Introduction
Sponges are filter-feeding basal organisms with a long evolutionary history (convincingly documented from lower Cambrian to recent) and are regarded by many scientists as one of the most primitive living animals. These sessile benthic organisms with rapid larval settlement are recognized as dynamic multicellular systems capable of living in various types of aquatic environments, usually comprising a significant portion of benthic communities (Bond, Reference Bond1992; Carballo and Bell, Reference Carballo, Bell, Carballo and Bell2017). They can help build hard substrates for benthic communities and can positively influence the levels of primary production and nutrition cycles (Jiménez and Ribes, Reference Jiménez and Ribes2007; De Goeij et al., Reference De Geoeij, Moodley, Houtekamer, Carballeira and van Duyl2008; Mohamed et al., Reference Mohamed, Saito, Tal and Hill2010; Carballo and Bell, Reference Carballo, Bell, Carballo and Bell2017).
Their inhabiting ability, even in disturbed environments, as well as their conservative features that favor migration and reduce speciation, allows the poriferans to be considered potential tools for paleoecology and paleobiogeography analysis (Bond, Reference Bond1992; Carrera and Rigby, Reference Carrera and Rigby1999).
Many authors have been able to identify paleoecology and paleobiogeography patterns, even with considerable uncertainties, such as limited resolution, differential quality of preservation, and phylogenetic affinities (see Webby, Reference Webby1980; Rigby and Webby, Reference Rigby and Webby1988; Rigby and Chatterton, Reference Rigby and Chatterton1989; Carrera and Rigby, Reference Carrera and Rigby1999; Delecat et al., Reference Delecat, Arp, Reitner, Reitner, Queric and Arp2011; Ritterbusch et al., Reference Ritterbusch, Bottjer, Corsetti and Rosas2014, Reference Ritterbusch, Rosas, Corsetti, Bottjer and West2015; Li et al., Reference Li, Feng, Janussen and Reitner2015; Botting et al., Reference Botting, Zhang and Muir2017; Muir et al., Reference Muir, Botting, Beresi, Carballo and Bell2017).
When those uncertainties are reduced, it is possible to elucidate some generalized patterns of sponges, as noted by Muir et al. (Reference Muir, Botting, Carrera, Beresi, Harper and Servais2013) regarding the glaciation effects on shallow-water sponges during the Paleozoic. In addition, with the identification of distribution and migration patterns, one can infer oceanic currents, paleogeographic dispositions, and climatic features (Carrera and Rigby, Reference Carrera and Rigby1999). According to Carrera and Rigby (Reference Carrera and Rigby1999), paleobiogeographic studies of Paleozoic sponges are not very common. Here, we describe a new species of the genus Teganiella, Teganiella finksi n. sp., found in Pennsylvanian black shale of the Mecca Quarry in the United States of America. This discovery expands the chronologic distribution and allows us to classify the genus as an endemic group of Laurentia in low latitudes associated with arid climate conditions linked to local hypersaline periods. Our interpretation also suggests that the unknown Form A of Court Creek, Illinois, described by Rigby and Bitter (Reference Rigby and Bitter2005) is a poorly preserved Teganiella finksi.
Geologic setting
The Pennsylvanian system in eastern Indiana is restricted to the Carbondale Group, which ranges in thickness from 79 m to 143 m but averages slightly more than 91 m. It is subdivided into the Linton, Petersburg, and Dugger formations (Burger and Wier, Reference Burger, Wier and Shaver1970, Reference Burger, Wier and Shaver1986). The Linton Formation is the lowermost unit in the Carbondale Group, and deposits attain 24 m in thickness. This formation is subdivided into five members: Coxville Sandstone, Colchester Coal, Mecca Shale, Velpen Limestone, and Survant Coal. Another undefined unit is composed of shale, clay, and sandstone. This formation extends along the eastern edge of the Illinois Basin (Fig. 1).
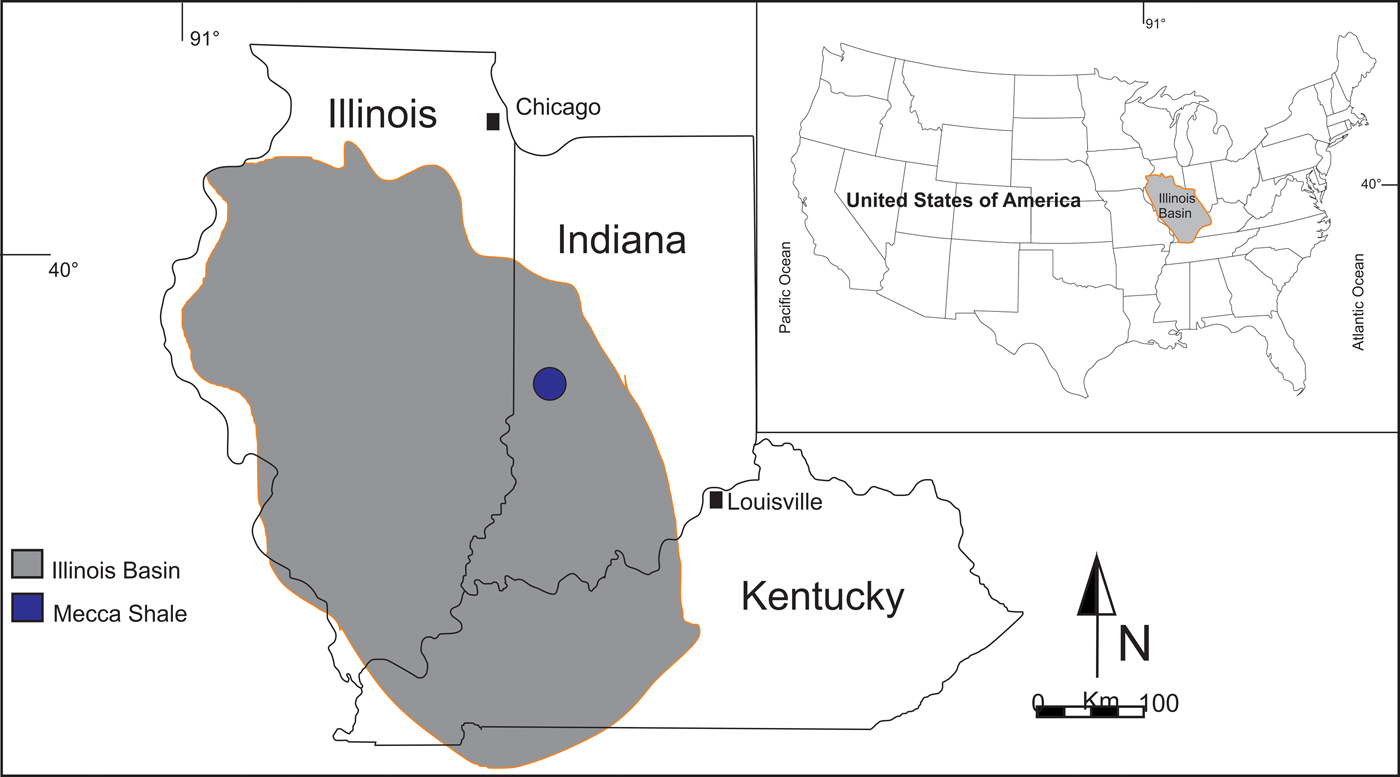
Figure 1. The locality of the Mecca Shale Member of the Linton Formation, USA. The map was drawn using Corel Draw x7.
The Mecca Quarry (MQ) Shale, which overlies the Colchester Coal Member, is a fossiliferous bedded sheety shale (0.3 m to 2.1 m thick) rich in heavy metals such as vanadium, zinc, and molybdenum (Woodland, Reference Woodland, Zangerl and Richardson1963; Vine, Reference Vine1966; Coveney, Reference Coveney1977). The type section is exposed at 0.8 km southeast of Mecca, Parke County, Indiana, and has been subdivided into four levels (A, B, C, D; Fig. 2). It is considered one of the most widespread black shales of the Pennsylvanian age in the United States midcontinent, ranging from Illinois to Indiana, and is correlated with the black shale of the Verdigris Formation and its equivalents in Iowa, Nebraska, Oklahoma, Kansas, and Missouri (Zangerl and Richardson, Reference Zangerl and Richardson1963; Wanless and Wright, Reference Wanless and Wright1978; Fig. 1). The shale often shows sheets thinner than a millimeter (Wier, Reference Wier1950, Reference Wier1965). Zangerl and Richardson (Reference Zangerl and Richardson1963) observed that the MQ has a great fossil content known as ‘Mecca Fauna,’ which is not equally distributed and well preserved throughout the layers. The Mecca Fauna is composed of marine or at least brackish water animals, comprising invertebrates such as Porifera; articulate and inarticulate Brachiopoda; Mollusca (Cephalopoda and Pelecypoda); Annelida; Arthropoda (Crustacea and Echinodermata); and vertebrates (Acanthodii and Elasmobranchii). It also has plant remains previously identified as Neuropteris sp., Omphalophloios cyclostigma White, Reference White1898, Syringodendron sp., Calamites sp., and seaweeds (see Zangerl and Richardson, Reference Zangerl and Richardson1963).

Figure 2. Lithologic section of Mecca Quarry Shale Member with A, B, C, D layers, Linton Formation, Indiana. Outcrop picture taken by Zangerl and Richardson (Reference Zangerl and Richardson1963).
Two models of paleoenvironments have been proposed for the MQ. The first characterized it as an offshore deepwater model, as observed in other shales of midcontinent Pennsylvanian cyclothems (Evans, Reference Evans1967; Heckel, Reference Heckel1977). However, Zangerl and Richardson (Reference Zangerl and Richardson1963) conducted a major paleoecologic study in the Mecca Quarry and by combining the shale description (including geochemical analyses) with the fossil content, they assigned a shoreline lagoon with marine influence model, as seen in the southwest of the Florida Peninsula. The detailed geochemical studies of heavy metals of the MQ conducted by Coveney and Martin (Reference Coveney and Martin1983) also corroborate the paleoenvironment proposed by Zangerl and Richardson (Reference Zangerl and Richardson1963). Considering the context and the presence of reticulosan sponges, we consider the paleoenvironment to be shoreline lagoon with a very brief marine incursion.
The sponge samples analyzed in this work came from levels B and D of the outcrop along the side of a small ravine approximately 150 meters north-northeast of the Mecca Shale Member type section near the Rockville-Clinton highway (U.S. 41; Fig. 2).
Materials and methods
In 1960, Rainer Zangerl and Eugene Richardson conducted a large and very detailed paleoecological study of Pennsylvanian black shale of the Logan and Mecca Quarries of eastern Indiana, collecting over 16.7 m2 of these shales and reassembling them at the Field Museum of Natural History in Chicago. In 1963, Matthew Nitecki, an invertebrate paleontology curator at the Field Museum, identified 43 samples resembling sponges from Mecca Shale. He sent 34 of these samples to the American Museum of Natural History paleospongiologist Robert Finks, who identified the samples as possible sponges and stored them.
We reanalyzed all 43 samples and selected six well-preserved samples to describe the species. The specimens were measured using an electronic caliper associated with an Olympus SZ51 stereomicroscope. The photographs were taken with a Canon EOS Rebel T2i digital camera. The sponge body and spicules were analyzed using an environmental scanning electron microscope (ESEM) Philips XL30 housed at the Faculty of Earth Sciences in Sosnowiec, Poland.
Aiming to delimit the spicule composition, we also performed energy dispersive X-ray spectroscopy (EDS) at Centro de Microscopia Eletrônica (CME), Federal University of Paraná and at the Federal University of Rio Grande do Sul.
Repository and institutional abbreviation
Specimens used in this study are housed in the American Museum of Natural History (AMNH), New York.
Systematic paleontology
Phylum Porifera Grant, Reference Grant and Todd1836 Class uncertain ‘Reticulosa’ sensu Botting and Muir, Reference Botting and Muir2013
Remarks
Erected by Reid (Reference Reid1958) as an extinct group of the Hexactinellida class and designated by Finks and Rigby (Reference Finks, Rigby and Kaessler2004) as an order. According to Finks and Rigby, reticulosans are thin-walled sponges composed of stauractin, pentactins or hexactins, and paraclavules. However, Botting and Muir (Reference Botting and Muir2013) recognized Reticulosa as a paraphyletic group, being at least a stem group of Hexactinellida, Silicea, and Demospongiae. For this paper, we follow the classification of Botting and Muir (Reference Botting and Muir2013).
Family Teganiidae de Laubenfels, Reference de Laubenfels and Moore1955 Genus Teganiella Rigby, Reference Rigby, Dutro and Pfeffertkorn1986
Type species
Teganiella heathi Rigby, Reference Rigby, Dutro and Pfeffertkorn1986 by original designation. Bashkirian, United States of America.
Teganiella finksi new species Figures 3–7

Figure 3. Pennsylvanian Teganiella finksi n. sp. (1) AMNH 84173 and (4) AMNH 84181, well-delimited specimens with poorly preserved spicules; pyritized complete sponge with lower portion composed mainly of minor stauractin; (2) AMNH 84194 and (3) AMNH 84176, sponge with pores. Scale bars = 10 mm. Red arrows mark the pores.

Figure 4. Holotype of Teganiella finksi n. sp., AMNH 84179. (1) Completed and well-defined sponge with possibly a flat osculum on top; (2) details of the triaxon hexactin; (3) the lower portion of the sponge formed by hexactin and minor stauractin. (1) Scale bar = 10 mm; (2, 3) scale bars = 1 mm. Red arrows = hexactins; green arrows = autodermalia; gray arrow = stauractin.
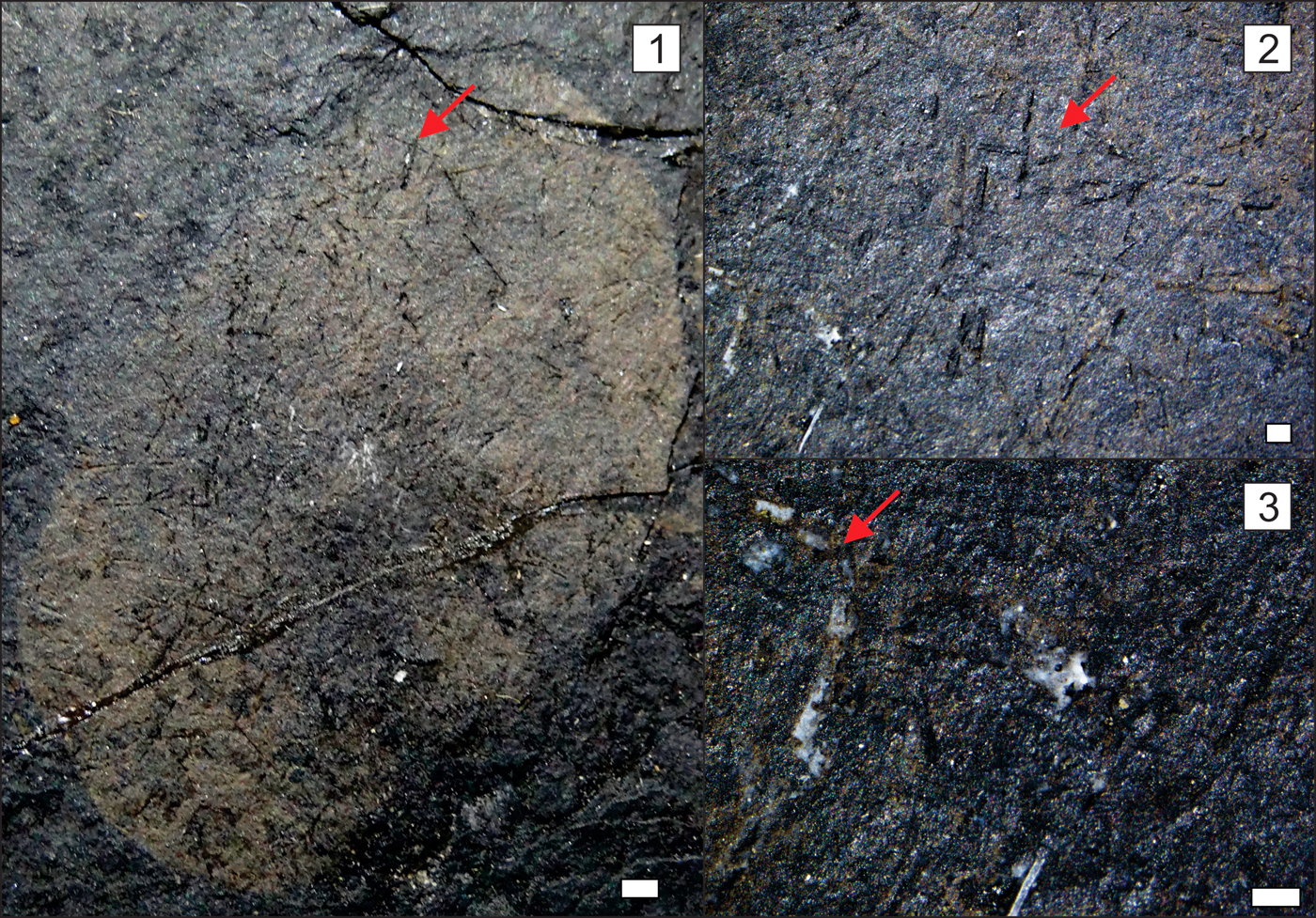
Figure 5. Paratype of Teganiella finksi n. sp., AMNH 84172. (1) Complete sponge with isolate spicules; (2, 3) details of the triaxon hexactin and tiny spicules of dermalia. (1) Scale bar = 1 mm; (2) scale bar = 250 µm; (3) scale bar = 200 µm. Red arrows = hexactins.

Figure 6. Paratype of Teganiella finksi n. sp., AMNH 84174. (1) Well-defined sponge with external layer preserved and isolate spicules imbibed. It is also possible to observe the dermal and gastral layer; (2) details of the spicules imbibed on the layer; (3) details of the triaxon hexactin and diactins. (1) Scale bar = 1 mm; (2, 3) scale bars = 200 µm. Red arrows = hexactins; green arrow = possible gastral layer; blue arrow = diactins.
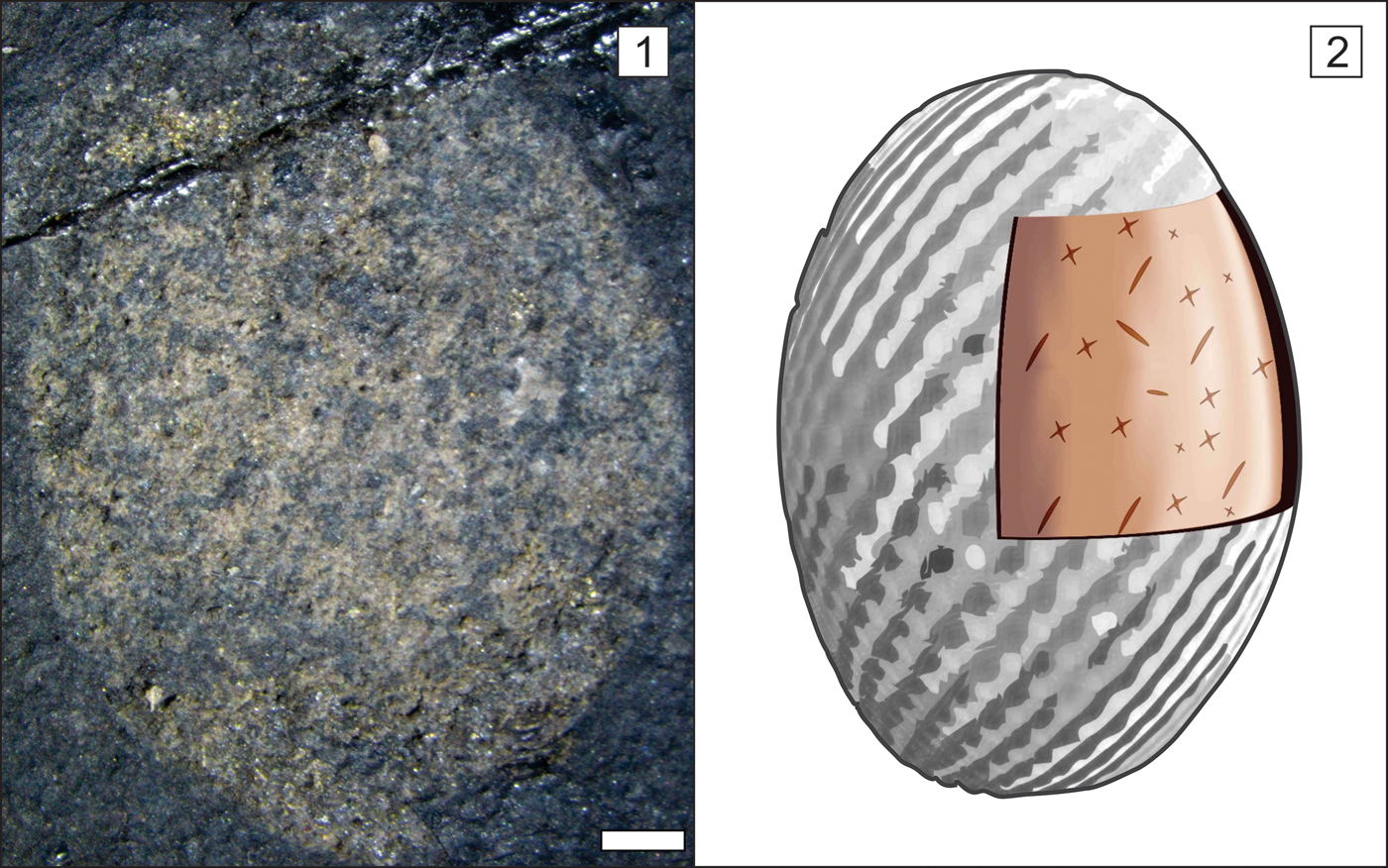
Figure 7. Teganiella finksi n. sp. (1) Paratype AMNH 84177, rounded specimen with isolated hexactin in the lower portion. (2) Reconstruction of Teganiella finksi n. sp., marking the osculum absence, the random porous presence, hexactins, stauractins, and diactins. Scale bar = 1 mm.
Holotype
AMNH 84179, well-delimited ovoid sponge with diactins, hexactins, and small openings preserved; upper Moscovian, Mecca Shale, Linton Formation, Indiana, United States of America.
Diagnosis
Small, coarse Teganiella sponge with numerous ostia in the dermal layer. Spicules are small triaxons, mainly hexactin with no arrangement. Diactin and possible stauractin are also present.
Occurrence
The type section is exposed at 0.8 km southeast of Mecca, Parke County, Indiana.
Description
Sponges are small, flat, rounded to vase shaped, measuring 9 mm to 50 mm in height and 8 mm to 29 mm in width (Figs. 3–7). Coarse openings with no regular pattern and diameters of 0.05–0.07 mm are observed (Fig. 3.2, 3.3); however, these openings can be a taphonomical artifact. Almost all patches of spicules are triaxon hexactins preserved in 3D and/or as impressions, with rays 0.1–0.2 mm long and 0.02 mm in diameter (Fig. 4.2). However, diactins with rays 0.2–0.3 mm long and 0.02 mm in diameter and perhaps minor stauractins have also been found (Fig. 4.3). Spicules are present but difficult to identify due to a film that obscures the skeletal details. Most of the specimens are higher than they are wide, and osculum delimitation is not visible. No microscleres were found. The holotype AMNH 84179 (Fig. 4.1–4.3) is approximately 39 mm high and 25 mm wide. It has an ovoid profile with well-defined margins with patches of hexactins in autodermalia with rays that are 0.1 mm long and have diameters of 0.02 mm. On the upper edge, small openings are presented. We also observed fragments of diactins, most likely rhabdodiactins, with rays 0.05 mm long. However, these megascleres may be a taphonomic artifact because the other types of spicules are intact. The paratypes are AMNH 84172, 84174, and 84177. The first, AMNH 84172, is 23.07 mm high and 14.36 mm wide with well-defined margins and numerous diactin impressions (probably a taphonomical artifact; Fig. 5.1–5.3). The diactin rays vary from 0.25 to 1.08 mm long and 0.12 to 0.18 mm in diameter. Fewer tiny impressions of pores and hexactins or possible stauractins are also observed. The paratype AMNH 84174 has well-defined margins and is 19.67 mm high and 20.05 mm wide, with numerous loose diactins (rays varying from 0.19 to 1.03 mm long and from 0.017 to 0.106 mm in diameter; Fig. 6.1–6.3). In this specimen, tiny hexactins with rays varying from 0.17 to 0.28 mm and smaller pores with diameters varying from 0.07 to 0.09 mm are observed. The last paratype, AMNH 84177, is the smallest specimen with a well-defined margin and few impressions of diactins, hexactins, and ostia (Fig. 7). The specimen is 10.51 mm high and 8.31 mm wide. All the other samples, despite having well-defined margins and few diactins, hexactins, and ostia, are poorly preserved.
Etymology
In honor of Robert Finks, the renowned paleospongiologist of the American Museum of Natural History.
Taphonomy
Considering the description of Zangerl and Richardson (Reference Zangerl and Richardson1963), Teganiella finksi n. sp. is rare, isolated, parallel to the bedding planes, and assigned as parautochthonous. Sponges were intensively affected by diagenesis, showing two distinct modes of preservation. The majority have a prominent and coarse dermal layer with numerous openings, obscuring the internal skeletal structure, while the other specimens show well-defined hexactins and diactins. These two modes of preservation had already been identified by Rigby (Reference Rigby, Dutro and Pfeffertkorn1986) in the original description of the genus Teganiella heathi; however, the majority of the Rigby (Reference Rigby, Dutro and Pfeffertkorn1986) specimens were well defined, with spicules and oscula on the upper edge. The occurrence of two patterns of preservation shared by the species in distinct paleoecologic settings can be only a taphonomic bias related to a regional factor. The skeleton patterns obscured by an oxide film in dysaerobic conditions are normally related to the preservation of pyritized soft tissues and spicules. At least until now, no soft tissues have been recognized in any species. No taphonomic information is provided by Rigby and Mehl (Reference Rigby and Mehl1994) in the description of Teganiella ovata.
Energy-dispersive X-ray spectroscopy
Figures 8 and 9 show EDS spectra recorded from Teganiella finksi n. sp. from seven distinct samples, which are mainly enriched in zinc (Zn), oxygen (O), silica (Si), aluminum (Al), and sulfur (S). The sponge body and spicules are mainly composed of aluminosilicates. However, in most cases, the spicules were diagenetically transformed into ZnS (sphalerite). Zn and other elements, such as molybdenum (Mo), chromium (Cr), vanadium (V), nickel (Ni), and copper (Cu), occur in high concentrations (>1,000 ppm) in Mecca Shale (see Zangerl and Richardson, Reference Zangerl and Richardson1963). Sphalerite replacement of spicules has not been described, and combined with others, heavy metals can corroborate the shoreline lagoon with marine influence (e.g., Coveney and Martin, Reference Coveney and Martin1983; Wilkin and Barnes, Reference Wilkin and Barnes1997). The presence of ZnS is common in shale-hosted massive sulfide deposits (see Magnall et al., Reference Magnall, Gleeson, Stern, Newton, Poulton and Paradis2016). In a particular sample, the presence of BaSO4 (barite) was detected, a curious fact because it may register the biochemical activities of the sponge (see Ilan et al., Reference Ilan, Gugel and Van Soest2004; Keren et al., Reference Keren, Mayzel, Lavy, Polishchuk, Levy, Fakra, Pokroy and Ilan2017). However, considering the chemically unusual sediment of Mecca Shale, we believe that it is only a taphonomic artifact.
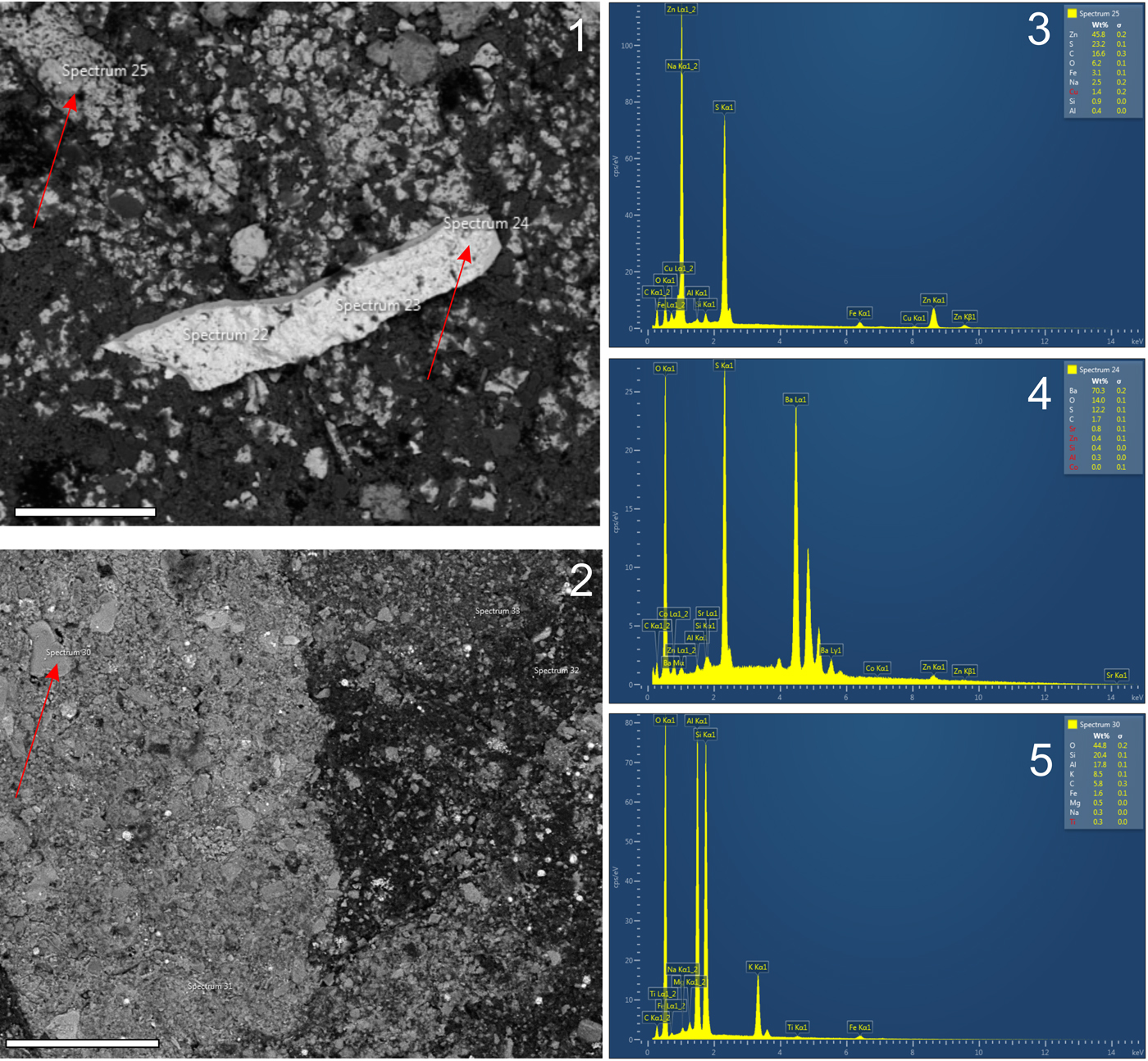
Figure 8. (1, 2) Environmental scanning electron microscope (ESEM) photomicrographs. (3–5) Energy-dispersive X-ray spectroscopy (EDS) spectra showing the elemental composition of the spicules (spectra 25) and sponge body (spectra 24 and 30). Scales bars = 100 µm.

Figure 9. Elemental composition map of Teganiella finksi n. sp. (sample AMNH 84179), showing a predominance of aluminum and silica. (1) Scale bar = 500 µm; (2–7) scale bars = 800 µm.
Remarks
The genus Teganiella comprises two species: Teganiella heathi Rigby, Reference Rigby, Dutro and Pfeffertkorn1986 (type species, from early Pennsylvanian [Bashkirian], Heath Formation, Montana) and Teganiella ovata Rigby and Mehl, Reference Rigby and Mehl1994 (from Middle Devonian, Red Hills [RH], Nevada). These two species are small, globular, or egg shaped with a skeletal net composed of thin, delicate hexactins or hexactine-derived spicules (as stauractins). Long rays, possible rhabdodiactins, and small pores are also presented. According to Rigby and Mehl (Reference Rigby and Mehl1994), Teganiella ovata has root tufts; however, the specimens vertically and diagonally flattened do not show such a structure. Teganiella heathi is up to 18 mm across with a skeletal net formed by thin hexactins (rays 0.8–1 mm long) and small pores of 0.3–0.7 mm in diameter, while Teganiella ovata is 23 mm high and 16 mm wide with a skeleton of hexactin or hexactin-derived spicules (stauractin; rays 0.4–0.5 mm) and the wall perforated by small pores (0.3–0.4 mm in diameter). Teganiella finksi n. sp. differs from Teganiella heathi and Teganiella ovata in size range of the overall body (from 11 to 36 mm high and 8 to 25 mm wide), in length of hexactin rays (0.1–0.2 mm), and in diameter of dermal openings (0.05–0.07 mm). Rigby and Bitter (Reference Rigby and Bitter2005) reported the occurrence of reticulosan sponges and unknown fossils assigned as Form A from the Pennsylvanian black shale in Court Creek, Illinois. These unknown specimens, 8–9 mm in diameter, were flattened, with an outer skeletal layer with irregularly placed ostia. Rigby and Bitter (Reference Rigby and Bitter2005) described them as a possible sponge with finer inhalant and coarser exhalant openings; however, due to uncertainty (e.g., lack of spicules), the researchers were prevented from ratifying the specimens as sponges. Considering the reported characteristics, even without the spicules preserved, we suggest that the sponge-like Form A described by Rigby and Bitter (Reference Rigby and Bitter2005) from Knox County may also be Teganiella finksi n. sp., though poorly preserved. This finding may also contribute to the possible consideration of Pennsylvanian black shale in Court Creek, Illinois, as an extension of Mecca Quarry Shale because its fossil content is similar, for example, the presence of conodont Gondolella pohli von Bitter and Merril (Reference von Bitter and Merrill1998) and Teganiella finksi n. sp.
Discussion
Paleoecology
The history of the genus Teganiella, which is now composed of three species, began during the Givetian (Middle Devonian, Denay Limestone Formation; Fig. 10), followed by Serpukhovian (Mississippian, Heath Formation); the species described here is from the Middle Pennsylvanian Mecca Shale and possibly from the Pennsylvanian black shale in Court Creek, Illinois. All species of Teganiella can be defined as parautochthonous because they did not show any signs of significant transportation. The specimens described here are moderately preserved without osculum, a root tuft, or a clear spicule pattern.

Figure 10. Teganiella species and their distinct preservation. (1) Teganiella ovata Rigby and Mehl (Reference Rigby and Mehl1994); (2) Teganiella heathi Rigby (Reference Rigby, Dutro and Pfeffertkorn1986); (3) Teganiella finksi n. sp. Photographs 1 and 2 are courtesy of Mark Florence and Kallie Moore, respectively. Scale bars = 1 cm.
The oldest species, Teganiella ovata (Fig. 10), was identified by Rigby and Mehl (Reference Rigby and Mehl1994) in the Middle Devonian dolomitic lime mudstone in Red Hills (RH), Nevada. According to Schultze (Reference Schultze2010), the RH comprises unusual Middle Devonian fauna with fishes, sponges, conulariids, bivalves, ammonoids, brachiopods, and echinoderms. This fauna lived in shallow, open marine water with a high terrestrial contribution located approximately 28°S latitude under arid climatic conditions (Scotese, Reference Scotese2001). After fishes, sponges are the most common fossil, comprising sixteen different forms. Among these forms, specimens from the family Hintzespongiidae Finks, Reference Finks and Broadhead1983 (Cyathophycus simpsonenis Rigby and Mehl, Reference Rigby and Mehl1994) and Teganiidae (Bulbospongia sp., Rufuspongia sp., and Taleolaspongia) occur associated with Teganiella ovata (see Rigby and Mehl, Reference Rigby and Mehl1994). According to the latter authors, Teganiella ovata specimens were preserved flattened as complete individuals, isolated or arranged in pairs, concordant to bed plane. As with fishes and conulariids, the sponges were substituted by hematite. Schultze (Reference Schultze2010) suggested the presence of dysaerobic conditions, perhaps hypersaline conditions, and rapid burial during the preservation process at RH. He also believed that the sponges preserved there were endemic at the species and/or genus level.
The first recognized teganiellid sponge was the Late Mississippian Teganiella heathi (Fig. 10) described by Rigby (Reference Rigby, Dutro and Pfeffertkorn1986) from the Serpukhovian Bear Gulch (BG) limestones of the Heath Formation, Montana. The Bear Gulch Lagerstätte preserves a highly productive tropical marine bay that was deposited at approximately 12°N latitude and experienced monsoonal climatic conditions (Witzke, Reference Witzke, McKerrow and Scotese1990; Grogan and Lund, Reference Grogan and Lund2002; Lund et al., Reference Lund, Greenfest-Allen and Grogan2012). The paleoenvironment assigned to this formation consisted of shallow marine and brackish water shales and limestone lenses of less turbid or deeper water (Grogan and Lund, Reference Grogan and Lund2002). Considering the detailed work of Grogan and Lund (Reference Grogan and Lund2002), the benthic habitat at BG was not permanently anoxic, and repeated events may have caused death by asphyxiation and rapid burial. The authors noted that paleoclimatic conditions and paleocirculation at Bear Gulch Bay indicated moments of hypersalinity linked to an arid climate regime and freshwater influx. The fossil content at BG comprises a diverse biota of invertebrates, vertebrates, and algae. Among the invertebrates, the sponge Teganiella heathi was found as an agglomerate of complete sponges concordant to the bedding plane and sometimes with the root tuft preserved (Rigby, Reference Rigby, Dutro and Pfeffertkorn1986) and was associated with other sponges, such as Ursaspongia tulipa Finks and Rigby, Reference Finks, Rigby and Kaessler2004 and Dictyospongiinae.
The latest discovery, Teganiella finksi n. sp., has been found in Mecca Shale (Middle Pennsylvanian) in Indiana. These sponges inhabited shoreline lagoons with marine influence located approximately 12°S latitude under a tropical climate regime. No primary evidence of hypersaline conditions has been observed. The sponges are rare, normally isolated, and preserved concordantly to the bedding plane at levels B and D. The MQ has a great fossil content of marine or brackish water animals, comprising invertebrates (such as cephalopods, orbiculoid brachiopods, oligochaete worms, and bivalves), vertebrates (sharks, conodonts, and palaeoniscoids), and plants (Zangerl and Richardson, Reference Zangerl and Richardson1963; Fig. 10). Unlike the other Teganiella species, Teganiella finksi n. sp. occurs alone, with no other associated sponges. Combining the data of Zangerl and Richardson (Reference Zangerl and Richardson1963) with the associated spicules and EDS analyzed here, we can conclude that Teganiella finksi n. sp. were buried in an oxygen-deficient, probably anoxic, microenvironment.
Considering the environmental context of the three species and their paleoecological features, even with three occurrences, we can deduce that Teganiella occupied shallow water limited to quiet water facies, showing a trend toward more restricted environmental conditions over time. There are many potential mechanisms that might lead sponges to migrate to more closed-inlet conditions, such as hydrodynamic effects, predator distributions, or a specific habitat requirement. If we ponder the heavy metal composition of the MQ, we can imagine a specific nutrient composition. However, we cannot rule out any other factors.
Paleobiogeography
Before identifying paleobiogeographic patterns or the distribution of specific sponge groups, it is necessary that we keep in mind three key factors: their incapability of movement, their quick larval settlement, and the discontinuity of fossil records. Considering these characteristics, the paleogeographic distribution of each species of Teganiella, and their similar characteristics, we conclude that the genus remained associated with periequatorial conditions (temperature dependent) under arid climates and lived in somewhat different paleoenvironments with localized factors, such as hypersaline periods, and they represent an endemic genus for Laurentia.
During the time of Teganiella’s appearance, the North American Plate was moving to the north (Scotese, Reference Scotese2001; Wicander and Monroe, Reference Wicander and Monroe2012). The oldest species appeared at 28°S latitude. Then a new species reappeared after the Frasnian-Fammenian mass extinction under favorable paleoenvironmental conditions in the paleoequatorial area of Laurentia at 12°N latitude. From the Mississippian to Pennsylvanian (approximately 12°N to 12°S), the tectonic movements are reduced, and the distribution could be due to possible eastern paleocurrents (Fig. 11).

Figure 11. Paleogeographic map of the Teganiella genus distribution through Givetian, Serpukhovian, and Moscovian. (1, 2) Teganiella ovata Rigby and Mehl (Reference Rigby and Mehl1994); (3, 4) Teganiella heathi Rigby (Reference Rigby, Dutro and Pfeffertkorn1986); (5, 6) Teganiella finksi n. sp. It is possible to observe the paleoequatorial distribution of the genus and the paleogeographic distribution of the land masses. The map was based on the Paleomap Paleoatlas Project (Scotese, Reference Scotese2016) using Gplates.
Conclusions
The discovery of a new species from the Middle Pennsylvanian from Mecca, Indiana, provides insight into the chronological extension of the Teganiella, an endemic genus of Laurentia. Compared with the associated fauna, the poriferan occurrence at the MQ is considered rare. The characters identified and current taxonomy of the Porifera phylum do not allow us to designate a class due to the homoplasic possibility of spicule development and lack of microscleres.
However, the morphological characters (body and spicule measures) and taphonomic similarity allow us to classify a genus level. In addition, the paleogeographical context for this new finding shows a low-latitude setting, with a notable scarcity of these reticulosan sponges recorded to date in the Pennsylvanian Basin of the United States. Teganiella finksi n. sp. is the least widespread genus throughout its stratigraphic range.
Acknowledgments
This work had financial support from the Programa de Formação em Recursos Humanos em Geologia da Petrobras (PFRH-PB 240), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Collection Grant (AMNH), and 1219 fund. We are thankful for the support of the American Museum of Natural History (AMNH, New York) and the Field Museum (Chicago), especially to B. Hussaini, M. Hopkins, N. Landmann, and P. Mayer, for letting us analyze and study the sponge samples. We also thank the Universidade Federal do Rio de Janeiro (UFRJ) and Universidade Federal de Santa Catarina for their help during all work phases. P. Mayer (Field Museum), E. Zeiger (Field Museum), and M. Zatoń (University of Silesia) kindly improved the language of the text, which is greatly acknowledged. We thank M. Florence, K. Moore, C. S. Silva, and D.R. Briske for the pictures provided for this article. We thank N.P. Gauer for the EDS analyses. The article benefited from constructive reviews of two journal referees.













