species
Cythere nitida Lilljeborg, Reference Lilljeborg1853 (designated by Sars, Reference Sars1866).
Xestoleberis oppoae Yasuhara, Okahashi, and Cronin, Reference Yasuhara, Okahashi and Cronin2009c
Figure 15.5–15.9
- Reference Yasuhara, Okahashi and Cronin2009c
Xestoleberis oppoae Yasuhara, Okahashi, and Cronin, p. 927, pl. 22, figs. 1–6.
Holotype
Adult female LV, USNM PAL 536985 (National Museum of Natural History, Washington DC, USA), northwestern Atlantic Ocean, Quaternary.
Remarks
This is the second record of this species that was originally discovered in the northwestern Atlantic Ocean.
Discussion
We found that ODP Site 925 yielded an ostracode fauna with tropical deep-sea faunal elements that are distinct from higher latitude faunas. The genera Zabythocypris, Aratrocypris, Hemiparacytheridea, Chejudocythere, and deep-sea Semicytherura were found here (note that they are not abundant; see Fig. 18), but they have not been reported from mid to high latitudes of the Atlantic Ocean. These genera are known from Recent and Quaternary sediments in the low-latitude Atlantic (Cronin, Reference Cronin1983; Yasuhara et al., Reference Yasuhara, Okahashi and Cronin2009c), Pacific (Maddocks, Reference Maddocks1969; Coles et al., Reference Coles, Ayress, Whatley, Whatley and Maybury1990; Corrége, Reference Corrége1993; Ayress et al., Reference Ayress, Whatley, Downing and Millson1995), and Indian oceans (Maddocks, Reference Maddocks1969; Ayress et al., Reference Ayress, Whatley, Downing and Millson1995). O'Hara et al. (Reference O'Hara, Rowden and Bax2011) reported a similar pattern for bathyal ophiuroids in the Pacific, Southern, and Indian oceans around Australia, finding a tropical bathyal fauna that was distinct from that from higher latitudes.
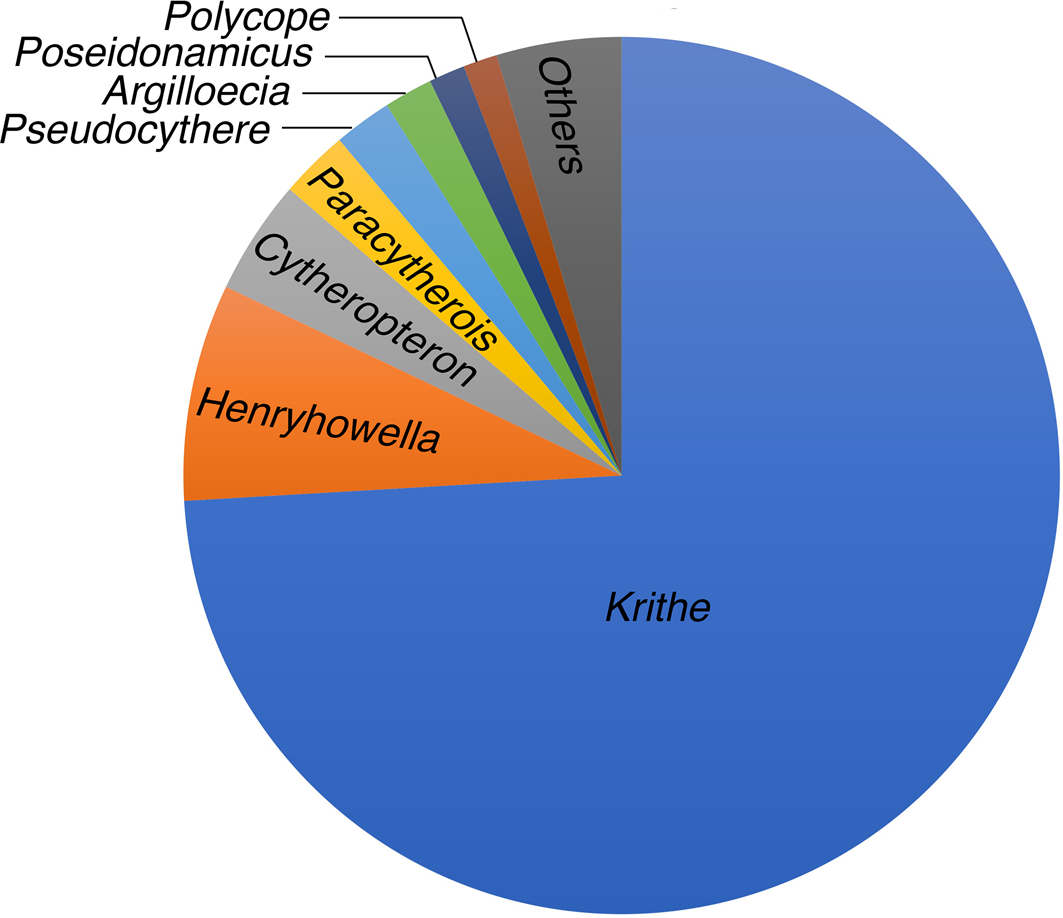
Figure 18. Pie chart showing the faunal composition of the ODP 925 ostracodes. All samples studied are lumped to calculate relative abundances of genera. Zabythocypris, Aratrocypris, Hemiparacytheridea, Chejudocythere, and Semicytherura are not abundant (<1% in total, among 5008 ostracode specimens that we picked and counted) and not among the top eight genera shown in the pie chart.
We suggest that this global distribution of tropical deep-sea fauna may be a Tethyan legacy. Many of these tropical deep-sea genera (Aratrocypris, Hemiparacytheridea, Chejudocythere, Semicytherura) are known from Cretaceous chalks in western Europe (Whatley et al., Reference Whatley, Witte and Coles1989; Coles et al., Reference Coles, Ayress, Whatley, Whatley and Maybury1990; Ayress and Dorn, Reference Ayress and Dorn2012). This suggests that they were widespread in the Tethys during the warm Cretaceous and Paleogene periods, even as they are limited to the tropics in the deep water of today's oceans.
It is puzzling that a characteristic deep-sea, low-latitude fauna exists even though the deep sea in the tropics does not have distinct environmental conditions from those of the deep sea in higher latitudes. Deep-sea temperature, particulate organic carbon flux, and seasonality do not change much at the tropical-extratropical boundary (O'Hara et al., Reference O'Hara, Rowden and Bax2011; Yasuhara and Danovaro, Reference Yasuhara and Danovaro2016; Sweetman et al., Reference Sweetman, Thurber, Smith, Levin, Mora, Wei, Gooday, Jones, Rex, Ingels, Ruhl, Frieder, Danovaro, Würzberg, Baco, Grupe, Pasulka, Meyer, Dunlop, Henry and Roberts2017). Deep-sea temperature has latitudinal structure, but it is mostly between colder polar and warmer temperate regions, rather than between tropical and temperate regions (Yasuhara and Danovaro, Reference Yasuhara and Danovaro2016). Particulate organic carbon flux from sea-surface production is the major food source of deep-sea benthos, but this carbon flux and its seasonality change depending on the distance from the coast, water depth, and presence of upwelling, rather than latitude (Watling et al., Reference Watling, Guinotte, Clark and Smith2013; Sweetman et al., Reference Sweetman, Thurber, Smith, Levin, Mora, Wei, Gooday, Jones, Rex, Ingels, Ruhl, Frieder, Danovaro, Würzberg, Baco, Grupe, Pasulka, Meyer, Dunlop, Henry and Roberts2017).
According to published fossil occurrences, Tethyan tropical genera were distributed widely, not only in the tropics, but also in mid–high latitudes during the Paleogene (Whatley and Coles, Reference Whatley and Coles1991). As climates cooled over the Cenozoic, however, they progressively disappeared from mid–high latitude regions. For example Chejudocythere, Aratrocypris, and Semicytherura do not occur after the Oligocene in the mid–high latitude North Atlantic, according to the compilation by Whatley and Coles (Reference Whatley and Coles1991), suggesting their distributions contracted to the tropics by the Neogene. They may have persisted in the deep ocean in the tropics because their distributions included the relatively warm waters of the uppermost bathyal zone. Such water depths may have been a refuge for their populations, as has been suggested for other deep-sea organisms (Zezina, Reference Zezina1997; Eme et al., Reference Eme, Anderson, Myers, Roberts and Liggins2020). This is consistent with the deep-sea source-sink hypothesis that suggests that abyssal populations are a demographic sink, with source populations located in shallower bathyal zones (Rex et al., Reference Rex, McClain, Johnson, Etter, Allen, Bouchet and Waren2005). In this sense, bathyal (< ~3000 m) biogeography (Fig. 19) should be more similar to that of the shelf (Spalding et al., Reference Spalding, Fox, Allen, Davidson, Ferdaña, Finlayson, Halpern, Jorge, Lombana, Lourie, Martin, McManus, Molnar, Recchia and Robertson2007; Costello et al., Reference Costello, Tsai, Wong, Cheung, Basher and Chaudhary2017) rather than that of deeper abyssal plain (Fig. 19) (UNESCO, 2009; Watling et al., Reference Watling, Guinotte, Clark and Smith2013), as indicated by O'Hara et al. (Reference O'Hara, Rowden and Bax2011). In fact, a recent study showed that deep-sea latitudinal diversity gradient is unclear in abyssal depths (Woolley et al., Reference Woolley, Tittensor, Dunstan, Guillera-Arroita, Lahoz-Monfort, Wintle, Worm and O'Hara2016). ODP Site 925 is situated in the lower bathyal North Atlantic province (BY4 of Watling et al., Reference Watling, Guinotte, Clark and Smith2013), which is widely distributed in the North Atlantic from the equatorial Atlantic in the south to Cape Cod in the northwestern Atlantic and the Faeroe Islands in the northeast Atlantic to the north (Fig. 19). This biogeographic scheme is largely based on oceanographic proxies because of sparse deep-sea biological data available. It is possible that the BY4 province can be further divided into latitudinal sub-provinces because (some or all of) the tropical deep-sea genera found in ODP Site 925 are not known from the northern part of the BY4 province (Yasuhara et al., Reference Yasuhara, Okahashi and Cronin2009c; Yasuhara and Okahashi, Reference Yasuhara and Okahashi2014, Reference Yasuhara and Okahashi2015).

Figure 19. Deep-sea benthic biogeographic provinces. Top panel for lower bathyal (BY) provinces (801–3500 m) and bottom panel for abyssal (AB) provinces (3501–6500 m). From Watling et al. (Reference Watling, Guinotte, Clark and Smith2013). ODP Site 925 is indicated.
The progressive restriction of certain genera to the deep tropics implies that the standard tropical-high and extratropical-low latitudinal diversity gradient (Rex et al., Reference Rex, Stuart and Coyne2000; Yasuhara et al., Reference Yasuhara, Hunt, Cronin and Okahashi2009b) in the deep sea was less pronounced when these taxa had wider latitudinal ranges in the Paleogene. An increase in the relative diversity of the deep tropics is also seen in the benthic foraminiferal fossil record at ca. 37 Ma and, since then, the standard deep-sea foraminiferal latitudinal diversity gradient has been persistent until today (Thomas and Gooday, Reference Thomas and Gooday1996; Culver and Buzas, Reference Culver and Buzas2000; Yasuhara et al., Reference Yasuhara, Huang, Hull, Rillo, Condamine, Tittensor, Kučera, Costello, Finnegan, O'Dea, Hong, Bonebrake, McKenzie, Doi, Wei, Kubota and Saupe2020).
Acknowledgments
We thank R.P.P. Wong, M.G.Y. Lo, C. Sanford, and the staff of the Electron Microscope Unit in the University of Hong Kong for continuous support; S. Whittaker for help in SEM imaging in the National Museum of Natural History; C.A. Alvarez Zarikian and an anonymous reviewer for valuable comments; and B. Hunda, T.M. Cronin, and J. Kastigar for editing. Samples used for this research were provided by the International Ocean Discovery Program (IODP). The work described in this article was partially supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (project codes HKU 17300720, HKU 17302518), the Seed Funding Programme for Basic Research of the University of Hong Kong (project codes 201811159076, 201711159057), the Small Equipment Grant of the University of Hong Kong, the Faculty of Science RAE Improvement Fund of the University of Hong Kong, the Seed Funding of the HKU-TCL Joint Research Centre for Artificial Intelligence, Smithsonian Postdoctoral Fellowship, and Smithsonian Marine Science Network Postdoctoral Fellowship (to MY); the Peter Buck Postdoctoral Fellowship of the National Museum of Natural History, Smithsonian Institution (to HHMH); and the 45th Round of the Post-doctoral Fellow Scheme of the University of Hong Kong (to YH).
Appendix 1
Detailed information of the specimens used for the present study. All specimens from late Quaternary sediments. Core samples are specified by standard ODP notation (hole and core #/section #/interval [cm]). USNM PAL, catalog numbers of the National Museum of Natural History; No., MY's personal catalog number. T, type (P, paratype; H, holotype); V, valve (L, left; R, right); A, adult; J, juvenile (A-1, adult minus one juvenile).






















