Introduction
The genus Retrotapes was erected by del Río (Reference del Río1997), describing four Miocene Patagonian species and proposing the inclusion of some Cenozoic Chilean and Antarctic taxa within it (species that were previously included in the genus Eurhomalea Cossmann, Reference Cossmann1920). Several authors confirmed the assignments of del Río (Reference del Río1997) for the Chilean taxa and proposed additional inclusions of species into the genus from Chile (Griffin and Nielsen, Reference Griffin and Nielsen2008; Nielsen and Valdovinos, Reference Nielsen and Valdovinos2008; Nielsen, Reference Nielsen2013) and from Antarctica (Beu and Taviani, Reference Beu and Taviani2014). Alvarez et al. (Reference Alvarez, del Río and Marenssi2014) reassigned three Eocene species from La Meseta Formation to Retrotapes that previously were considered as species of Eurhomalea by Zinsmeister (Reference Zinsmeister1984) and Stilwell and Zinsmeister (Reference Stilwell and Zinsmeister1992), and recognized the validity of the previous assignments of Chilean and Antarctic species as Retrotapes.
Jukes-Browne (Reference Jukes-Browne1909) emphasized the necessity to erect a new genus in order to separate Eurhomalea exalbida (Dillwyn, Reference Dillwyn1817) and E. lenticularis (Sowerby, Reference Sowerby I1835) from E. rufa (Lamarck, Reference Lamarck1818) (type species of Eurhomalea Cossmann, Reference Cossmann1920). Later, Ramorino (Reference Ramorino1968) erected a new species, E. salinensis, from Valparaíso Bay, and stated that E. lenticularis and E. salinensis have several differences that allowed them to be distinguished from E. rufa. Del Río (Reference del Río1997) erected the genus Retrotapes, including E. exalbida and E. lenticularis in it, based on the presence of a concave lunule bounded by a deep groove, non-divergent teeth, some of them bifid, and a ventral margin of the hinge plate curved behind the teeth. Moreover, according to the illustrations and descriptions of Ramorino (Reference Ramorino1968) that match with the characters of Retrotapes, Alvarez et al. (Reference Alvarez, del Río and Marenssi2014) included E. salinensis within this genus. Eurhomalea, only represented by its type species E. rufa, is characterized by its thin hinge plate with slightly curved margin and thin and divergent cardinal teeth, some of them entire and others slightly grooved. It is also characterized by the absence of an escutcheon and the presence of a very narrow lunule, bounded by a very shallow groove, which in some adult specimens could be absent, as illustrated by del Río (Reference del Río1997).
The genus Retrotapes also has been accepted by other authors (Gordillo, Reference Gordillo2006; Beu, Reference Beu2009), but some have questioned its validity (Lauriat-Rage et al., Reference Lauriat-Rage, Carriol, Lozouet, Giret and Leyrit2002; Huber, Reference Huber2010). Lauriat-Rage et al. (Reference Lauriat-Rage, Carriol, Lozouet, Giret and Leyrit2002) compared some hinge plate characters of Retrotapes and Frigichione Fletcher, Reference Fletcher1938 (Miocene, Kerguelen Island), and proposed that the latter taxon is a senior synonym of Retrotapes. Frigichione permagna (Tate, Reference Tate1900) (type species of Frigichione Fletcher, Reference Fletcher1938, pl. 1, fig. 3) differs from Retrotapes because of its thicker and subtriangular shells, slightly developed escutcheon, straight and short lunule that is bounded by a very shallow groove, and a hinge plate with straight margin and thicker cardinal teeth.
Huber (Reference Huber2010, p. 717), based on a misinterpretation of Jukes-Browne (Reference Jukes-Browne1909), Ramorino (Reference Ramorino1968), and del Río (Reference del Río1997), synonymized Eurhomalea and Retrotapes considering unnecessary the creation of the latter one, at least to include the extant species R. exalbidus and R. lenticularis.
Gallardo et al. (Reference Gallardo, González, Mena, Lomovasky, Morriconi and Clasing2003) analyzed the allozyme variation of 12 loci of Retrotapes exalbidus, R. lenticularis (included in Eurhomalea by these authors), and of Eurhomalea rufa, and they concluded that R. lenticularis and R. exalbidus are grouped together in a clade with a high support value of 100 bootstrap frequencies. This close connection between these two extant species and among other fossil taxa of Retrotapes, as well as its separation from Eurhomalea rufa, also was supported by the results of the geometric morphometric analysis performed by Alvarez et al. (Reference Alvarez, del Río and Marenssi2014), which supports the validity of Retrotapes.
There is some doubt about the inclusion of Retrotapes lenticularis into the subfamily Tapetinae. Recent phylogenetic proposals of Veneridae would seem to indicate that R. lenticularis is closely related to the subfamilies Venerinae and Chioninae, and not with Tapetinae, as traditional classification suggested (Kappner and Bieler, Reference Kappner and Bieler2006; Mikkelsen et al., Reference Mikkelsen, Bieler, Kappner and Rawlings2006). In both mentioned works, the studied specimen is the same (FMNH 301912), and it was described with a crenulated inner ventral margin (Kappner and Bieler, Reference Kappner and Bieler2006), a character that matches with the inclusion of it in Venerinae or Chioninae. However, none of the several shells studied in the present contribution has a crenulated inner ventral margin, which on the contrary is smooth, as in the rest of species of the Retrotapes and Eurhomalea. In the mentioned papers, R. lenticularis is the sister taxon of Tawera spissa (Deshayes, Reference Deshayes1835) with high values of support in all searches. Thus, it is possible that the tissues sample used to perform the molecular analysis would be of a Tawera species, for example T. gayi (Hupé, Reference Hupé and Gay1854), which inhabits the same locations as R. lenticularis and has a crenulated inner ventral margin. Unfortunately, specimen FMNH 301912 has no associated valves (J. Gerber, Field Museum of Natural History, personal communication, 2013), so the characteristics of its shell could not be corroborated. This is why until doubts about this specimen are clarified, R. lenticularis should be considered as a Tapetinae.
The main goal of the present contribution is to perform a cladistic analysis of the genus Retrotapes to test its monophyly and study relationships with others taxa of the subfamily. In addition, a revision of the extant and fossil species of Chile is carried out to have a complete knowledge of the systematics of the genus, continuing with the analysis started by del Río (Reference del Río1997), who studied the fossil species of Patagonia (Argentina), and Alvarez et al. (Reference Alvarez, del Río and Marenssi2014), who analyzed the fossil taxa from Antarctica.
Materials and methods
The studied Tapetinae come from the marine Cenozoic outcrops of Argentina, Antarctica, and Chile, known as San Julián (late Oligocene), Centinela (late Oligocene–early Miocene), 25 de Mayo (late Oligocene–early Miocene), Monte León (early Miocene), Carmen Silva (middle Miocene), and Puerto Madryn (late Miocene) formations from Argentina; La Meseta Formation (Eocene) from Antarctica; and Loreto (late Eocene), Guadal (late Oligocene–early Miocene), Navidad (early Miocene), Bahía Inglesa (late Miocene–late Pliocene), Coquimbo (late Miocene–late Pliocene), La Cueva (early Pliocene), and Tubul (Pliocene–Pleistocene) formations from Chile. Geological settings of the Retrotapes taxa from Argentina are summarized in del Río (Reference del Río1997), and those from Antarctica in Alvarez et al. (Reference Alvarez, del Río and Marenssi2014). Fossiliferous localities from Chile are displayed in Figure 1.
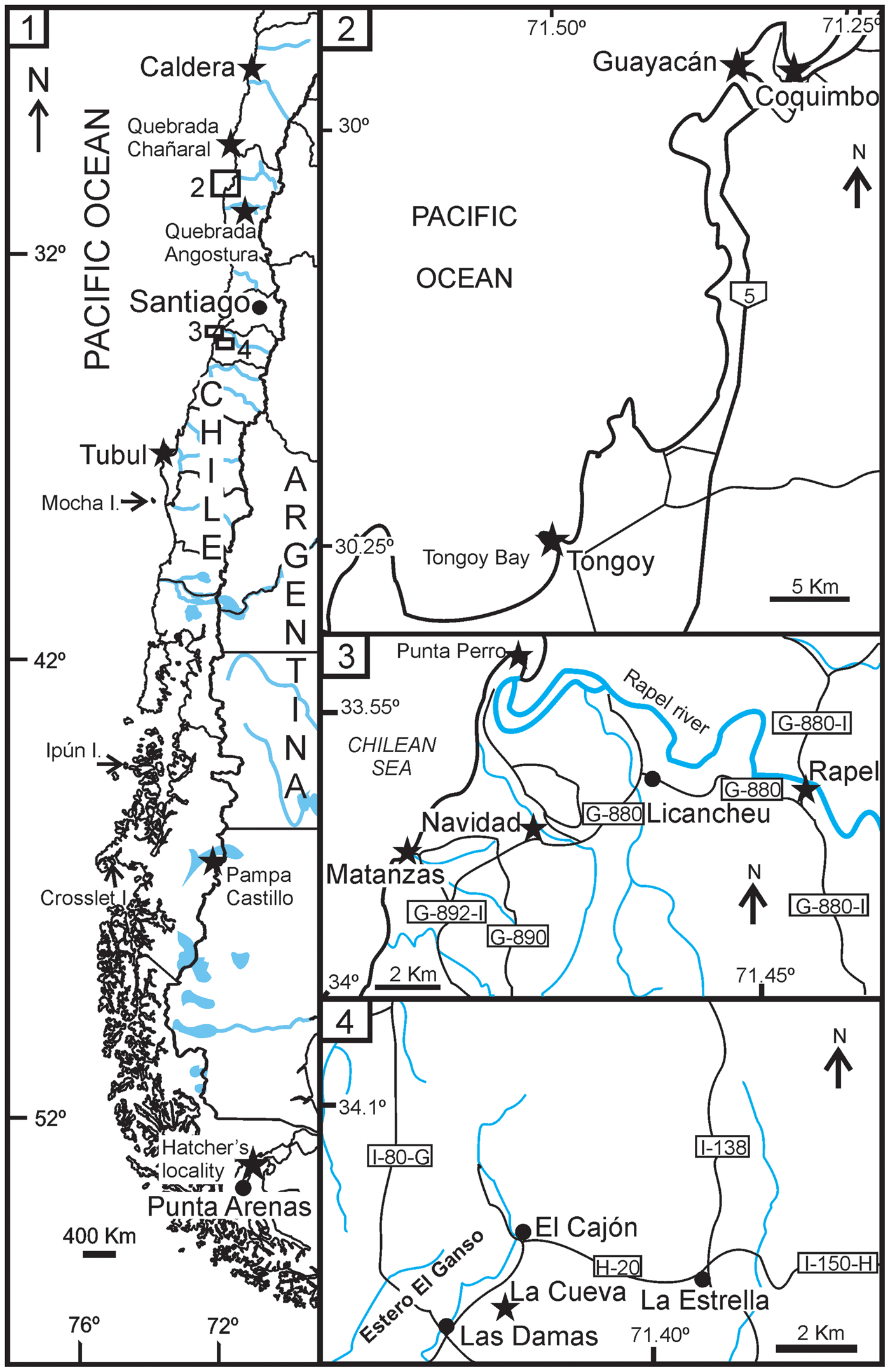
Figure 1. (1) Geographic location of the samples; (2) area of Coquimbo and Tongoy; (3) area of Navidad; (4) area of La Estrella. The black stars mark the localities.
Specimens collected by J.B. Hatcher from the Loreto Formation (late Eocene) come from the outcrops exposed to the north of Punta Arenas. The late Oligocene–early Miocene sediments that contain the studied material are exposed at Pampa Castillo (Guadal Formation).
Navidad Formation exposures are located at Navidad, Rapel Norte, Punta Perro, and Matanzas. Recent papers indicated a late Miocene–early Pliocene age for this unit based on foraminifera fauna (Finger et al., Reference Finger, Encinas, Nielsen and Peterson2003, Reference Finger, Nielsen, DeVries, Encinas and Peterson2007; Encinas, Reference Encinas2006; Encinas et al., Reference Encinas, Le Roux, Buatois, Nielsen, Finger, Fourtanier and Lavenu2006), but the molluscan fauna recorded there is reworked (Finger et al., Reference Finger, Nielsen, DeVries, Encinas and Peterson2007) and belongs to the early Miocene (DeVries and Frassinetti, Reference DeVries and Frassinetti2003; Nielsen and Glodny, Reference Nielsen and Glodny2009). Later, Finger et al. (Reference Finger, Encinas and Nielsen2013) revised those assignations and reidentified several foraminifera, indicating an early Miocene–middle Miocene age for this unit. Outcrops from Ipún Island have an early Miocene–middle Miocene age (Frassinetti, Reference Frassinetti2004; Nielsen and Encinas, Reference Nielsen and Encinas2014) and the suggested age for the sediments from Crosslet Island is middle Miocene–late Miocene (Frassinetti, Reference Frassinetti2006). Late Miocene–late Pliocene sediments that contain the fossil material studied are exposed at Caldera (Atacama Region, Bahía Inglesa Formation) (Guzmán et al., Reference Guzmán, Marquardt, Ortlieb and Frassinetti2000; Le Roux et al., Reference Le Roux, Achurra, Henríquez, Carreño, Rivera, Suárez, Ishman, Pyenson and Guststein2016).
Pliocene beds that contain the fossil material studied are exposed at Coquimbo, Tongoy, Guayacán, Quebrada de Chañaral, and Quebrada Angostura (Coquimbo Formation; late Miocene–late Pliocene), La Cueva and Estero del Ganso (La Cueva Formation; early Pliocene), Tubul, Cerro Las Lomas, and Tubul River (Tubul Formation; Pliocene–Pleistocene).
The Recent fauna analyzed comes from the Tropical East Pacific, Magellanic, Argentinean, and Caribbean Malacological Provinces.
Geographic and stratigraphic distributions of each studied species, fossils and extant, are summarized in Supplementary Data Set 1, and the materials of other taxa used for comparison and phylogenetic analysis are summarized in Supplementary Data Set 2. The methodology applied to perform the phylogenetic analysis is described in the corresponding section.
Repositories and institutional abbreviations
Material included in the present contribution is housed at: División Paleoinvertebrados, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires (MACN-Pi and exCIRGEO-PI); División Invertebrados, Museo Argentino de Ciencias Naturales Bernardino Rivadavia, Buenos Aires, Argentina (MACN-In); Cátedra de Paleontología de la Universidad de Buenos Aires, Buenos Aires, Argentina (CPBA); Museo de La Plata, Argentina (MLP); Repositorio Antártico de Colecciones Paleontológicas y Geológicas del Instituto Antártico Argentino, San Martín, Buenos Aires, Argentina (IAA-Pi); Colección Paleoinvertebrados, Museo de Historia Natural, Santiago, Chile (SGO.PI); Field Museum of Natural History, Chicago, USA (FMNH); Natural History Museum Rotterdam, Netherlands (NMR); South Australian Museum, North Terrace, Adelaide, Australia (SAM); Samling Paleobiologi, Naturhistoriska Riksmuseet, Stockholm, Sweden (PZ-NRM Mo); Paleontological Research Institution, Cornell University, Ithaca, New York, USA (PRI); Auckland Museum, Auckland, New Zealand (AM); Natural History Museum, London, United Kingdom (NHMUK); Natural History Museum of Denmark (Zoology), Copenhagen, Denmark (ZMUC).
Phylogenetic analysis
Characters
A matrix of 80 characters was developed (Supplementary Data Sets 1 and 2), describing the whole shell morphology, including: shape (13), hinge (30), umbo (1), lunule (6), nymph (3), escutcheon (5), pallial sinus (7), muscles scars (7), and sculpture (8).
Some characters concerning the shell shape, hinge plate, pallial line, and muscles scars were based on Mikkelsen et al. (Reference Mikkelsen, Bieler, Kappner and Rawlings2006) and Pérez et al. (Reference Pérez, del Río and Nielsen2013), but their states were completely modified. In addition, 25 continuous characters were built based on ratios and inclinations of some structures used in classic systematic works to compare different taxa (e.g., height/length of the valve, length of the lunule and nymph, pallial sinus inclination, teeth inclination, teeth length) (Fig. 2).

Figure 2. Measures and angles used to build continuous characters. (1) Length from the umbo to the posterior margin; (2) length of the nymph; (3) length of the lunule; (4) height of the shell; (5) length of the shell; (6) distance between the ventral margin and the pallial line; (7) width of the pallial sinus; (8) height of the nymph; (9) width of the teeth; (10) length of the teeth; (11) inclination of the teeth with respect to the horizontal axis; (12) inclination of the dorsal margin measured as a tangent that passes through the umbo and the contact between the dorsal and posterior margins; (13) inclination of the abductor muscles; (14) inclination of the dorsal side of the pallial sinus, measured as a tangent that joins the apex of the pallial sinus and the contact of it with the posterior abductor muscle scar.
In order to minimize the lack of information, most of the reviewed materials were studied first hand. The taxon Retrotapes andrillorum Beu and Taviani, Reference Beu and Taviani2014 (McMurdo Sound, Antarctica, Miocene) was not included in the analysis because it was only reviewed through published pictures and some inner characters of the shell were not visible due to the sedimentary matrix that fills it, increasing therefore the amount of missing data in the matrix. The taxa R. difficilis (Ortmann, Reference Ortmann1899) and R. scutatus (Ihering, Reference Ihering1907) also were not included because only a few characters of the hinge were useful in both species due to the incompleteness of the specimens. The same problem occurred with Frigichione permagna (Tate, Reference Tate1900), however this taxon was considered in the analysis to test the synonymy with the genus Retrotapes del Río, Reference del Río1997 proposed by some authors. The percentage of missing entries is 2.92%.
Ingroup
In addition to the type species of the genera Frigichione and Eurhomalea (F. permagna and E. rufa), 10 of the 13 known species of the genus Retrotapes were included to build the matrix: R. antarcticus (Sharman and Newton, Reference Sharman and Newton1894), R. robustus (Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992), R. newtoni (Wilckens, Reference Wilckens1911), R. ninfasiensis del Río, Reference del Río1997, R. striatolamellatus (Ihering, Reference Ihering1897), R. fuegoensis del Río, Reference del Río1997, R. navidadis (Philippi, Reference Philippi1887), R. fuenzalidae (Philippi, Reference Philippi1887), R. lenticularis, and R. exalbidus. Other austral taxa that share some features with Retrotapes were included: Atamarcia sulcifera (Marwick, Reference Marwick1927) (type species of Atamarcia), Eumarcia fumigata (Sowerby, Reference Sowerby II1853) (type species of Eumarcia), and Katelysia scalarina (Lamarck, Reference Lamarck1818) (type species of Katelysia).
Outgroup
Several genera of the subfamily Tapetinae were included to build the matrix considering only their type species in most of the cases, namely: Gomphina undulosa (Lamarck, Reference Lamarck1818), Neotapes undulatus (Born, Reference Born1778), Polititapes aureus (Gmelin, Reference Gmelin and Gmelin1791), P. virgineus (Linnaeus, Reference Linnaeus1767), Venerupis corrugata (Gmelin, Reference Gmelin and Gmelin1791), Ruditapes philippinarum (Adams and Reeve, Reference Adams, Reeve and Adams1850), R. decussatus (Linnaeus, Reference Linnaeus1758), Protapes gallus (Gmelin, Reference Gmelin and Gmelin1791), Marcia opima (Gmelin, Reference Gmelin and Gmelin1791), Paleomarcia tatei (Fletcher, Reference Fletcher1938), Paphia rotundata (Linnaeus, Reference Linnaeus1758), Tapes literatus (Linnaeus, Reference Linnaeus1758), Notopaphia elegans (Deshayes, Reference Deshayes1854), and Irus carditoides (Lamarck, Reference Lamarck1818).
Search
A phylogenetic analysis was performed following the maximum parsimony criterion using the TNT 1.5 software (Goloboff et al., Reference Goloboff, Farris and Nixon2008), through a heuristic search of 100 replicates of Wagner trees (with addition of random sequences) followed by TBR branch swapping algorithm holding 10 trees per replicate. Characters 1 to 25 were considered as continuous. The methodology of character weighting was implied weighting (Goloboff, Reference Goloboff1993), performing 100 searches for k values between 1 and 100. The support measures were estimated by resampling using frequency differences under Bootstrap (BS) (Felsenstein, Reference Felsenstein1985) and Jackknife (JK) (Farris et al., Reference Farris, Albert, Källersjö, Lipscomb and Kluge1996), with a p = 8 (equivalent to removing 10% of the characters) (Goloboff et al., Reference Goloboff, Farris, Källersjö, Oxelman, Ramírez and Szumik2003) and performing 1,000 pseudo-replicates.
In this analysis, Retrotapes is represented by 10 species, whereas the other genera included are only represented by one or two species, which generates an oversampling of Retrotapes. This design of the matrix could cause Retrotapes to direct the transformations of the characters in such a way that the phylogenetic relationships would be spurious. To solve this problem, two different analyses were performed. The first one was performed to test the relationships of Retrotapes with the other genera, reducing the active taxa of Retrotapes to only three terminals: its type species R. ninfasiensis (Puerto Madryn Formation; late Miocene) and the extant taxa R. lenticularis and R. exalbidus, whose assignment to the genus was recently questioned by Huber (Reference Huber2010). The other search was performed to test the inner relationships of the genus including the 10 taxa mentioned above.
Results
Analysis with the reduced matrix
Each search performed with a different k value (k between 1 and 100) resulted in a single topology, obtaining ranges of k where the recovered topologies are similar to each other. The trees have different topologies for k values 3, 9, and 22 (Fig. 3). The BS and JK values were calculated and informed on each topology (Fig. 3). The tree obtained for the k value 22 is the most abundant topology, and it is the same one obtained in an exploratory search performed without implied weighting, it also has the best BS and JK values, therefore the discussion is based on it.

Figure 3. Topologies recovered at different k values from the performed analysis with the reduced matrix. (1) k = 3–8; (2) k = 9–21; (3) k = 22–100. BS values are informed over the branches; JK values are informed under the branches; only values over 50 are informed.
In all the performed searches, Frigichione is recovered as a basal taxon, and Eurhomalea is closely related with Venerupis and Ruditapes. Meanwhile, Retrotapes is monophyletic and closely related to Atamarcia and Paleomarcia.
On the topology with the k value of 22, the genus Retrotapes is supported by 13 synapomorphies: (character 2 [c2]) Vertical adductor muscle scar (1.953–1.957), (c3) posterior adductor muscle scar slightly oriented backwards (1.919–1.926), (c4) Tooth 3a slightly tilted backwards (1.943–1.960), (c5) Tooth 1 strongly sloped backwards (2.101–2.113), (c6) Tooth 3b sub-horizontal (2.226), (c14) umbo position (0.881–0.884), (c17) space of the hinge plate occupied by 3a tooth (0.808–0.828), (c25) ratio between length and height of the nymph (0.975–0.990), (c30) high hinge plate, (c50) comarginal elements of the sculpture spaced towards the umbo and closer to each other towards the ventral margin of the disk, (c56) lunule bounded by a deep groove, (c63) edge between dorsal and posterior margins rounded, and (c70) presence of marked growth ribs and thin ribs interspersed among them.
Analysis with the complete matrix
Each search performed with a different k value (k between 1 and 100) resulted in a single topology, obtaining ranges of k in which the recovered topologies are similar to each other. The trees have different topologies for k values 2, 7, and 57 (Fig. 4). The BS and JK values were calculated and informed on each topology (Fig. 4). The tree obtained for k value 7 is the most abundant topology and has the best BS and JK values, therefore the discussion is based on it. The latter phylogenetic tree was temporally calibrated with the timePaleoPhy() function of the package paleotree (Bapst, Reference Bapst2012) for R (R Core Development Team, 2018) using the ‘mbl’ calibration and a minimum branch length of 0.1 My (Fig. 5). This setting was chosen in order to recover the most conservative age estimation for each branch. As a result, ghost lineage lengths are mostly a consequence of the age of its sister-branch (Pérez and Ezcurra, Reference Pérez and Ezcurra2018).

Figure 4. Topologies recovered at different k values from the performed analysis with the complete matrix. 1, k = 2–6; 2, k = 7–56; 3, k = 57–100. BS values are informed over the branches; JK values are informed under the branches; only values over 50 are informed.

Figure 5. Time calibrated phylogenetic tree performed with the topology recovered at k = 7 from the performed analysis with the complete matrix. Quat. = Quaternary, Plio. = Pliocene, Pleisto. = Pleistocene. Age axis in million years.
In all the performed searches, Retrotapes is recovered as a monophyletic group and the Eocene Antarctic R. newtoni is recovered as the basal-most taxon. In most of the searches (k range from 1 to 56) the suboval (R. newtoni, R. fuegoensis, R. fuenzalidae) and subtriangular (R. robustus) taxa are successive sister taxa to a group of subquadrate shells in which are included the type species R. ninfasiensis and the extant taxa R. exalbidus and R. lenticularis, the latter being a sub-rounded one. That group of subquadrate shape is recovered in all the searches.
Systematic paleontology
Family Veneridea Rafinesque, Reference Rafinesque1815
Subfamily Tapetinae Gray, Reference Gray1851
Genus Retrotapes del Río, Reference del Río1997
Type species
Retrotapes ninfasiensis del Río, Reference del Río1997 (Puerto Madryn Formation, late Miocene) (Fig. 6.6–6.9).
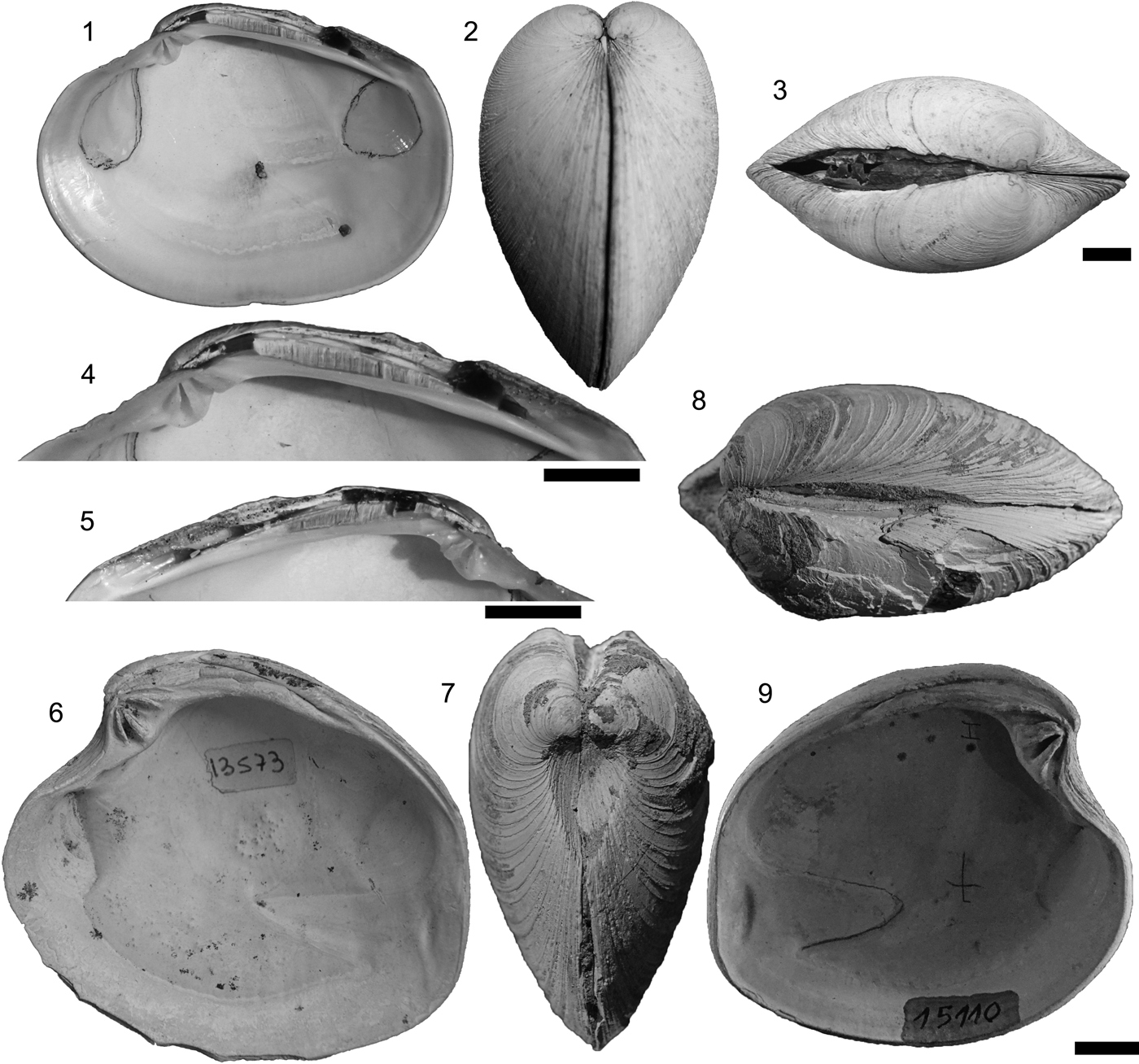
Figure 6. (1–5) Eurhomalea rufa (Lamarck, Reference Lamarck1818): (1, 4, 5) MACN-In 37805: right valve interior view, and right and left hinge plate (Caldera, Chile, Recent). (2, 3) MACN-In 24780: anterior and dorsal views (Tongoy Bay, Chile, Recent). (6–9) Retrotapes ninfasiensis del Río, Reference del Río1997: (6) CPBA 13573 (Holotype), a right valve, interior view (Cerro Prismático, Puerto Madryn Formation, Argentina); (7, 8) CPBA 15090: anterior and dorsal views (Punta Norte, Puerto Madryn Formation, Argentina); (9) CPBA 15110 (Paratype), a left valve, interior view (Fondeadero Ninfas, Puerto Madryn Formation, Argentina). Scale bar (1–9) 1 cm.
Other included species
Retrotapes antarcticus, R. newtoni, and R. robustus, La Meseta Formation (Eocene, Marambio Island, Antarctica); R. difficilis (Ortmann, Reference Ortmann1899), Loreto Formation (late Eocene, Punta Arenas, Chile), R. scutatus (Ihering, Reference Ihering1907), San Julián (late Oligocene) and Centinela (late Oligocene–early Miocene) formations (Santa Cruz, Argentina); R. fuegoensis, Carmen Silva Formation (middle Miocene, Isla Grande de Tierra del Fuego, Argentina); R. striatolamellatus, early Miocene sediments of Centinela (late Oligocene–early Miocene) and Monte León formations (early Miocene) (Santa Cruz, Argentina); R. navidadis, Navidad (early Miocene) and Guadal formations (late Oligocene–early Miocene) and sediments from Ipún and Crosslet islands (Chile); R. andrillorum Beu and Taviani, Reference Beu and Taviani2014, McMurdo Sound (early Miocene, Antarctica); R. fuenzalidae, La Cueva (early Pliocene) and Tubul (Pliocene–Pleistocene) formations (Chile); R. exalbidus (Dillwyn, Reference Dillwyn1817) (Pliocene–Recent, from Chiloé, 42°S (Chile), Eastern Pacific Ocean, to Rio Grande do Sul, 32°S (Brazil), Western Atlantic Ocean); and R. lenticularis (Sowerby, Reference Sowerby I1835) (Pliocene–Recent, Eastern Pacific Ocean between 14°S in Perú and 33°S in Chile).
Occurrence
Eocene–Recent.
Retrotapes difficilis (Ortmann, Reference Ortmann1899) new combination
Figure 7.1–7.4
- 1899
Venus difficilis Ortmann, p. 428.
- 1902
Venus difficilis; Ortmann, p. 135, pl. 28, fig. 1.
- 1907
Marcia difficilis; Ihering, p. 350.
Holotype
A left valve (PRI 72689) from Punta Arenas, Chile (Loreto Formation) (Ortmann, Reference Ortmann and Scott1902, pl. 28, fig. 1; Fig. 7.1–7.4).
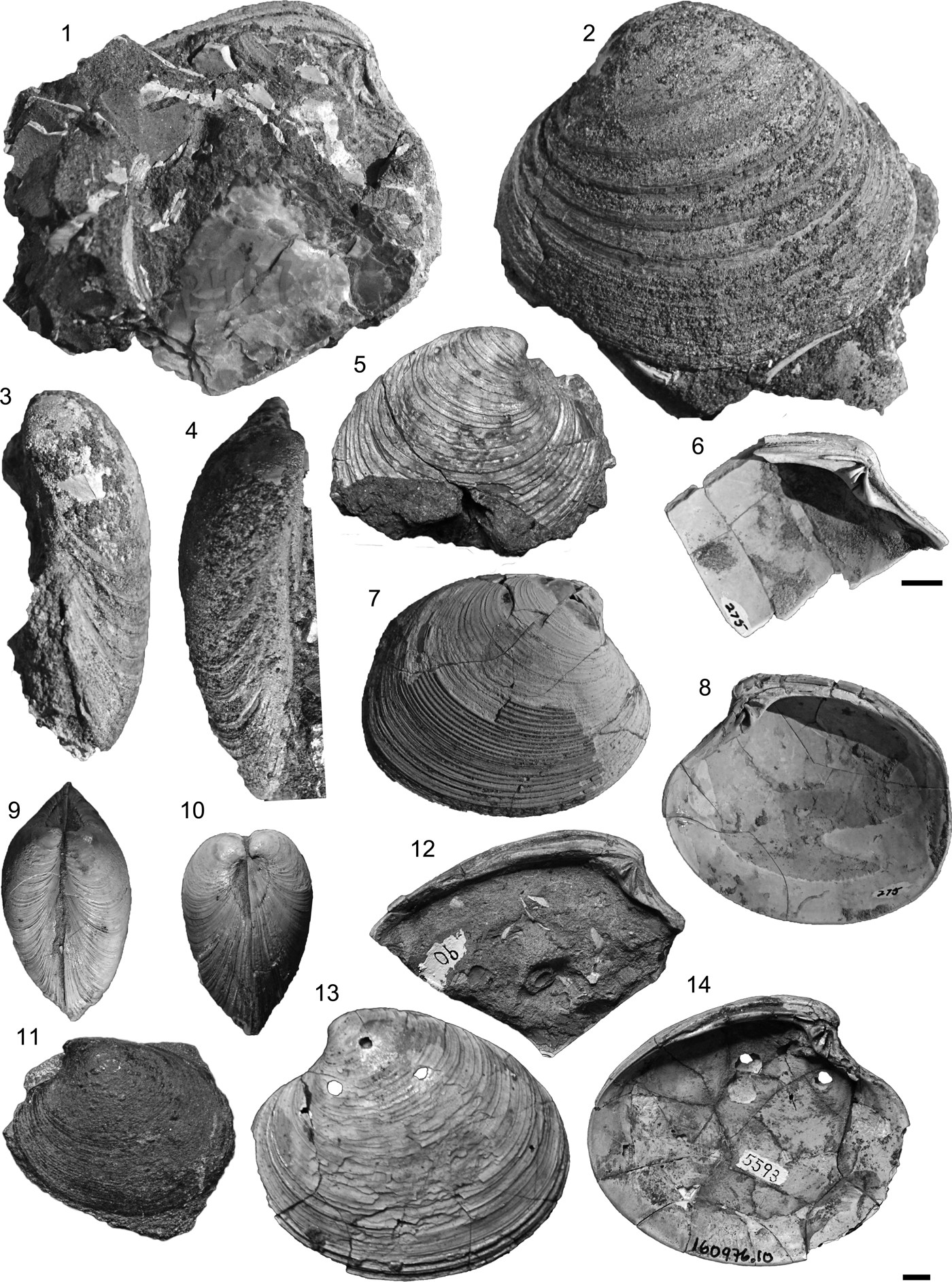
Figure 7. (1–4) Retrotapes difficilis (Ortmann, Reference Ortmann and Scott1902), PRI 72689 (Holotype) left valve, interior, lateral, anterior, and dorsal views (Punta Arenas, Loreto Formation). (5–11) Retrotapes navidadis (Philippi, Reference Philippi1887): (5) SGO.PI 134 (Holotype) right valve, lateral view (Navidad, Navidad Formation); (6–8) MACN-Pi 6355, (6) left hinge plate, (7) right valve, lateral view, (8) right valve, interior view (Navidad, Navidad Formation); (9, 10) MACN-Pi 6356, articulated specimen: dorsal and anterior views (Navidad, Navidad Formation); (11) SGO.PI 4292 (holotype of Eurhomalea? navidadiformis Frassinetti and Covacevich, Reference Frassinetti and Covacevich1999), lateral view (Pampa Castillo, Guadal Formation). (12–14) Retrotapes fuenzalidae (Philippi, Reference Philippi1887): (12) SGO.PI 90 (Paralectotype) left hinge plate (La Cueva, La Cueva Formation); (13, 14) SGO.PI 5593, left valve, lateral, and interior views (estero del Ganso, La Cueva Formation). Scale bar (1–14) 1 cm.
Diagnosis
Shell subtriangular to suboval shaped, umbo small and slightly curved, lunule slightly concave and bounded by a shallow groove.
Occurrence
Horizon III, Loreto Formation, late Eocene, Punta Arenas, Chile.
Description
Shell subtriangular to suboval shaped, convex, medium to large sized, longer than high. Umbo small, slightly curved, placed at anterior 0.25 of length. Anterior, dorsal, and ventral margins convex, posterior slightly convex. Lunule concave and bounded by a shallow groove. Escutcheon wide, wider in the left valve, with comarginal sculpture similar to that of rest of the shell. Nymph short and smooth. Hinge plate narrow, curved behind the cardinal teeth, which are not divergent and do not exceed the ventral margin of the hinge plate. Right hinge with tooth 3a slightly sloped backwards; tooth 1 thick and sloped backwards; tooth 3b thick, bifid and sub-horizontal. Left hinge with tooth 2a subtriangular, high, curved, and ventrally thickened; tooth 2b thick, rectangular, asymmetrically bifid and tilted backwards; tooth 4b lamellar, sub-horizontal, and separated from the nymph by a groove. Shell sculptured with fine comarginal ribs, which are closer to each other towards ventral margin of the disk.
Materials
Six specimens, MACN-Pi 432, PRI 66447, 66448, 72686, 72687, 72688 (Supplementary Data Set 1).
Measurements
Holotype PRI 72689: 71.35 mm length, 63.60 mm height.
Remarks
The specimens of the Ortmann and Ihering collections, from Punta Arenas, which are poorly preserved, are very similar to adult specimens of the Antarctic Eocene taxon Retrotapes robustus (Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992). Unfortunately the lack of inner characters, as well as complete hinge plates, did not allow determination of whether these two taxa are synonyms or not, so it has been decided to keep R. robustus as a valid taxon. Retrotapes difficilis would be the oldest species of the genus in the American Continent.
This taxon differs from R. antarcticus, R. newtoni (Eocene; Antarctica) and the South American species of the genus by its subtriangular shape, small umbo, and its slightly concave lunule, bounded by a shallow groove.
Retrotapes navidadis (Philippi, Reference Philippi1887)
Figure 7.5–7.11
- 1887
Venus navidadis Philippi, p. 120, pl. 14, fig. 4.
- 1887
Venus lamelligera Philippi, p. 121, pl. 14, fig. 6.
- 1907
Marcia navidadis; Ihering, p. 304.
- 1974
Venus navidadis; Frassinetti, p. 47, fig. 4.
- 1979
“Venus” (Marcia) navidadis; Tavera, p. 80, pl. 13, fig. 20.
- 1997
Retrotapes navidadis; del Río, p. 77.
- 1999
Eurhomalea? navidadiformis Frassinetti and Covacevich, p. 36, pl. 7, figs. 2, 3.
- 2006
Retrotapes navidadis; Frassinetti, p. 65, fig. 6.
- 2014
Retrotapes navidadis; Alvarez et al., p. 62.
Holotype
Venus navidadis Philippi, a right valve (SGO.PI 134) from Navidad (Navidad Formation, Chile) (Philippi, Reference Philippi1887, pl. 14, fig. 4; Fig. 7.5).
Emended diagnosis
Shell thin, subquadrate shaped, medium sized, sculptured with thin comarginal ribs.
Occurrence
Matanzas, Navidad, Rapel Norte, Punta Perro, (Navidad Formation, early Miocene, Chile), and Pampa Castillo (Guadal Formation, late Oligocene–early Miocene, Chile) and outcrops from Ipún and Crosslet islands (Chile).
Description
Shell thin, subquadrate shaped, medium sized. Umbo small, placed at anterior 0.25 of length. Dorsal margin slightly convex, posterior margin truncated, straight to slightly convex, ventral and anterior margins rounded. Lunule concave and bounded by a deep groove. Escutcheon narrow, wider in the left valve, with comarginal sculpture similar to that of rest of the shell. Nymph short and smooth. Hinge plate narrow, curved behind the cardinal teeth, which are not divergent and do not exceed the ventral margin of the hinge plate. Right hinge with tooth 3a lamellar, slightly sloped backwards or vertical; tooth 1 thin, slightly lower than tooth 3a, with a groove in its posterior area and tilted backwards; tooth 3b rectangular, thick, bifid and horizontal. Left hinge with all its cardinal teeth sloped backwards; tooth 2a triangular, thin, curved, higher than the other teeth, and ventrally thickened; tooth 2b thick, triangular, asymmetrically bifid; tooth 4b lamellar, sub-horizontal, and separated from the nymph by a groove. Dorsal-posterior region of right valve with a groove for the insertion of left valve. Adductor muscle scars isomyarian and deep; anterior pedal retractor scar placed below the anterior margin of the hinge plate and separated from the adductor muscle scar; posterior pedal retractor scar joined to the posterior adductor muscle scar. Pallial sinus short, with dorsal and ventral margins straight, dorsally oriented, with apex slightly sharpened. Shell sculptured with high comarginal ribs, which are closer to each other towards ventral margin of the disk, and with very fine radial ribs.
Materials
Thirty-five specimens, MACN-Pi 433, 6353–6357, SGO.PI 92, 99, 130, 5096–5103, 5475, 5572, 6148, 6156, 6165. One external cast, SGO.PI 4292 (Holotype, Eurhomalea? navidadiformis Frassinetti and Covacevich). Four internal casts SGO.PI 4413, 4342, 5144, 5145. Thirteen fragmented shells, SGO.PI 5572 (Supplementary Data Set 1).
Measurements
Holotype SGO.PI 134: 51.40 mm length, 43 mm height.
Remarks
The specimens studied and identified by Ortmann (Reference Ortmann and Scott1902, p. 141, pl. 27, fig. 12) as Venus navidadis Philippi (Reference Philippi1887) and later named by Ihering (Reference Ihering1907, p. 304) as Marcia ortmanni, belong to young specimens of Retrotapes striatolamellatus (Ihering, Reference Ihering1907) (del Río, Reference del Río1997).
Eurhomalea? navidadiformis Frassinetti and Covacevich (Reference Frassinetti and Covacevich1999, p. 36, pl. 7, figs. 2, 3) (Fig. 7.11) is considered as a junior synonymy of Retrotapes navidadis (Philippi, Reference Philippi1887) because it was erected based on external casts from Pampa Castillo, which have the same proportions, shape, lunule, and sculpture as those of the latter taxon. This assignment is doubtful because the lack of inner characters and because the Guadal Formation is supposed to be of Atlantic origin (Frassinetti and Covacevich, Reference Frassinetti and Covacevich1999; Encinas et al., Reference Encinas, Folguera, Bechis, Finger, Zambrano, Pérez, Bernabé, Tapia, Riffo, Buatois, Orts, Nielsen, Valencia, Cuitiño, Oliveros, De Girolamo Del Mauro, Ramos, Folguera, Contreras Reyes, Heredia, Encinas, Iannelli, Oliveros, Dávila, Collo, Giambiagi, Maksymowicz, Iglesia Llanos, Turienzo, Naipauer, Orts, Litvak, Alvarez and Arriagada2018), which would indicate a more southern distribution than previously known for the species.
The presence of Retrotapes navidadis in the outcrops of Crosslet Island (Frassinetti, Reference Frassinetti2006) extended its stratigraphic range from the late Oligocene–early Miocene to the middle Miocene–late Miocene.
Retrotapes navidadis differs from the rest of the subquadrate species of the genus (e.g., R. antarcticus, R. andrillorum, R. ninfasiensis, R. striatolamellatus, R. exalbidus) by its smaller size, thinner shells, and curved 2a tooth. Its subquadrate shape separates it from the subtriangular species (R. robustus and R. difficilis) and from the sub-rounded R. lenticularis.
Retrotapes fuenzalidae (Philippi, Reference Philippi1887)
Figure 7.12–7.14
- 1887
Venus fuenzalidae Philippi, p. 125, pl. 19, fig. 3.
- 1887
Venus colchaguensis Philippi, p. 122, pl. 17, fig. 4.
- 1969
Eurhomalea fuenzalidai; Herm, p. 128, pl. 12, figs. 15, 16.
- 1974
Eurhomalea colchaguensis; Frassinetti, p. 47.
- 2013
Retrotapes fuenzalidae; Nielsen, p. 52, pl. 9, figs. a–h.
- 2014
Retrotapes fuenzalidae; Alvarez et al., p. 64.
Syntype
Fragmented left valve (SGO.PI 90) from La Cueva (La Cueva Formation, Chile) (Philippi, pl. 19, fig. 3; Fig. 7.12).
Emended diagnosis
Shell medium sized, suboval in shape, thin, laterally compressed.
Occurrence
Pliocene beds of La Cueva and Estero del Ganso (La Cueva Formation, Chile) and Tubul (Tubul Formation, Chile).
Description
Shell thin, suboval, medium sized, laterally compressed. Umbo small, placed at anterior 0.25 of length. Dorsal, ventral, anterior, and posterior margins convex. Lunule slightly concave and bounded by a deep groove. Escutcheon wide, wider in the left valve, with comarginal sculpture similar to that of rest of the shell. Nymph short and smooth. Hinge plate wide, curved behind the cardinal teeth, which are not divergent and do not exceed the ventral margin of the hinge plate. Right hinge with tooth 3a lamellar, and sloped backwards; tooth 1 thin, tilted backwards; tooth 3b rectangular, thin, bifid and horizontal; dorsal-posterior region of right valve with a groove for the insertion of left valve. Left hinge with all its cardinal teeth sloped backwards; tooth 2a triangular, straight, thin; tooth 2b thick, triangular, asymmetrically bifid; tooth 4b lamellar, slightly curved, sub-horizontal, and separated from the nymph by a groove. Adductor muscle scars isomyarian and shallow; anterior pedal retractor scar placed below the anterior margin of the hinge plate and separated from the adductor muscle scar; posterior pedal retractor scar joined to the posterior adductor muscle scar. Pallial sinus short, linguiform. Shell sculptured with low comarginal ribs, which are closer to each other towards ventral margin of the disk.
Materials
Seven specimens, SGO.PI 120 (Holotype, Venus colchaguensis Philippi), SGO.PI 4863–4866, 5091, 5593. (Supplementary Data Set 1).
Measurements
SGO.PI 5593: 69.4 mm length, 28.1 mm height.
Remarks
This species is assigned to Retrotapes because of its wide escutcheon, wider in the left valve, lunule bounded by a deep groove, hinge plate wide, curved behind the teeth, which are tilted backwards.
The suboval shape of Retrotapes fuenzalidae distinguishes it subquadrate taxa (e.g., R. antarcticus and R. exalbidus), from the subtriangular taxa (R. robustus and R. difficilis) and from the sub-rounded R. lenticularis. Its shape and the presence of a slightly concave lunule make it very similar to R. fuegoensis del Río, Reference del Río1997 (Carmen Silva Formation, middle Miocene, Tierra del Fuego, Argentina), but its smaller size and 3a tooth strongly sloped backwards allows differentiation from them.
There is some controversy regarding the synonyms of this taxon. Herm (Reference Herm1969) validated the specific epithet fuenzalidai and Nielsen (Reference Nielsen2013) fuenzalidae. Here, the proposal of Nielsen (Reference Nielsen2013) was considered as correct, consequently the valid name of this taxon is Retrotapes fuenzalidae (Philippi, Reference Philippi1887).
Retrotapes exalbidus (Dillwyn, Reference Dillwyn1817)
Figure 8.1–8.5
- 1795
Venus exalbida Chemnitz, p. 225, pl. 202, fig. 1974 [not binomial].
- 1817
Venus exalbida Dillwyn, p. 170.
- 1842
Venus hanetiana d'Orbigny, p. 123, pl. 13, figs. 3–6.
- 1854
Venus subalbicans Hupé, p. 339.
- 1863
Venus exalbida; Reeve, p. 14, pl. 3, fig. 13.
- 1887
Venus subalbicans; Philippi, p. 122.
- 1887
Venus araucana Philippi, p. 117, pl. 17, fig. 6.
- 1887
Venus coquimbana Philippi, p. 125, pl. 19, fig. 2.
- 1887
Venus hupeana Philippi, p. 132, pl. 26, fig. 1.
- 1902
Marcia exalbida; Dall, p. 360.
- 1907
Marcia exalbida; Ihering, p. 297.
- 1938
Samarangia exalbida; Lamy and Fischer-Piette, p. 614.
- 1944
Samarangia exalbida; Carcelles, p. 287, pl. 12, figs. 93, 94.
- 1954
Eurhomalea exalbida; Keen, p. 54.
- 1957
Venus araucana; Tavera and Veyl, p. 170, pl. 4, fig. 13c.
- 1960
Eurhomalea exalbida; Powell, p. 182.
- 1969
Eurhomalea coquimbana; Herm, p. 127, pl. 12, fig. 9 (non figs. 10, 11).
- 1970
Samarangia exalbida; Castellanos, p. 250, pl. 22, figs. 4, 5.
- 1974
Eurhomalea araucana; Frassinetti, p. 47, figs. 1, 2.
- 1994
Eurhomalea exalbida; Ríos, p. 288, pl. 99, fig. 1412.
- 1995
Eurhomalea araucana; Frassinetti and Covacevich, p. 54, text-fig. 3c, pl. 1, fig. 18.
- 1997
Eurhomalea araucana; Frassinetti, p. 74, pl. 2, fig. 6.
- 1997
Retrotapes exalbida; del Río, p. 80, figs. 22–24.
- 2008
Retrotapes exalbidus; Griffin and Nielsen, p. 257, pl. 1, figs. 2–4, pl. 16, figs. 1–3.
- 2008
Retrotapes exalbidus; Nielsen and Valdovinos, p. 206, fig. 12.
- 2010
Eurhomalea exalbida; Huber, p. 373.
- 2014
Retrotapes exalbidus; Alvarez et al., p. 63. figs. 5.7–5.12.
- 2015
Eurhomalea exalbida; Forcelli and Narosky, p. 160.
Holotype
Venus exalbida Dillwyn (ZMUC-BIV-388) one left and one right valve, from Malvinas Islands (Recent) (Chemnitz, Reference Chemnitz1795, pl. 202, fig. 1974; Fig. 8.1–8.3).

Figure 8. (1–5) Retrotapes exalbidus (Dillwyn, Reference Dillwyn1817): (1–3) ZMUC-BIV-388 (holotype); (1) left valve, interior view; (2) right valve, interior view; (3) right valve, lateral view (Malvinas Islands, Argentina, Recent). (4, 5) MACN-In 21170, left and right hinge plate (San Matías Gulf, Argentina, Recent). (6–10) Retrotapes lenticularis (Sowerby, Reference Sowerby I1835), NHMUK 20160316/1-2 (syntype): (6) left valve, interior view; (7) right valve, interior view; (8) right valve, lateral view; (9, 10) left and right hinge plate (Valparaiso Bay, Chile, Recent). Scale bar (1–10) 1 cm.
Diagnosis
Shell subquadrate, thin; hinge plate thin; lunule bounded by a shallow groove; reduced fold groove in the posterior area of the right valve; pallial sinus triangular, with dorsal margin sub-horizontal to ventrally oriented.
Occurrence
Pliocene beds of Coquimbo, cerro Las Lomas, and La Cueva (Coquimbo and La Cueva Formation, Chile), and Plio-Pleistocene beds of Tubul Formation. Puerto San Julián (coastal ridges, Pleistocene, Argentina). Puerto Quequén, San Matías Gulf, Ushuaia, Puerto Deseado (Recent, Argentina).
Description
Shell thin, subquadrate shaped, large sized. Umbo small, placed at anterior 0.25–0.20 of length. Dorsal margin slightly convex, posterior margin truncated, straight to slightly convex, ventral and anterior margins rounded. Lunule concave and bounded by a shallow groove that is deeper through the ventral margin. Escutcheon narrow, wider in the left valve, with comarginal sculpture similar to that of rest of the shell. Nymph short and smooth. Hinge plate narrow, curved behind the cardinal teeth, which are not divergent and do not exceed the ventral margin of the hinge plate. Right hinge with tooth 3a lamellar, sub-vertical; tooth 1 thin, with the same height of tooth 3a, asymmetrically bifid with the posterior area larger than anterior, and sloped backwards; tooth 3b rectangular, thin, bifid and sub-horizontal; dorsal-posterior region of right valve with a groove for the insertion of left valve. Left hinge with all its cardinal teeth tilted backwards; tooth 2a triangular, thin, higher than the other teeth; tooth 2b thick, rectangular, asymmetrically bifid with larger posterior area; tooth 4b lamellar, slightly curved or straight, sub-horizontal, and separated from the nymph by a groove. Adductor muscle scars isomyarian and deep; anterior pedal retractor scar placed below the anterior margin of the hinge plate and separated from the adductor muscle scar; posterior pedal retractor scar joined to the posterior adductor muscle scar; 11–13 small pedal elevator muscle scars under the hinge plate. Pallial sinus short, triangular, with dorsal margin straight, horizontal or ventrally oriented, and ventral margin straight or slightly curved, with apex sharpened. Shell sculptured with high comarginal ribs, which are closer to each other towards ventral margin of the disk.
Materials
Two hundred twenty five specimens, MLP 26523, SGO.PI 122 (Syntype, Venus araucana Philippi), 114 and 125 (Syntypes, Venus araucana Philippi), 164 (Holotype, Venus coquimbana Philippi), 983, 1308–1310, 1317, 5009, 5088–5090, 5160, MACN-Pi 6302–6304, 6320, MACN-In 19822, 21069, 21170 (Supplementary Data Set 1).
Measurements
Holotype ZMUC-BIV-388: 76.2 mm length, 59.75 mm height.
Remarks
The synonymy proposed with Venus subalbicans Hupé (Reference Hupé and Gay1854, p. 339) and Venus araucana Philippi (Reference Philippi1887, p. 117, pl. 17, fig. 6) by Griffin and Nielsen (Reference Griffin and Nielsen2008) and Nielsen and Valdovinos (Reference Nielsen and Valdovinos2008) confirmed the presence of R. exalbidus in the Pliocene of the central region of Chile, nearby Concepción, which expanded its geographical and stratigraphical ranges.
Griffin and Nielsen (Reference Griffin and Nielsen2008, p. 257, pl. 1, figs. 2–4) also proposed a synonymy with Venus aerea Hupé (Reference Hupé and Gay1854, p. 338), but this taxon has a cancellate sculpture similar to that observed in the subfamily Chioninae (e.g., Ameghinomya chiloensis [Philippi, Reference Philippi1887]), which rejects the synonymy with R. exalbidus.
The specimens of Venus coquimbana Philippi (Reference Philippi1887, p. 125, pl. 19, fig. 2), as well as those described by Herm (Reference Herm1969, p. 127, pl. 12, fig. 9), are similar to R. exalbidus in shape, pallial sinus, hinge, and sculpture, and therefore V. coquimbana is considered here as a junior synonym of R. exalbidus. It is important to note that the hinge plates figured by Herm (Reference Herm1969, pl. 12, figs. 10, 11) have divergent teeth and very small and low umbones, which differ from those of V. coquimbana, but are very similar to that observed in Eurhomalea rufa. The synonymy proposed here extends the range of R. exalbidus to the north of Chile during the Pliocene.
D'Orbigny (Reference d'Orbigny1842) erected the species Venus hanetiana (Coquimbo and Horcón formations, Pliocene, Chile) based on internal casts that were assigned to the genus Retrotapes by Griffin and Nielsen (Reference Griffin and Nielsen2008). In these casts, two different morphotypes are recognized. One is mediolaterally wide, with well-developed muscle scars, and the other is mediolaterally compressed with shallow muscle scars. These two morphotypes are similar to those observed in R. exalbidus, in which flat and globoid morphotypes were also recognized (Alvarez and Pérez, Reference Alvarez and Pérez2016). This evidence, plus the presence of R. exalbidus in the same region, allows synonymy of V. hanetiana with R. exalbidus.
Retrotapes exalbidus differs from the other species of the genus by its thinner shell, shallower medial sulcus of the lunule, and shallower groove of the posterior area of the shell. Its subquadrate shape distinguishes it from the extant subcircular R. lenticularis, from suboval fossil taxa (R. newtoni, R. fuegoensis, R. fuenzalidae, and R. scutatus), and from subtriangular taxa (R. difficilis and R. robustus). Among the subquadrate taxa, it is more similar to the Antarctic species R. andrillorum Beu and Taviani (McMurdo Sound, Miocene) and R. antarcticus (Sharman and Newton, Reference Sharman and Newton1894) (La Meseta Formation, Eocene, Marambio Island), even sharing the same intraspecific variation discussed earlier with the latter species.
Retrotapes lenticularis (Sowerby, Reference Sowerby I1835)
Figure 8.6–8.10
- 1835
Venus lenticularis Sowerby, p. 42.
- 1887
Venus buchanani Philippi, p. 127, pl. 22, fig. 2.
- 1902
Samarangia lenticularis; Dall, p. 361.
- 1968
Eurhomalea salinensis Ramorino, p. 218, pl. 3, fig. 2, pl. 9, figs. 2, 3.
- 1969
Eurhomalea lenticularis; Herm, p. 128, pl. 13, figs. 1–4.
- 1997
Retrotapes lenticularis; del Río, p. 80, figs. 19–21.
- 2010
Eurhomalea lenticularis; Huber, p. 373.
- 2014
Retrotapes lenticularis; Alvarez et al., p. 64.
- 2014
Retrotapes salinensis; Alvarez et al., p. 64.
Syntype
One right and one left valve (NHMUK 20160316/1-2) from Valparaíso Bay (Recent, Chile) (Fig. 8.6–8.10).
Diagnosis
Shell thick, subquadrate to subcircular. Sculptured with comarginal ribs similar to other species of the genus, and with very fine radial ribs.
Occurrence
Pliocene beds of La Cueva (La Cueva Formation, early Pliocene, Chile), Caldera (Bahía Inglesa Formation, late Miocene–late Pliocene, Chile), Guayacán, Tongoy, and Quebrada de Chañaral (Coquimbo Formation, late Miocene–late Pliocene, Chile). Puerto San Antonio and Valparaíso (Recent, Chile).
Description
Shell thick, subquadrate to subcircular. Umbo small, placed at anterior 0.25 of length. Dorsal margin slightly convex, posterior slightly convex, ventral and anterior margins rounded. Lunule concave and bounded by a shallow groove that is deeper through the ventral margin. Escutcheon narrow, wider in the left valve, with comarginal sculpture similar to that of the rest of the shell. Nymph narrow and smooth. Hinge plate narrow, curved behind the cardinal teeth, which are not divergent and do not exceed the ventral margin of the hinge plate. Right hinge with tooth 3a lamellar, subvertical; tooth 1 thin, triangular, higher than tooth 3a, asymmetrically bifid with the posterior area larger than anterior, and sloped backwards; tooth 3b triangular, thick, bifid, and sub-horizontal; dorsal-posterior region of right valve with a groove for the insertion of left valve. Left hinge with all its cardinal teeth tilted backwards; tooth 2a triangular, thin, higher than the other teeth, and curved forward; tooth 2b thick, triangular, curved, asymmetrically bifid with larger posterior area; tooth 4b lamellar, slightly curved, sub-horizontal, and separated from the nymph by a groove. Adductor muscle scars shallow; anterior pedal retractor scar placed below the anterior margin of the hinge plate and separated from the adductor muscle scar; posterior pedal retractor scar joined to the posterior adductor muscle scar; up to 13 small pedal elevator muscle scars under the hinge plate. Pallial sinus short, triangular, with dorsal margin straight, ventrally oriented, and ventral margin curved, with apex rounded. Shell sculptured with low comarginal ribs, which are closer to each other towards ventral margin of the disk, and with radial ribs of nanometric thickness.
Materials
Forty valves, SGO.PI 158 and 166 (Syntotypes, Venus buchanani Philippi) 1022, 1094, 1115, 1118, 1123, 1144, 1233, 1256–1258, 1272, 1286, MACN-Pi 6358, MACN-In 12175, 12861. Four hinges SGO.PI 5593 (Supplementary Data Set 1).
Measurements
Syntype NHMUK 20160316/1-2: 77.23 mm length, 71.43 mm height.
Remarks
The current distribution of this taxon is between 24°S and 33°S on the coast of Chile (Bernard, Reference Bernard1983), but Paredes and Cardoso (Reference Paredes2003) published some small valves (7.4 mm length) from Independencia Bay (Perú; 14°S) as Retrotapes exalbidus, which were reassigned to R. lenticularis by Alvarez et al. (Reference Alvarez, del Río and Marenssi2014), which has expanded to the north the known distribution of this species. The specimens of Venus buchanani Philippi, Reference Philippi1887 (Guayacán, Coquimbo Formation) have exactly the same characters as R. lenticularis (Sowerby, Reference Sowerby I1835), and this species is therefore synonymized with it.
Ramorino (Reference Ramorino1968) erected a new taxon, Eurhomalea salinensis, to include some small shells from Valparaíso Bay, Chile. Alvarez et al. (Reference Alvarez, del Río and Marenssi2014) included this species in the genus Retrotapes del Río, Reference del Río1997 based on the characters described and illustrated by Ramorino (Reference Ramorino1968). A further revision of this species allowed it to be synonymized with R. lenticularis. As Ramorino (Reference Ramorino1968) mentioned, both species have exactly the same cardinal teeth, pallial sinus, and lunule bounded by a deep groove. The principal difference referred by the author to separate them is the larger shells of R. lenticularis, which also has a more concave lunule, but these characters probably indicate that R. salinensis is based on juvenile specimens of R. lenticularis. Similar differences are observed during the ontogeny of other species of Retrotapes, such as R. striatolamellatus, in which young specimens have a slightly concave lunule and the adults have the most concave lunule of the genus. Another difference is the sculpture of comarginal ribs in R. lenticularis, which is smooth in R. salinensis sensu Ramorino (Reference Ramorino1968), but, as the same author illustrated, the comarginal sculpture is clearly visible in it.
In order to have access to some pictures of the holotype of R. salinensis, Professor Bernardita Campos Maia from the Malacology Lab of the Valparaíso University where Dr. Ramorino worked was contacted. She sent some photos of specimens of that species from the personal collection of Dr. Ramorino without catalogue number. Moreover, through the contact with Dr. Ramorino himself, she confirmed (B. Campos Maia, personal communication, 2018) that the holotype of R. salinensis deposited in the Museum of Montemar, catalogue number 2716, has been lost.
Discussion
It is necessary to start the discussion talking about the phylogenetic position recovered for those taxa that were synonymized with Retrotapes by previous authors. As was previously mentioned, Lauriat-Rage et al. (Reference Lauriat-Rage, Carriol, Lozouet, Giret and Leyrit2002) synonymized Retrotapes del Río, Reference del Río1997 with Frigichione Fletcher, Reference Fletcher1938. In our analysis, the type species F. permagna (Tate, Reference Tate1900) was included, and in all the searches is recovered in a basal position, between the outgroup and Gomphina undulosa (Lamarck, Reference Lamarck1818). This result coincides with the systematic history of the genus Frigichione, which was originally included in Cyclininae Frizzell, Reference Frizzell1936, and later recovered as a basal Chioninae in the phylogenetic analysis performed by Harte (Reference Harte1998).
This background and the obtained results lead to rejection of the synonymy with Retrotapes, as proposed Alvarez et al. (Reference Alvarez, del Río and Marenssi2014), and that Frigichione is not a Tapetinae. This conclusion is reinforced by the position recovered for G. undulosa, which is the sister taxon of a clade that includes all the rest of the studied Tapetinae. This result agrees with that obtained by Mikkelsen et al. (Reference Mikkelsen, Bieler, Kappner and Rawlings2006) and Chen et al. (Reference Chen, Li, Kong and Zheng2011) based on molecular characters, in which G. undulosa was recovered as a Pitarinae Stewart, Reference Stewart1930. Thus, neither F. permagna nor G. undulosa can be considered as Tapetinae.
As was mentioned before, most of the species that are included now within Retrotapes were previously included within the genus Eurhomalea. In all the performed searches, there is no close relationship between Retrotapes and Eurhomalea. These results agree with those obtained on the geometric morphometric analysis of Alvarez et al. (Reference Alvarez, del Río and Marenssi2014). The genus Eurhomalea is only represented by its type species, E. rufa, which is closely related to Venerupis and Ruditapes.
The genus Retrotapes is a monophyletic group, which is closely related to Atamarcia Marwick, Reference Marwick1927 (Miocene, New Zealand) and Paleomarcia Fletcher, Reference Fletcher1938 (Miocene, Kerguelen Island), forming a major clade that also included Katelysia scalarina (Recent, South Australia), Paphia rotundata (Recent, Indian Ocean), and Marcia opima and Protapes gallus (Recent, Indo-Pacific Region).
Among the Retrotapes species, the suboval Eocene Antarctic R. newtoni, the most ancient species of the clade (Alvarez et al., Reference Alvarez, del Río and Marenssi2014), is recovered basal to the rest of the species, and other suboval and subtriangular species are successive sister taxa to a group of subquadrate-shaped ones (Fig. 5). With high values of k (high level of homoplastic characters), this subtriangular- and suboval-shaped species grouped together in a sister clade to the subquadrate taxa. The subquadrate group is divided into two lineages, one comprised of Miocene Patagonian species that includes the type species R. ninfasiensis (late Miocene, Chubut Province) and R. striatolamellatus (early Miocene, Santa Cruz Province) (Fig. 5), and is characterized by its strongly concave lunule and by having the most sloped backwards tooth 3a of all species. The other lineage includes taxa with slightly concave lunule and tooth 3a vertical or slightly slanted backwards. In this latter group most of the studied Chilean species are recovered, including R. lenticularis and R. exalbidus, which are closely related to the Miocene Chilean R. navidadis and the Eocene Antarctic R. antarcticus (Fig. 5). These results contradict the proposal of Huber (Reference Huber2010) in which the mentioned extant taxa are assigned to Eurhomalea and the genus Retrotapes is considered valid only as a fossil taxon. In addition, the close relationship between R. exalbidus and R. antarcticus was discussed by Alvarez and Pérez (Reference Alvarez and Pérez2016) who studied the similarities between the two morphotypes that these species shared.
Based on its paleontological record, the genus Retrotapes possibly has its origin in the Eocene of Antarctica, where it is represented by three species: R. newtoni, R. antarcticus, and R. robustus. This last taxon has many characters (e.g., shape, hinge plate, lunule) in common with the late Eocene Chilean R. difficilis (Loreto Formation, Punta Arenas), which is included in the genus in the present contribution and is the most ancient record for the genus in southern South America. From this extreme Austral region, the genus diversified along both the Atlantic and Pacific coasts of the South American Continent. On the Atlantic side, it is represented by R. navidadis (late Oligocene–early Miocene, Pampa Castillo), R. scutatus and R. striatolamellatus (early Miocene, Santa Cruz Province), R. fuegoensis (middle Miocene, Tierra del Fuego Province), and R. ninfasiensis (late Miocene, Chubut Province). On the Pacific side, it is represented by R. navidadis (early Miocene, Navidad), R. fuenzalidae (Pliocene, La Cueva, Estero del Ganso and Tubul), and by the two extant taxa. Retrotapes lenticularis was present in the Pliocene beds of the La Cueva and Coquimbo formations in central and northern Chile, and today inhabits the seashore from this zone reaching to southern Perú. In this contribution, new synonyms are assigned to Retrotapes exalbidus, extending its previously known Pliocene record from central to northern Chile (Coquimbo, La Cueva, and Tubul formations); this taxon is extinct in these areas today, and its distribution is restricted from Chiloé Island (South of Chile) to Rio Grande do Sul (South of Brazil).
Conclusions
The genus Retrotapes del Río, Reference del Río1997 is a monophyletic group and is not closely related to Frigichione Fletcher, Reference Fletcher1938 and Eurhomalea Cossmann, Reference Cossmann1920, which rejects the synonymies proposed by some previous authors. Retrotapes is closely related to other Austral taxa, such as Paleomarcia Fletcher, Reference Fletcher1938 (Kerguelen Island), Atamarcia Marwick, Reference Marwick1927 (New Zealand), and Katelysia Römer, Reference Römer1857 (South of Australia).
Retrotapes was represented in Chile by three species: R. difficilis (late Eocene), R. navidadis (late Oligocene–early Miocene), and R. fuenzalidae (Pliocene). At present times, it is represented by two species, R. exalbidus (Pliocene–Recent), and R. lenticularis (Pliocene–Recent).
The synonymies proposed in the present contribution expand the known geographic distribution of R. exalbidus during the Pliocene through the north of Chile. Moreover, the validity of R. salinensis (Ramorino, Reference Ramorino1968) is rejected by considering it as a juvenile of R. lenticularis.
The phylogenetic position here recovered for R. exalbidus and R. lenticularis confirmed that these two extant taxa belong to Retrotapes, as opposed to the assignments and proposals of Huber (Reference Huber2010).
Acknowledgments
I especially thank C.J. del Río for her assistance, patience, and helpful comments and corrections regarding my PhD Thesis. The author is indebted to the curators who facilitated access to paleontological and biological collections: C.J. del Río and M. Longobucco (MACN-Pi and exCIRGEO-PI), A. Tablado and M. Romanelli (MACN-In), M. Tanuz (CPBA), C. Amenabar (IAA-Pi), A. Riccardi (MLP), and C. Salazar and S. Soto (SGO.PI). I also want to thank those curators from several collections that sent me pictures: A. Salvador (NHMUK), G. Dietl (PRI), C. Franzén-Bengtson and J. Hagström (PZ-NRM Mo), S. Hannam (MA), M. Binnie (T), J. Gerber (FMNH), T. Schiøtte (ZMUC-BIV), and J. Trausel and F. Slieker (NMR). I thank M.B. von Baczko, who improved the language. I especially thank S. Nielsen and A. Beu for their helpful comments and suggestions as reviewers, and to J. Jin for his suggestions as editor that improved this work. The use of TNT software was facilitated by the Willi Hennig Society. CONICET is acknowledged for the post-graduate grant given to me. This research was also supported by ANPCyT-PICT 57.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.dv15kp5










