Introduction
Fossil submarine cave faunas are relatively rare, especially in the Paleozoic (for reviews see Kobluk, Reference Kobluk1981; Taylor and Palmer, Reference Taylor and Palmer1994; Taylor and Wilson, Reference Taylor and Wilson2003). This paper describes an Upper Ordovician (Katian) cave system underneath a carbonate hardground with trepostome bryozoans attached to the ceiling. The bryozoans contain abundant borings, bioclaustrations, and organic remains within their zoaria. The dominant bryozoan species, the massive calcitic Stigmatella personata, is also found on the upper exposed surface of the hardground, enabling us to directly compare exposed and cryptic, upside-down varieties of this bryozoan. Study of this fossil assemblage thus gives us early examples of cave-dwelling bryozoans that are elsewhere known from the Jurassic (Palmer and Fürsich, Reference Palmer and Fürsich1974; Wilson, Reference Wilson1998), Neogene (Rosso et al., Reference Rosso, Sanfilippo, Ruggieri, Maniscalco and Vertino2015), and Recent (Harmelin, 1986, 1997, Reference Harmelin2000; Rosso et al., Reference Rosso, Sanfilippo, Taddei Ruggiero and Di Martino2013). New information is gained about bioerosion during the Ordovician Bioerosion Revolution (Wilson and Palmer, Reference Wilson and Palmer2006; Buatois et al., Reference Buatois, Mángano, Olea and Wilson2016), preservation by bioclaustration (Taylor, Reference Taylor1990) within bryozoan skeletons, and the distribution of preserved bryozoan polypides called “brown bodies” (Boardman, Reference Boardman1999).
Geological setting
Locality
The carbonate hardground and associated cave fauna were found and collected in 1999 south of Maysville, Mason County, Kentucky (38.609352°N, 83.810973°W; College of Wooster location C/W-10). It was exposed for many meters on both sides of US Highway 68, 0.2 km south of its junction with US Highway 62/KY 1236. The cave portion of the hardground was destroyed by later road work, but parts of the hardground itself are still in place. The hardground is within the Corryville Formation of the Upper Ordovician Katian Stage (Fig. 1). It is ~2.2 meters above road level. The bryozoans at this location were previously described as “reefs” by Cuffey (Reference Cuffey1998), who believed the outcrops had been destroyed by road construction between 1992 and 1998. Cuffey (Reference Cuffey1998) at the time of his collections, did not note the caves or pendant bryozoans (Fig. 2), so they were likely not exposed during his visits.

Figure 1 Location of the hardground within the Corryville Formation.

Figure 2 Large colony of Stigmatella personata (NMW 2017.9G.1.1) growing downwards from the hardground (black triangle), both the bryozoan and substrate extensively bored (white arrows). Black arrow indicates the way up of sediments.
Stratigraphy
The Corryville is a formation (sometimes referred to as a member of the Grant Lake Formation) within the type Cincinnatian Series exposed in southeastern Indiana, southwestern Ohio, and central Kentucky. It is Katian (Late Ordovician) in age and within the Cincinnatian Sequence C3 (Patzkowsky and Holland, Reference Patzkowsky and Holland1996). In the North American stage terminology it is Maysvillian.
The Corryville consists of very fossiliferous limestones (packstones and wackestones) and shales deposited on a carbonate-siliciclastic ramp under considerable storm influence (Holland, Reference Holland1993). The sediments accumulated in a deep subtidal environment (between fair-weather wavebase and storm wavebase) on the paleocontinent Laurentia ~20°S of the paleoequator (Holland and Patzkowsky, Reference Holland and Patzkowsky2007; Vogel and Brett, Reference Vogel and Brett2009, fig. 2).
Hardground and cave development
Hardgrounds are synsedimentarily cemented, in situ, rocky seafloors (Wilson and Palmer, Reference Wilson and Palmer1992; Taylor and Wilson, Reference Taylor and Wilson2003). They are common throughout the Cincinnatian Series, especially in limestones formed in subtidal paleoenvironments (Palmer, Reference Palmer1982). Typical hardgrounds, such as the Corryville example in this study, are only a few centimeters thick and have less-indurated sediments above and below. They are thus commonly found as shelf-like projections from outcrops or loose slabs, allowing easy access to their bored and encrusted surfaces.
This Corryville hardground was at some point exposed on the seafloor and currents washed away large patches of soft clay underneath it, forming cavities 5–20 cm high (based on field measurements) with the hardground as a solid roof. Bryozoans then occupied the ceilings of the cavity below the hardgrounds as well as the hardground upper surfaces, producing the cryptic and exposed communities we see today on the tops and bottoms of the Corryville hardground slabs (Fig. 3). The same phenomenon has been documented with hardgrounds exposed in the Middle Jurassic of Utah (Wilson, Reference Wilson1998). We do not know how long these small caves remained open and connected to normal marine circulation, but we suspect a substantial interval because the cryptic bryozoans hanging pendantly from the ceilings grew to considerable sizes—up to 93 mm in diameter and 50 mm high.

Figure 3 Reconstruction of living positions of bryozoan colonies of Stigmatella personata.
Materials and methods
The specimens (NMW 2017.9G.1-7) forming the basis of this work were collected from exposures in 1999 (38.609352°N latitude, 83.810973°W). Longitudinally oriented thin sections were prepared to examine internal structures and photographed using a Canon 70D on a Leica Z6 microscope. Colony measurements are given in Table 1.
Table 1 Measurements of Stigmatella personata colonies; mean averages, ranges, number of measurements given in brackets.

Repository and institutional abbreviation.—NMW - Amgueddfa Cymru – National Museum Wales, Cardiff, UK.
Systematic paleontology
Phylum Bryozoa Ehrenberg, Reference Ehrenberg1831
Class Stenolaemata Borg, Reference Borg1926
Superorder Palaeostomata Ma, Buttler, and Taylor, Reference Ma, Buttler and Taylor2014
Order Trepostomata Ulrich, Reference Ulrich1882
Family Heterotrypidae Ulrich, Reference Ulrich1890
Genus Stigmatella Ulrich and Bassler, Reference Ulrich and Bassler1904
Type species
Stigmatella crenulata Ulrich and Bassler, Reference Ulrich and Bassler1904
Stigmatella personata Ulrich and Bassler, Reference Ulrich and Bassler1904
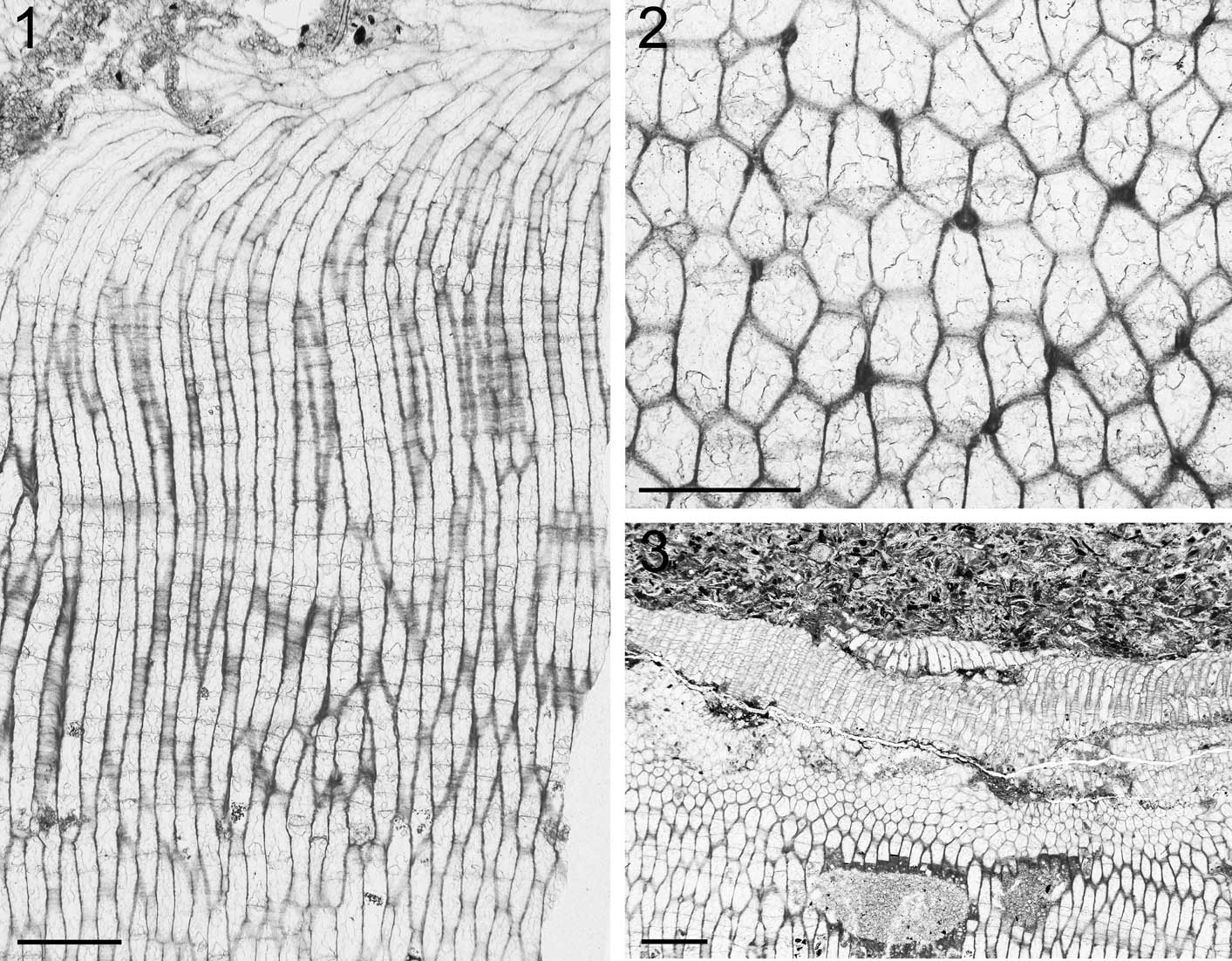
Figure 4 (1) NMW 2017.9G.2, Long zooecial chambers of Stigmatella personata colony growing downwards, longitudinal section; (2) NMW 2017.9G.1.2, polygonal zooecial chambers of S. personata with styles in walls, tangential section; (3) NMW 2017.9G.1.2, Monticulipora colony growing downwards overgrown by S. personata colony. Scale bars (1, 3)=1 mm; (2)=0.5 mm.

Figure 5 Stigmatella personata: (1) NMW 2017.9G.2.2, long zooecial tubes showing a gradual change in orientation; (2) NMW 2017.9G.1.2, localized development of styles in autozooecial wall; (3) NMW 2017.9G.2.2, large multi-layered colonies formed by self-overgrowth; (4) NMW 2017.9G.1.2, overgrowths marked by sediment infilling the zooecial chambers in the older part of the colony; (5) NMW 2017.9G.2.2, localized overgrowth; (6) NMW 2017.9G.2.2, intracolonial nature of the overgrowths recognized by uninterrupted zooecial chambers adjacent to those overgrowing part of the colony. Longitudinal sections; scale bars=1 mm.
1904 Stigmatella personata Ulrich and Bassler, Reference Ulrich and Bassler1904, p. 35.
1925 Stigmatella personata lobata Reference DyerDyer, p. 72.
1964 Stigmatella personata Ulrich and Bassler; Reference Utgaard and PerryUtgaard and Perry, p. 82.
1973 Stigmatella personata lobata Dyer; Reference FritzFritz, p. 17.
Holotype
USNM 43.201 from the Richmond Formation, Hanover, Ohio (Ulrich and Bassler, Reference Ulrich and Bassler1904).
Diagnosis
Zoaria branching or massive, formed by multiple overgrowths. Maculae composed of macrozooecia. Long autozooecia with diaphragms along length. Styles present in patches, mesopores absent.
Occurrence
Ordovician, Katian, midwestern USA (Ohio, Indiana, Kentucky).
Description
The zooaria are massive in form and multi-laminar with the new parts of the colony overgrowing older ones. They range in size and are up to 93 mm in diameter and 50 mm in height. Monticules of clusters of larger autozooecial chambers form regular conical structures on colony surface. Endozones restricted to short recumbent autozooecia budded from exterior basal colony walls. In exozones, autozooecial boundaries polygonal in cross-section. Autozooecia in disordered pattern on colony surface. Zooecial walls thin, cortex thickness regular throughout exozone; microstructure poorly preserved. Zooecia polygonal in transverse section, 0.23 mm maximum mean diameter and 0.38 mm maximum mean diameter in monticules. Thin basal diaphragms abundant in some parts of zooecial chambers and lacking in others, spaced on mean average 0.19 mm. Styles are observed in localized patches. Mesopores are absent.
Remarks
Stigmatella personata was originally described by Ulrich and Bassler (Reference Ulrich and Bassler1904) as a smooth branching colony characterized by having no mesopores and few styles. Dyer (Reference Dyer1925) proposed S. personata lobata, which differed in colony morphology, forming irregular lobate masses covered by low monticules. Utgaard and Perry (Reference Utgaard and Perry1964) regarded the differences between S. personata lobata and the type species as variations in growth form. Fritz (Reference Fritz1973) however disagreed and considered there was a significant difference in the numbers of styles between the two. The colonies from the Corryville Formation show variation of style number within the colony. Encrusting trepostome colonies are known to develop erect branches, so species cannot be distinguished by external morphological form.
Bryozoan colonies
Taxonomy and form
The bryozoan colonies are recognized as having lived on an exposed hardground surface and having inhabited small caves beneath it. The Corryville Formation is estimated to have been deposited below the fair-weather wavebase and above the storm wavebase, and so well within the photic zone (Holland and Patzkowsky, Reference Holland and Patzkowsky2007). We investigated as to whether there were differences between those that grew upwards in presumably well-lit waters and those that grew downwards from the ceilings in the darker, light-restricted caves. There would have been differences in the water currents in the two environments and colony growth may have been affected by gravity.
Taxonomically no differences were recognized between the upwards-growing, exposed bryozoans and those living pendantly in the caves. All the large colonies are identified as the trepostome Stigmatella personata Ulrich and Bassler, Reference Ulrich and Bassler1904 (Fig. 4.1, 4.2) regardless of their growth orientation. Only one other encrusting trepostome species is recognized; one small colony of Monticulipora is observed growing partly over a small single layer of S. personata and the celling of the cave (Fig. 4.3). This colony is in turn overgrown by a large S. personata.
There are few discernible anatomical differences between the bryozoan colonies from the two locations. The pendant, cave-dwelling S. personata on average has longer zooecial tubes than its exposed equivalent, possibly caused by environmental factors in the different locations. A maximum zooecial length of 22.5 mm and was measured in colonies growing downwards and 16.5 mm in those growing upwards. The mean average of the downward growth is 10.6 mm (range 3.7–22.5 mm; SD 4.96 mm) and upward 5.6 mm (range 0.89–16.6 mm; SD 4.14 mm).
The spacing between diaphragms in the zooecial chambers was measured to determine if there is a difference between the colonies in different orientations. The diaphragms are commonly widely spaced, but in some locations are found closer together. The intervals between the first five diaphragms from the zooecial opening at the colony surface or at the point of overgrowth were measured over 200 times in colonies growing up and growing down (~420 measurements). The results showed there was only a difference of 0.005 mm in growth direction, a mean average of 0.196 mm recorded for the colonies in caves and 0.191 mm for those on the hardgrounds. However, within one colony the distances are variable as can be seen in Figure 4.1.
Growth and overgrowths of colonies
The autozooecial chambers in the colony generally grew long, up to 22.5 mm, and straight, but can be seen to have localized changes in direction of colony growth (Figs. 4.1, 5.1). Thickening of the zooecial walls and the localized development of styles can also occur (Fig. 5.2), sometimes accompanying a change in growth direction. These developments may be controlled by external microenvironmental effects.
Discussion
The colonies of Stigmatella personata are multi-layered because of self-overgrowth (Fig. 5.3). The intracolonial nature of the overgrowths is recognized in thin section (Fig. 5.4) by uninterrupted zooecial chambers adjacent to those overgrowing part of the colony, which has sediment that infills zooecia. This suggests the zooids in the overgrown part of the colony died and the living zooids overgrew them. The area of overgrowth can be localized or extend over most of the colony (Fig. 5.3, 5.5, 5.6). The death of parts of the colony may be due to localized predation, although no evidence can be seen in the skeletal walls. Modern-day cyclostome bryozoans are predated by a variety of organisms, including echinoderms, nudibranch sea slugs, pycnogonids, small crustaceans, and fish (Hayward and Ryland, Reference Hayward and Ryland1985; Lidgard, Reference Lidgard2008). The colonies living in the cave environment may have had some protection from predation compared to those growing in exposed environments. This may be the reason that longer autozooecial tubes are recognized in the bryozoans growing down in caves.
In colonies growing upwards and those growing down, the overgrowths are marked by sediment infilling the zooecial chambers in the older part of the colony (Fig. 5.4). Lev et al. (Reference Lev, Key and Lighthart.1993) observed ‘clay drapes’ on the top of colony-wide bands in colonies of Prasopora from the Middle Ordovician Martinsburg Formation of south-central Pennsylvania. They suggested that these represent a turbidity episode in which fine-grained particles smothered the colony. This was then followed by a regeneration of zooids. In the colonies from Kentucky, we consider that an influx of sediment may not be responsible for killing parts of the colony by smothering because often only localized sections are affected. Modern colonies are known to be able to clean the surface of sediment. Polypides in the colony generate water currents to feed and to remove sediment and waste (Lidgard, Reference Lidgard1981), and there are examples of zooids cleaning the colony using tentacles (Dick, Reference Dick1984). It is probable that the areas that had chambers infilled with sediment had no living animals inside.
Sediment could have entered the cave environment during a storm episode, but why was it retained on the colony surface and not dislodged due to gravity? Alternatively, it is possible that dead parts of the colony had become covered in biofilms. Gerdes et al. (Reference Gerdes, Kaselowsky, Lauer, Mawatari and Scholz2005) recognized that bryozoans represented a common settling ground for a wide range of epizoic biofilms, including cyanobacteria and fungi. Sediment may have adhered to the surface of the biofilm, trapping it between the layers when the colony overgrew.
Borings
All colonies in both growth orientations have been extensively bored (Fig. 6.1–6.6). The borings are located throughout the colony and can also be seen in the hardground substrate (Fig. 2). They all have a similar form: straight with a cylindrical cross section. Two different types are recognized, but both are identified as Trypanites Magdefrau, Reference Mägdefrau1932. This trace fossil, which is known from throughout the Phanerozoic, was extremely common during the Late Ordovician when it was a significant bioeroder of hard substrates (Wilson and Palmer, Reference Wilson and Palmer2006). The two types are found in the exposed and cave environment.

Figure 6 Trypanites borings: (1) NMW 2017.9G.1.2, colony extensively bored by Trypanites type A; (2) NMW 2017.9G.2.2, Trypanites type A overgrown by Stigmatella colony; (3) NMW 2017.9G.1.3, Trypanites type A polygonal in transverse section; (4) NMW 2017.9G.3.2, Trypanites type A infilled with micrite and containing cylindrical tubes of calcite, possible evidence of boring animal; (5) NMW 2017.9G.1.2, Trypanites type B cutting through multiple layers of Stigmatella colony; (6) NMW 2017.9G.1.2, Trypanites type B infilled with sediment containing dolomite rhombs and fragments of cryptostome bryozoans. (1, 2, 4–6) longitudinal sections; scale bars=1 mm; (3) tangential section; scale bar=0.5 mm.
Trypanites boring Type A
These are the smaller of the two varieties (Fig. 6.1) and have a slightly different structure, depending upon the whether they are boring into the bryozoan colonies or into the hardground substrate. The tubes range in diameter from 0.74 mm to 1.62 mm, with a mean diameter of 1.25 mm, and the maximum length measured is 16 mm.
These borings are confined to a single overgrowth within the bryozoan colonies. The borings are often covered over by subsequent layers of the colony (Fig. 6.2). This suggests that the borings were not occupied by living organisms at this time; possibly they had died in a storm-sedimentation event.
Type A borings appear circular in cross section in hand specimens with straight sides to the tube. When observed in transverse sections of the bryozoan colony (Fig. 6.1), the borings are bounded by the calcite walls, which creates a polygonal rather than circular structure (Fig. 6.3). Even though calcite colony walls were eroded in the boring process, excavating the cylindrical cavity parallel to the walls was the way of least resistance for the borer. This was also observed by Wyse Jackson and Key (Reference Wyse Jackson and Key2007) in Ordovician bryozoans from Estonia. They found the borings oriented roughly perpendicular to the colony surface minimized intersection with skeletal walls of the bryozoan zooecia. The strength of the calcite diaphragms within the bryozoan colony also resulted in a structure with a stepped appearance when they appear to form a more resistant barrier and cause the burrow to be offset to the adjacent chamber (Fig 6.2). There are some Type A borings that cut through zooecial chambers at a 90˚ angle.
Type A borings are commonly infilled with micrite. Some contain an additional cylindrical tube of calcite (Fig. 6.4). These appear to be similar to the ‘ghosts’ of organic material described by Wyse Jackson and Key (Reference Wyse Jackson and Key2007). They interpreted the ghosts as the sparry cement-filled cast of the boring organism that was killed by infilling of matrix into the larger boring it had excavated. This may have occurred during a storm event that buried the host colony. This is consistent with evidence for the colonies being covered in sediment and disrupting the growth.
Trypanites boring Type B
The second variety has a larger tube size; these tubes range in diameter from 2.4 mm to 3.2 mm, with a mean diameter of 2.9 mm and the maximum length measured is 39 mm. These cut through several layers of overgrowth (Fig. 6.5). The borings are infilled with various sediments, some containing numerous dolomite rhombs and others with larger fossil fragments, including cryptostome bryozoans, brachiopod shell, and echinoderm fragments (Fig. 6.6). There are no cylindrical calcite ‘ghosts’ present in these structures.
Bioclaustration
The primary bryozoan in this study, Stigmatella personata, hosts several bioclaustration structures (Figs. 7.1, 7.2). Bioclaustration was originally described by Palmer and Wilson (Reference Palmer and Wilson1988) as a process by which soft-bodied symbionts are entombed within the growing skeletons of other organisms. Taylor (Reference Taylor1990) expanded the definition to include the embedment of skeletal organisms as well. There are only four bioclaustration ichnotaxa formally described in Paleozoic bryozoans: Anoigmaichnus odinsholmensis Vinn et al., Reference Vinn, Wilson, Mõtus and Toom2014, from the Middle Ordovician (Darriwilian) of Estonia; Catellocaula vallata Palmer and Wilson, Reference Palmer and Wilson1988, in Upper Ordovician (Katian) trepostomes; Caupokeras calyptos McKinney, Reference McKinney2009, in Middle Devonian fenestrates (see also Suárez Andrés, Reference Suárez Andrés2014); and Chaetosalpinx tapanilai Ernst et al., Reference Ernst, Taylor and Bohatý2014, in Middle Devonian cystoporates.

Figure 7 Bioclaustration: (1) NMW 2017.9G.2.2, void with thickened wall overgrown by colony; (2) NMW 2017.9G.1.2, thickened wall grown around tubular structure. Longitudinal sections; scale bars=1 mm.
The bioclaustration structures in S. personata are flat-bottomed tubular structures. They appear to represent soft-bodied fouling organisms that spread across the apertures of three to six zooecia, effectively halting their growth. Neighboring zooecia grew up and eventually overtopped them, forming keyhole-like cross-sections, as in Figure. 7.1. Patches of sparry calcite and sediment appear to be additional examples of “ghosts” of soft tissues (Fig. 7.1). In some bioclaustration structures, there is a hint of a recrystallized skeleton for the embedded organism (Fig. 7.2).
The bioclaustration structures in S. personata do not fit the descriptions of current ichnotaxa, but with only cross-sections, we do not have enough information to erect a new ichnotaxon. We also cannot identify the symbiont that left these features. We know enough, though, to classify these bioclaustrations within the category Impedichnia (Tapanila, Reference Tapanila2005) because they impeded the normal growth of Stigmatella personata.
Organic remains
Remains found within the living chambers of Cincinnatian trepostomes have been interpreted as preserved indications of polypides. These were first identified by Cummings and Galloway in Reference Cummings and Galloway1915, who recognized brown granular material that was present in autozooids, but never in mesopores. The degenerated cells that result from the normal degeneration-regeneration cycles of living bryozoan polypides are known as brown bodies (Boardman, Reference Boardman1999). The fossilized remains of organic material that may have been brown bodies are referred to as brown deposits (Boardman and Cheetham, Reference Boardman and Cheetham1983; Key et al., Reference Key, Wyse Jackson, Miller and Patterson2008). In one specimen from Kentucky growing upwards on the exposed surface, brown deposits are found on the upper surfaces of diaphragms (Fig. 8). These do not have any indications of polypide anatomy similar to those recognized in other lower Paleozoic trepostomes from North America (Boardman, Reference Boardman1999).

Figure 8 NMW 2017.9G.3.2, Longitudinal section growing upwards showing the dark remains of organic material (black arrows) above diaphragms in zooecial chambers (white arrow indicating air bubbles). Longitudinal section; scale bar=1 mm.
Conclusions
This cave fauna is one of few submarine examples known in the Paleozoic. The distribution of organisms in and outside the caves supports the hypothesis that early cave-dwelling organisms were little differentiated from their exposed counterparts (see Taylor and Wilson, Reference Taylor and Wilson2003). Mesozoic cave and other cavity faunas, in contrast, usually had distinct polarization between cryptic and exposed communities (Palmer and Fürsich, Reference Palmer and Fürsich1974; Palmer and Wilson, Reference Palmer and Wilson1990; Taylor and Palmer, Reference Taylor and Palmer1994; Wilson, Reference Wilson1998). This fauna represents a community of large bryozoan colonies bored by two distinct organisms, with symbionts growing on the surface and biofilms developing on the dead parts of the colony.
Acknowledgments
We thank A.J. Valentine-Baars (Amgueddfa Cymru) for preparing the thin sections and J. Turner (Amgueddfa Cymru) for assistance with photography. The Luce Fund at The College of Wooster generously provided funds for the fieldwork. M.M. Key Jr., P.N. Wyse Jackson, and A. Ernst provided very helpful reviews.











