Introduction
The abrupt appearance of diverse small shelly fossils (SSFs) during the earliest Cambrian signals the initial stages of the Cambrian explosion (G. Li et al., Reference Li, Steiner, Zhu, Yang, Wang and Erdtmann2007; Maloof et al., Reference Maloof, Porter, Moore, Dud, Bowring, Higgins, Fike and Eddy2010). It therefore seems axiomatic that SSFs are of great importance for understanding the early rise of metazoan phyla and the origins of animal skeletogeny. Paleoecological reconstruction of SSF communities is a challenging task as the majority of SSFs are fragmentary or consist of isolated sclerites. A critical exception to this rule is the set of phosphatic SSFs in Orsten-type Lagerstätten, for example the Kuanchuanpu Biota in South China (ca. 535 Ma), which together have the potential to provide unique insights into the nature and significance of these fossils thanks to their high potential for exceptional preservation of both hard parts and soft tissues.
Carinachitids are an important component of early Cambrian SSFs in South China (Conway Morris and Chen, Reference Conway Morris and Chen1992). Their gently conical skeletal tubes exhibit several (three to five) transversely ribbed faces separated from each other by wide and deep corner sulci that usually bear fine transverse wrinkles (tw) (Fig. 1). To date, three genera and six species of carinachitids—Emeiconularia trigemme Qian in Qian et al., Reference Qian, Van Iten, Cox, Zhu and Zhuo1997; E. amplicanalis Liu et al., Reference Liu, Li, Shao, Wang, Yu, Han and Yang2005 (Fig. 1.1); Pentaconularia ningqiangensis Liu et al., Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011 (Fig. 2); Carinachites spinatus Qian, Reference Qian1977 (Figs. 3–5); C. tetrasulcatus Jiang in Luo et al., Reference Luo, Jiang, Wu, Song, Lin and Zhang1982; and C. curvatornatus Chen, Reference Chen1982 —have been reported from the Kuanchuanpu Formation and equivalent horizons in South China (Qian, Reference Qian1977; He, Reference He1987; Conway Morris and Chen, Reference Conway Morris and Chen1992; Qian et al., Reference Qian, Van Iten, Cox, Zhu and Zhuo1997; Liu et al., Reference Liu, Li, Shao, Wang, Yu, Han and Yang2005, Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011). These fossils collectively exhibit tri-, tetra-, or pentaradial symmetry in transverse sections (Liu et al., Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011), and these symmetries may have arisen independently in different lineages (Han et al., Reference Han, Kubota, Li, Ou and Wang2016a, Reference Han, Li, Kubota, Ou and Toshinob). In addition, the tube wall appears to have been flexible and composed of organic material and/or calcium phosphate (Conway Morris and Chen, Reference Conway Morris and Chen1992; Qian et al., Reference Qian, Chen, Feng, Xu and Liu1999).
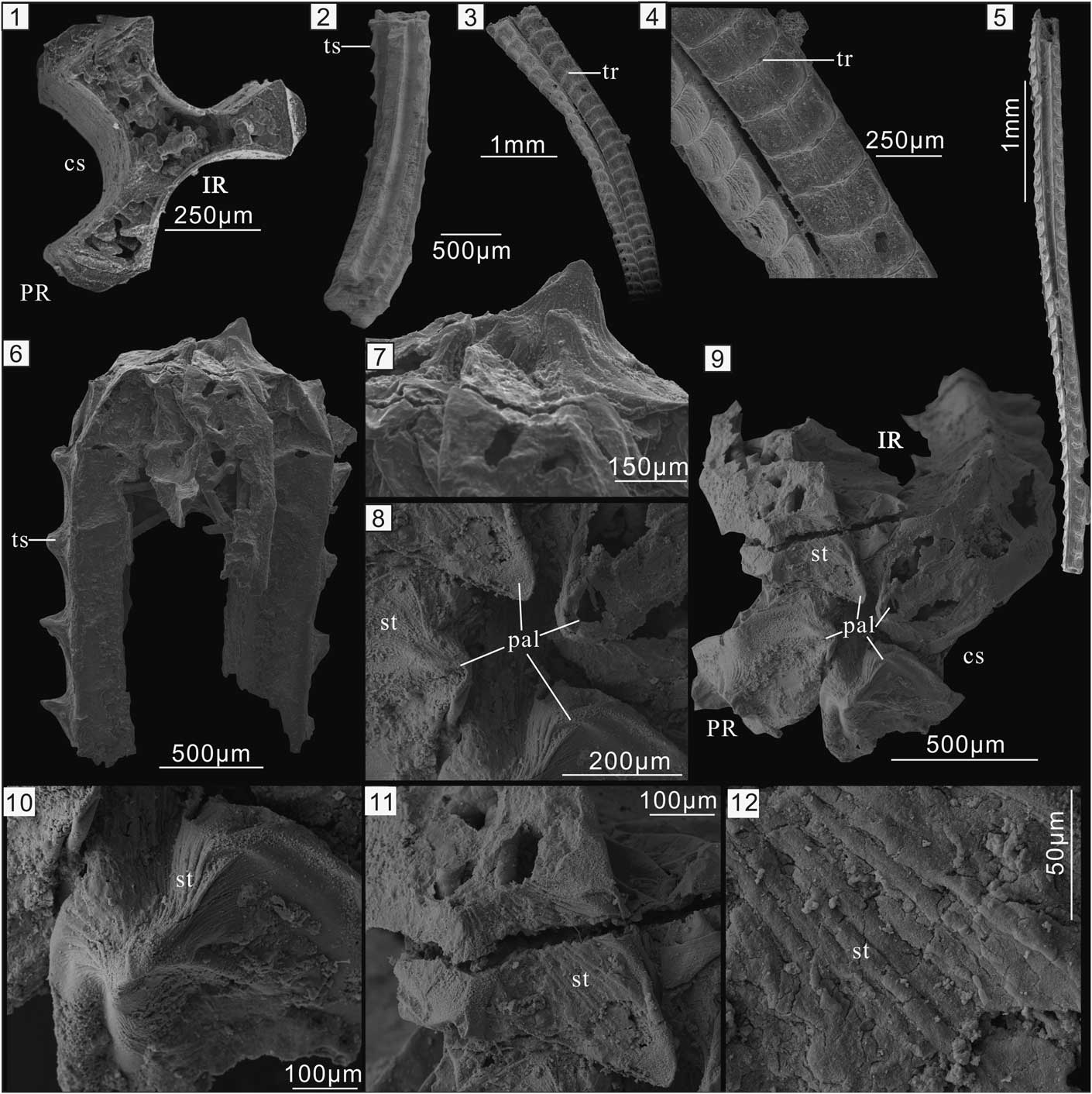
Figure 1 Carinachitids from the lower Cambrian Kuanchuanpu Formation in South China. (1) Triradiate Emeiconularia amplicanalis Liu et al., Reference Liu, Li, Shao, Wang, Yu, Han and Yang2005. (2) Pentamerous Pentaconularia ningqiangensis Liu et al., Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011. (1, 2) Courtesy of Y.H. Liu. (3–6) Tetraradiate Carinachites spinatus Qian, Reference Qian1977. (3, 4) ELISN93-157, showing the displacement between neighboring arcuate ribs, which are connected in the middle by striations. Both the faces and ribs widen slightly toward the apertural end of the skeleton. (5) ELISN93-45. (6–12) ELISN148-52. (6, 7) Lateral view of the tube. (8, 9) Close-up of the tube aperture shows one of the plicate apertural lobes being elevated above the others. (10) The elevated, plicate apertural lobes with converging striated folds. (11) Single plicate apertural lobe with converging striated folds and the corner sulci with parallel striations. (12) Close-up of (11) showing secondary cracks on the striated surface. IR=interradius; PR=perradius; cs=corner sulci; pal=plicate apertural lobes; st=striations; tr=transverse ribs; ts=thorn-like spines.

Figure 2 Micro-CT reconstruction of specimen ELISN148-52. (1a–1e) lateral view; (1f, 1g) oral view of the virtual cross sections. The sagittal positions of (1a–1e) are indicated respectively by ‘1a,’ ‘1b,’ ‘1c,’ ‘1d,’ ‘1e,’ and ‘1f’ in (1f, 1g). The horizontal levels of (1f, 1g) are respectively indicated by ‘1f’ and ‘1g’ in (1a–1f). White arrows indicate double-layered tube wall. cs=corner sulcus; fa=face; mmf=microbial-mediated filaments; pal=plicate apertural lobes; st=soft tissue; ts=thorn-like spines; PR=perradius; IR=interradius.

Figure 3 Cross sections and proposed orientation of the main radial symmetry planes in Cambrian carinachitids. (1, 2) Inferred triradial symmetry in Emeiconularia; (1) E. amplicanalis; (2) Emeiconularia trigemme with thickened faces sensu Qian et al., Reference Qian, Chen, Feng, Xu and Liu1999; (3) tetraradial symmetry in Carinachites; (4) pentaradial symmetry in Pentaconularia (Modified from Liu et al., Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011). cs=corner sulcus; fa=face; st=soft tissue; tb=tube; PR=perradius; IR=interradius.

Figure 4 3D reconstructions of Cambrian carinachitids with a hypothetical apical tip. (1–3) Lateral, oblique, and oral views, respectively, of Emeiconularia; (4–6) lateral, oblique, and oral views, respectively, of Carinachites spinatus; (7–9) lateral, oblique, and oral views, respectively, of Pentaconularia.

Figure 5 Peridermal tube of Olivooides multisulcatus from the Cambrian Kuanchuanpu Formation in South China. (1) Specimen ELISN19-20, aboral view showing five longitudinal rows of plicate corners (pc); (2–4) XX30-127. Oral view of the specimen. (2) Tube aperture with five plicate lobes (pl); (3) close-up of (2). (4) Diagnostic stellate ornament of the aboral ends. PR=perradii; IR=interradii.
Carinachitids, together with co-occurring hexangulaconulariids (Yue and Bengtson, Reference Yue and Bengtson1999; Van Iten et al., Reference Van Iten, Zhu and Li2010) and olivooids (see Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014), have been assigned to the order Conulariida of the subclass Conulata (He, Reference He1987; Qian et al., Reference Qian, Chen, Feng, Xu and Liu1999). Because carinachitids and hexangulaconulariids are very small (<5 mm long) and lack the facial midline and carina typical of many Ordovician and younger conulariids, they have been classified as protoconulariids (Qian et al., Reference Qian, Chen, Feng, Xu and Liu1999). The zoological affinities of Conulata have been controversial (Babcock et al., Reference Babcock, Feldmann, Hoffmann and Nitecki1986; Brood, Reference Brood1995), but the group is now generally assigned to the subphylum Medusozoa of the phylum Cnidaria (Bengtson and Yue, Reference Bengtson and Yue1997; Van Iten et al., Reference Van Iten, de Moraes Leme, Simões, Marques and Collins2006, Reference Van Iten, Zhu and Li2010, Reference Van Iten, Marques, Leme, Pacheco and Simões2014). Phylogenetic relationships among protoconulariids remain poorly understood as all previously collected specimens of carinachitids lack both the apical and apertural regions, and thus their complete morphology and growth patterns are unknown. Here we describe a tetramerous specimen of Carinachites spinatus that preserves the tube aperture. This specimen provides critical new insights into the morphology, systematic classification, and paleoautecology of carinachitids.
Materials and methods
Specimens of Carinachites spinatus were obtained from samples of phosphatic limestone collected from the Kuanchuanpu Formation in southern Shaanxi Province, South China, and digested in 10% acetic acid. Specimens ELISN148-52, ELISN93-45, ELISN93-157, ELISN19-20, ELISN23-240, and ELISN12-154 come from the Shizhonggou section in Ningqiang County, while specimen XX30-127 is from the Yangjiagou section in Xixiang County (for localities, see Steiner et al., Reference Steiner, Li, Qian, Zhu and Erdtmann2007, fig.1). All specimens were coated with gold and then imaged using an FEI Quanta 400 FEG scanning electron microscope (SEM). Micro-CT data for specimen ELISN148-52 were acquired at the Tohoku University (Fig. 2) and were processed using VG Studio 2.2 Max for 3D reconstructions. The terminology used herein mostly follows that of Conway Morris and Chen (Reference Conway Morris and Chen1992), Van Iten (Reference Van Iten1992a), and Han et al., Reference Han, Kubota, Li, Ou and Wang2016a.
Repository and institutional abbreviation
The figured specimens in this study are housed in the Early Life Institute (ELI), Northwest University, Xi’an, China.
Results
Tube morphology of Carinachites spinatus
Carinachitids are abundantly represented by tetramerous C. spinatus Qian Reference Qian1977 in the Kuanchuanpu Formation in the Shizhonggou section in Shaanxi Province. The tube of this species exhibits four prominent, equidimensional convex faces separated from each other by deep corner sulci (Figs. 1.3–1.6, 2.6). Each face usually bears a longitudinal series of arcuate transverse ribs that range in shape from simple welts to more complex folds (Conway Morris and Chen, Reference Conway Morris and Chen1992). The distance between adjacent ribs increases slightly toward the wide or oral end of the tube (Fig. 1.3). Near the facial midline, the region between any two adjacent ribs exhibits several, mutually parallel or irregular, longitudinal striated folds that in most cases are separated from each other by an inconspicuous shallow groove (Fig. 1.4).
The transverse ribs in some specimens are arcuate near the distal end and as wide as the faces (Fig. 1.3, 1.4), while in other specimens the ribs consist of a prominent, sharp, thorn-like spine (ts) such as those exhibited by specimens ELISN93-45 (Fig. 1.5) and ELISN148-52 (Fig. 1.6). The ribs on any two neighboring faces usually are located at the same levels above the apex, but some ribs exhibit longitudinal offset (Fig. 1.3, 1.4) (Conway Morris and Chen, Reference Conway Morris and Chen1992). In addition, the ribs of some specimens are offset along the facial midline (Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 6.1).
The apertural region, preserved only in the relatively large specimen ELISN148-52, is superficially dome-shaped (Fig. 1.6–1.9). The maximum diameter of the tube is approximately 1.3 mm, but near its apertural end the tube tapers rapidly, with the faces curving smoothly toward the longitudinal axis of the tube and becoming more or less perpendicular to it. Close to the longitudinal axis, the faces and intervening corner sulci are inclined toward the aboral end of the tube (Fig. 1.6, 1.7, 1.9). Present on each face, at the summit of the aperture, is a triangular tongue-shaped structure, and the distal ends of the faces almost meet near the longitudinal axis of the tube, leaving just a narrow central opening. One of the tongue-shaped structures projects much farther than the others beyond the aperture (Fig. 1.6). The tongue-shaped structures are not flat features but rather fold-like structures having two main, arched sides separated by two flanks. For this reason, we term the tongue-shaped structures ‘plicate apertural lobes’ (pal). The vertical abapertural side of the apertural lobes extends far into the apertural opening and exhibits a medial subtriangular groove bordered by two elevated flanks (Fig. 1.8, 1.9). The abapertural side is either flat or outwardly convex with a central ridge, and there are many longitudinal striations on the two sides. These striations converge on the tip of the lobes, and there are some oblique, irregular cracks on the striated surface (Fig. 1.11, 1.12). Situated peripheral to the apertural lobes are four longitudinal rows of nearly evenly spaced, nose-shaped, thorn-like spines aligned along the lateral sides of the faces. In addition, the distance between the apertural lobes and the marginal thorn-like spines is approximately equal to the distance between adjacent thorn-like spines. The adapertural side of the lobes, which is more or less perpendicular to the lateral faces, is concave aborally. The abapertural side, like the bridge of a nose, is inclined at approximately 30º to the lateral faces. The four corner sulci, which are substantially lower than the apertural lobes, follow the inward foldings of the adjacent faces and extend far into the tube cavity (Fig. 1.9). The external surface of the sulci is highly and irregularly folded and exhibits fine parallel striations. The summit of the four corner sulci is evidently lower than that of the faces (Fig. 1.9). Clearly, then, the tube aperture, including the inwardly folded portion, is a smooth continuation of the faces and corner sulci.
Tube wall and internal anatomy
Micro-CT observations confirm the presence of a narrow apertural opening and the continuation of the tube walls into the inwardly and downwardly folded apertural lobes (Fig. 2.1a–e). The tube wall of carinachitids generally exhibits a prismatic inner layer of uniform thickness and a granular outer layer that is much thinner in the corner sulci than in the faces (e.g., Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 8.19; Qian et al., Reference Qian, Van Iten, Cox, Zhu and Zhuo1997, plate 2, 1c, 3c; Liu et al., Reference Liu, Li, Shao, Wang, Yu, Han and Yang2005, plate 2, 1e, j; Liu et al., Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011, fig. 2f-g). The prismatic layer originally was thought to consist of overgrowths of diagenetic apatite, while the granular layer was thought to have been originally organic but later replaced by diagenetic apatite (Qian et al., Reference Qian, Chen, Feng, Xu and Liu1999). Although we are mindful of possible preservational artefacts, Micro-CT imaging revealed that the thickness of the tube wall in specimen ELISN148-52 appears to vary, and that the apertural walls are much thinner than the lateral tube walls (Fig. 2.1a, 2.1c, 2.1e). High magnification imaging revealed a single-layered wall in the apertural region (Fig. 2.1c) and bilayered lateral tube walls (white arrows in Fig. 2.1a, 2.1g). In addition, the facial walls are thicker than those of the corner sulci (Fig. 2.1a, 2.1g). Finally, both the thorn-like spines and the apertural lobes are hollow (Fig. 2.1b).
Present within the tube is a short, subcylindrical mass measuring ~200 µm in diameter and 400 µm in length. The upper part of this feature is in direct contact with the inward folds of the faces and corner sulci (Fig. 2.1b–e), and it is connected to the lateral tube wall by numerous fine, straight filaments (mmf) (Fig. 2.1a–g). No additional details of the subcylindrical mass can be discerned.
Discussion
Relic soft-tissue of Carinachites spinatus
Similar fine filaments commonly occur within associated fossils, including poorly preserved Carinachites (Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 7.9–7.11), other tubular microfossils (e.g., Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, figs. 7.21, 11.9, 11.12, 11.15), and egg envelopes with partly decayed embryos (e.g., Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, fig. 4.6–4.9). Because these internal filaments are generally interpreted as microbial in origin and derived from partly decomposed soft tissue (Yue and Bengtson, Reference Yue and Bengtson1999), we interpret the subcylindrical mass surrounded by these fine filaments in specimens of C. spinatus as remains of soft tissue that underwent partial decay.
Relic soft tissue similar to that near the tube aperture of Carinachites spinatus also is present in Hexaconularia (Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, fig. 7.18), Olivooides (e.g., P. Li et al., Reference Li, Steiner, Zhu, Yang, Wang and Erdtmann2007, fig. 4d; Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, figs. 10.3, 11.6, 11.12, 11.15), and Quadrapygites (e.g., Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, figs. 14.19, 15.13, 15.15). In addition, an intact, trumpet-shaped mass of relic soft tissue consisting of an upper calyx and a slender basal stalk extending to the aboral end has been documented in Olivooides (see Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, fig. 12.7–12.10). The presence of relic soft tissues in Olivooides embryos has been demonstrated convincingly by the discovery of exceptionally well-preserved, primary internal anatomy (Han et al., Reference Han, Li, Kubota, Ou and Toshino2016b). All of these relic soft-tissue structures are smaller in diameter than the surrounding tube wall. Whether the soft tissues of Carinachites spinatus reached the aboral end of the tube cannot be determined at present.
Growth of the tubes of Carinachites spinatus
The thorn-like spines on the transverse ribs of this taxon resemble the plicate apertural lobes in many respects including shape, size, surface ornament, spacing, and tip directions. Thus, it seems clear that the spines and lobes are substantially the same kind of structure and are simply located in different positions on the faces. The tube aperture was a kind of extracellular matrix that was most likely secreted by epithelial tissue at the oral end, as only in this area was the tube in direct contact with the soft body. If this hypothesis is correct, then one can make the following additional inferences: (1) the thorn-like spines most probably were derived from the plicate lobes and not vice versa. Delimited by the corner sulci, the transverse extension of the two flanks of the plicate lobes may have undergone ontogenetic transformation into the lateral face ribs. The abapertural groove of the plicate lobes was transformed into the middle groove or the central ridges between adjacent ribs. The converging striations on the plicate lobes are equivalent to the striated folds on the lateral thorn-like spines. (2) The distance between neighboring ribs on the same face is approximately equal to or less than the radius of the tube aperture and the depth of the inward portion of the plicate lobes. The displacement of the ribs reflects the displacement of the segmented faces and the corner sulcus and thus the migration of the entire tube aperture. (3) Following the previous inward portion of tube aperture, new inward portion skeleton was secreted by the epithelium of soft tissue at the oral region. The new skeleton may have been primarily attached with the epithelium, and afterward the new apertural parts may have detached with the epithelium of the oral region and been pushed onward and outward with centrifugal expansion, finally being displaced to the lateral side of the tube and becoming the lateral components of the lateral walls. (4) Periodic renewal of the tube aperture necessarily led to orally addition of iterated ribs and ‘segmentation’ of the faces. Together, these processes reflect the growth of the tube by apertural extension (Fig. 4.4–4.6).
The multiple thorn-like spines on specimens ELISN93-45 (Fig. 1.5) and ELISN148-52 (Fig. 1.6) indicate that the morphology of the ribs on the faces of a single individual is essentially uniform and constant, without gradual transformation from welts to arcuate ribs or other, more complex folds. Probably, this replacement began at the basal end of the tube and continued to the upper part without metamorphosis. If this hypothesis is correct, then the specimens of C. spinatus described in Conway Morris and Chen (Reference Conway Morris and Chen1992) are likely a mixture of several species. Specimens with sharp, thorn-like spines or arcuate ribs as well as welts should be reinterpreted as different species rather than different developmental stages, unless these variants can be shown to co-occur in the same individual of C. spinatus.
In addition to sequential adoral addition of ribs on the faces, growth of the Carinachites tube also involved increase in the diameter of the tube and the width of the corner sulci. Along the longitudinal axis, the corner sulcus expands gradually toward the aperture (Fig. 1.5). In the most complete specimen (ELISN93-45), which however lacks the apex and apertural margin, at least 35 ribs are present on each face (Fig. 1.5). If the tube could grow up to 1.3 mm in width, as indicated by specimen ELISN148-52 (Fig. 1.6), then we infer that a single face may have contained at least 130 ribs over a total length of approximately 25 mm. With regard to the apertural extension model mentioned in the preceding, neighboring ribs at the same level indicate that the plicate apertural lobes were replaced synchronously by new ones (Fig. 4.4–4.6). In contrast, longitudinal offset of ribs along the corner sulci may reflect diachronous replacement of previously formed plicate lobes, indicating that the tube opening was always more or less partly closed. The corner sulci are generally thinner and more flexible than the faces (Qian et al., Reference Qian, Van Iten, Cox, Zhu and Zhuo1997), and in most fragmentary specimens, the sulci exhibit secondary breakage. Probably in life the flexible corner sulci served as buffer zones that prevented tearing of the tube during diachronous replacement of the ribs on neighboring faces. The reason for longitudinal offset of the ribs along the facial midline (Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 6.1) remains unclear, though probably each apertural lobe was formed asynchronously by two adjacent subunits of soft tissue. It is important to note that longitudinal offset or asynchronous displacement of the ribs also can be seen on hexangulaconulariids, but it has never been observed in olivooids.
Tube morphology and growth of other carinachitids
Since the ribs most likely constitute displaced plicate lobes, the four uniform plicate lobes in tetraradiate Carinachites spinatus correspond to the four rows of lateral facial ribs. Similarly, triradiate Emeiconularia and pentamerous Pentaconularia ningqiangensis Liu et al., Reference Liu, Li, Shao, Zheng, Zheng, Wang, Wang and Wang2011 most likely possessed three (Fig. 4.1, 4.2) and five centripetal plicate lobes (Fig. 4.7–4.9), respectively. Similarly, specimens with arcuate ribs reflect the presence of a set of centripetal arcuate lobes in the apertural region. Nevertheless, the apertural region of Carinachites tetrasulcatus (Chen, Reference Chen1982) is difficult to reconstruct as its ribs are low and inconspicuous (Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 8.22). Probably the aperture of this species resembled a four-sided pyramid with deep, concave corner sulci similar to those of Hexaconularia sichuanensis He and Yang, Reference He and Yang1986 (e.g., Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, fig. 7.13–7.16, 7.19–7.21). We hypothesize that this species exhibited periodical growth similar to that of Carinachites spinatus. However, such eversion probably was possible only in organic or lightly sclerotized exoskeletons and not in those with thick and rigid hard parts such as tubes of Emeiconularia trigemme (Qian et al., Reference Qian, Van Iten, Cox, Zhu and Zhuo1997). Thus, secondary, subsequent thickening of the lateral walls, suggested by the double-layered wall structure (Fig. 2), is proposed here to resolve conflicts between the flexibility of the primary apertural wall and the rigidity of the thick secondary tube wall. The thin primary apertural wall in Carinachites spinatus, represented by the outer layer with fine transverse wrinkles, may have been rich in organic material. The outer layer may have undergone subsequent thickening on its inner surface by a mixture of inorganic materials (represented by the smooth granular layer), thus resulting in a double-layered structure similar to that of conulariids (Brood, Reference Brood1995; Ford et al., Reference Ford, Van Iten and Clark2016). This model could account for: (1) the flexibility of the external tube surface (as indicated by the striations and welts shown in Conway Morris and Chen, Reference Conway Morris and Chen1992) and the relative rigidity of the entire tube wall, (2) the high abundance of fragmentary specimens of carinachitids and the extremely rare preservation of their aperture, and (3) probable variation in mechanical properties between the different layers as reflected in the secondary cracks on the tube surface (Fig. 1.11, 1.12). However, because the relic soft tissue is much smaller in diameter than the host tube, the sides of the soft body may not have been in direct contact with the lateral tube wall. How the organic or inorganic material was deposited on the inner surface of the outer layer remains unknown.
Feeding habits of carinachitids
Despite the absence of in situ preservation of carinachitid tubes, it was originally assumed that carinachitids were solitary sessile forms having their aboral end attached to hard or firm substrates (He, Reference He1987) as in extant medusozoan polyps. A pelagic habit for carinachitids is unlikely as their skeletonized tube appears to have been too dense to float in seawater. However, because the soft body was almost entirely enclosed within the tube, filter-feeding on microorganisms seems highly likely. Besides the function of supporting the growing soft tissues, periodic tube growth and thickening of carinachitids reflects competition for ecological tiering among a varied benthos. In contrast to the reduced vestigial peridermal theca of cubopolyps and most scyphistomae, the thickening of the tube wall in carinachitids, and coeval tubular fossils (anabaritiids, hyolithelminths), indicate an adaptive strategy focusing on defense against predators such as cycloneuralians (e.g., Liu et al., Reference Liu, Xiao, Shao, Broce and Zhang2014a; Zhang et al., Reference Zhang, Xiao, Liu, Yuan, Wan, Muscente, Shao, Gong and Cao2015). Interestingly, suspension feeding by the hypostome rather than normal elongate tentacles with nematocysts has also been observed in extant polyps of Eudendrium (Hydrozoa) (Puce et al., Reference Puce, Bavestrello, Arillo, Azzini and Cerrano2002), in which the mucous-lined gastroderm plays a major role in capturing food particles such as zooplankton. Such behavior correlates with high concentrations of food particles and intense water movement, a scenario that seems compatible with the marine shelf environment favored by Cambrian small shelly fossils (Yin et al., Reference Yin, He, Qian and Xiao1999; Steiner et al., Reference Steiner, Li, Qian and Zhu2004, Reference Steiner, Li, Qian, Zhu and Erdtmann2007).
The asynchronous displacement of the ribs in Carinachites spinatus indicates that the flexible tube aperture may have opened to a greater extent in this taxon than in olivooids. Relative to the radius of the tube, both the width and height of the ribs on Emeiconularia amplicanalis are smaller than in E. trigemme. This fact indicates that the oral lobes of E. amplicanalis could only partially cover the tube aperture, thus allowing continual contact of the soft body with the ambient environment. Presumably, retractile tentacles in E. amplicanalis, if present, could protrude beyond the tube opening, thus enabling limited predatorial behavior.
Comparisons
Carinachitids versus coronate polyps
Carinachitid tubes resemble the chitinous periderm of coronate scyphozoans (i.e., Stephanoscyphus), which are sheathed in a cone-shaped tube showing well-developed longitudinal folds and horizontal annulations (Chapman, Reference Chapman1966; Werner, Reference Werner1966, Reference Werner1973). However, differences between them are also evident. In particular, Stephanoscyphus may be either solitary or colonial. The periderm of colonial forms is irregularly branched (Jarms, Reference Jarms1991), in some cases with a tube-in-tube structure (Werner, Reference Werner1966, fig. 13). By contrast, carinachitid tubes are exclusively solitary. Second, whereas Stephanoscyphus has an operculum that is separate from the tube, the tube aperture and lappets of carinachitids constitute a continuous extension of the rest of the tube. Third, the scyphozoan periderm, including the peridermal teeth or cusps inside the tube of Stephanoscyphus, is secreted by ectoderm of the lateral body wall. By contrast, the external layer of the carinachitid tube, except for the apex, was generated by epithelium of the oral part, and neither teeth nor cusps are present within the carinachitid tube. Fourth, Stephanoscyphus tubes are more or less circular in transverse cross section and uniform in thickness; by contrast, carinachitid tubes are polygonal and exhibit distinct faces and corner sulci. Fifth, an operculum with triangular cusps is absent in Stephanoscyphus (Werner, Reference Werner1966), while oral lobes or lappets are a consistent diagnostic feature of the tube of carinachitids and co-occurring olivooids. Finally, strobilation, a characteristic of Stephanoscyphus, has not been observed in carinachitids. In short, these comparisons suggest that carinachitids may only be distantly related to extant scyphozoans.
Comparison with extant hydrothecae
Extant, colonial thecate hydranths begin and complete their development within a small, capsule-like hydrotheca. The hydrotheca in some species, for example Sertulariella quadrata Nutting, Reference Nutting1900a, is square in transverse cross section and exhibits dense transverse striations or longitudinal folds (i.e., S. rugosa (Linnaeus, Reference Linnaeus1758)) similar to those of carinachitid tubes (Nutting, Reference Nutting1900a). The oral end of the hydrotheca has a protective operculum with or without a set of triangular, plate-like teeth or converging cusps capable of opening and closing (Crowell, Reference Crowell1991). Many species of Sertulariella, for example S. quadrata, S. rugosa, and S. peculiaris (Leloup, 1935 in Galea, Reference Galea2008), have an operculum with four triangular cusps (Nutting, Reference Nutting1900a; Chapman, Reference Chapman1966; Galea, Reference Galea2008) that are somewhat similar to the lobes of Carinachites spinatus. Symplectoscyphus (Millard, Reference Millard1975) and S. rathbuni (Nutting, Reference Nutting1900a) have three teeth similar to those of Emeiconularia (assuming our reconstruction is correct). Notably, the chitinous hydrotheca and operculum are secreted by glandular cells of the epidermis of the hydranth, especially the hypostome (Berrill, Reference Berrill1949), thus supporting the previously inferred oral formation of carinachitid tubes. In rare cases, the apertural teeth of the hydrothecae are folded inward as in solitary carinachitid tubes (Nutting, Reference Nutting1900b, pl. 14, fig. 6). Major differences between hydrothecae and carinachitids include: (1) the colonial habit of hydrothecae; (2) the absence of triangular cusps in the lateral walls of hydrothecae; and (3) the teeth in hydrothecae, which are sheet-like, with a free adaxial end, and thus are quite different from those of carinachitids.
Comparison among carinachitids, olivooids, hexangulaconulariids, and conulariids
Prior to conducting a cladistic analysis of relationships among extant and fossil taxa within Medusozoa, morphological comparisons among olivooids, carinachitids, hexangulaconulariids, and Paleozoic conulariids are necessary. As noted previously (He, Reference He1987), the similarities among the skeletons of olivooids, carinachitids, hexangulaconulariids, and Paleozoic conulariids are striking (Table 1). They include: (1) possession of a superficially cone-shaped tube that almost completely enveloped the soft tissue (Qian and Bengtson, Reference Qian and Bengtson1989; Sendino et al., Reference Sendino, Zágoršek and Vyhlasová2011); (2) tube with serially repeated transverse and longitudinal wrinkles (Qian and Bengtson, Reference Qian and Bengtson1989) representing periodic growth by oral addition (Brood, Reference Brood1995); (3) fine, regularly spaced longitudinal striations, ~5–10 µm in width, as one of typical features of Olivooides tubes (e.g., Yue and Bengtson, Reference Yue and Bengtson1999, fig. 2D; Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, fig. 12.13, 12.14), are present also on the corner surface of Carinachites tetrasulcatus (e.g., Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 9.15, 9.16); (4) tube tapered in the apertural region (Qian and Bengtson, Reference Qian and Bengtson1989), where the tube aperture is folded inward (e.g., Brood, Reference Brood1995; Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014); (5) all tubes exhibit distinct apical and abapical regions (Fig. 1.8) (Van Iten et al., Reference Van Iten, Zhu and Li2010), although the carinachitid apex is unknown yet; and (6) radial symmetry, a characteristic that is a link to the medusozoans, is well represented by all four families.
Table 1 Morphological comparisons among olivooids, hexangulaconulariids, carinachitids, and conulariids.
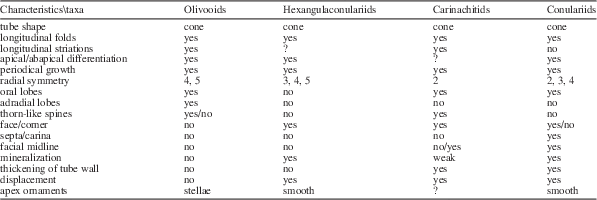
Apart from the mentioned similarities, additional specific similarities between carinachitids and olivooids are remarkable: (1) The cone-shaped tubes of these two taxa exhibit similar variation in the pattern of radial symmetry. Both of them exhibit rare pentaradial symmetry and dominant tetraradial symmetry. However, triradial symmetry is not known in olivooids. (2) The tube aperture in both Carinachites spinatus and Olivooides has four or five prominent plicate apertural lobes (usually termed ‘lobate folds’ in Yue and Bengtson, Reference Yue and Bengtson1999; Han et al., Reference Han, Kubota, Li, Ou and Wang2016a, Reference Han, Li, Kubota, Ou and Toshinob; termed ‘oral lobes’ in Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, in Olivooides multisulcatus Qian, Reference Qian1977) (Fig. 5). (3) Lateral ornaments on the tube wall, including plicate thorns (Fig. 5.1) (termed ‘plicate cornice’ in O. multisulcatus [see Han et al., Reference Han, Kubota, Li, Ou and Wang2016a, fig.2] and ‘triangular thickening’ by Steiner et al., Reference Steiner, Qian, Li, Hagadorn and Zhu2014, fig.10), are derived from the tube aperture (Yasui et al., Reference Yasui, Reimer, Liu, Yao, Kubo, Shu and Li2013). (4) As mentioned in the preceding, the soft tissues are always connected to the tube aperture. (5) There are similar patterns of tube formation except for lateral thickening, in both cases with tube formation mediated by soft tissue at the oral end (Yasui et al., Reference Yasui, Reimer, Liu, Yao, Kubo, Shu and Li2013; Liu et al., Reference Liu, Li, Shao, Zhang, Wang and Qiao2014b; Han et al., Reference Han, Kubota, Li, Ou and Wang2016a, Reference Han, Li, Kubota, Ou and Toshinob). (6) Rare preservation of the apertural end in olivooids and carinachitids as well as Paleozoic conulariids probably indicates that the newly secreted tube aperture was predominantly organic or weakly sclerotized and thus less resistant to decay than the lateral ribs.
Differences between carinachitids and olivooids also are evident. Although carinachitid tubes bear regular ribs, they were never compressed during diagenesis along the longitudinal axis as in olivooid tubes. This difference may be partially attributed to the presence in carinachitids of deeply concave corner sulci, outwardly bulging faces, and later ontogenetic thickening, thus providing stronger support for the soft body. The face-corner configuration in carinachitids also reflects an incipient differentiation of the meridian planes. The apertural lobes among different taxa of olivooids vary greatly in morphology. Thus, unlike Olivooides multisulcatus (Fig. 5.1–5.3), Quadrapygites and O. mirabilis Yue, 1984 in Xing et al., Reference Xing, Ding, Luo, He and Wang1984 lack clear differentiation in size between the principle apertural lobes and the adradial apertural lobes. By contrast, carinachitids exhibit only the principle apertural lobes. The corner sulci in carinachitids may correspond to the adradial apertural lobes in olivooids. In addition, carinachitids exhibit greater morphological variation on the faces than do olivooids, including variation in rib shape and height, displacement of ribs along the midline, and convergence of the striations. The facial ribs of carinachitids may represent a derived feature in comparison with the continuous transverse crests in olivooids. Finally, it has generally been accepted that the periderm of olivooids was organic and uniform in thickness. By contrast, the tubes of carinachitids, hexangulaconulariids, and conulariids, although showing some degree of flexibility, are relatively thick and slightly mineralized (e.g., Brood, Reference Brood1995; Qian et al., Reference Qian, Van Iten, Cox, Zhu and Zhuo1997; Leme et al., Reference Leme, Simoes, Marques and Van Iten2008; Ford et al., Reference Ford, Van Iten and Clark2016).
Similarities between carinachitids and hexangulaconulariids include: (1) sclerotization of the tube wall, (2) development of faces and corner sulci, (3) transverse ornament showing displacement/offset along the midline of the faces and corner sulci, and (4) sessile benthic mode of life on firm substrates or hard parts (e.g., Van Iten et al., Reference Van Iten, Muir, Simões, Leme, Marques and Yoder2016a). The displacement mechanism of carinachitids may have also been present in hexangulaconulariids (Conway Morris and Chen, Reference Conway Morris and Chen1992, fig. 11.12; Van Iten et al., Reference Van Iten, Zhu and Li2010, fig. 2e) and latest Ediacaran Paraconularia (e.g., Van Iten et al., Reference Van Iten, Marques, Leme, Pacheco and Simões2014, fig. 3c–d; Van Iten et al., Reference Van Iten, Leme, Pacheco, Simões, Fairchild, Rodrigues, Galante, Boggiani and Marques2016b). In this connection, it should be noted that relics of small soft parts extending along the tube axis of conulariids, originally interpreted as remains of an alimentary tract (Babcock, Reference Babcock1989), most likely represent polyps as previously suggested by Van Iten (Reference Van Iten1991) and supported by currently available material of carinachitids and the internal anatomy of Olivooides (e.g., Han et al., Reference Han, Kubota, Li, Ou and Wang2016a).
Differences between carinachitids and hexangulaconulariids also are evident. In particular, the pseudohexaradial symmetry of hexangulaconulariid tubes, which exhibit a fundamental bimerous tetraradial symmetry, reflects further morphological differentiation of the meridian planes within a framework of tetraradial symmetry. Such meridian plane differentiation, oriented perpendicular to the longitudinal axis, probably indicates unknown differentiation of soft part structures such as gonads, septa, and the vascular system. Finally, the apertural lobes of hexangulaconulariids are not triangular as in carinachitids and olivooids.
Carinachitids share detailed similarities with Paleozoic conulariids (except for Cambrian Baccaconularia in Hughes et al., Reference Hughes, Gunderson and Weedon2000) in face/corner sulcus differentiation and formation of apertural lobes (equivalent to the apertural lappets of Sendino et al., Reference Sendino, Zágoršek and Vyhlasová2011), and both taxa exhibit tri-, tetra- or pentaradial symmetry. However, biradial symmetry, common in conulariids, has not been observed in carinachitids. In addition, the corners of some conulariids, for example Eoconularia loculata (Wiman, 1895 in Jerre, Reference Jerre1994), are much thicker than the faces (Jerre, Reference Jerre1994), contrasting with the relatively thickened faces of carinachitid Emeiconularia trigemme (Fig. 2.1b). Moreover, in addition to plicate apertural lobes (Ford et al., Reference Ford, Van Iten and Clark2016), conulariids exhibit two other types of apertural lobes (Sendino et al., Reference Sendino, Zágoršek and Vyhlasová2011). Finally, the internal anatomy of the tube wall of conulariids is much more complex at the corners and midlines than in carinachitids, as summarized by Van Iten (Reference Van Iten1991, Reference Van Iten1992b). For example, there are eight types of internal midline structures (Bischoff, Reference Bischoff1978; Van Iten, Reference Van Iten1991, Reference Van Iten1992b; Jerre, Reference Jerre1994), including: (1) a single continuous (nonseriated) carina, and (2) a pair of continuous carinae (flanking the midline), (3) a pair of seriated carinae, (4) a single seriated carina (subsequently discovered by Hughes et al., Reference Hughes, Gunderson and Weedon2000 in Baccaconularia), and (5) the Y-shaped continuous single carina documented by Jerre (Reference Jerre1994) in Eoconularia loculata (Wiman). The corners may be: (1) nonthickened, (2) thickened without formation of a clear carina, (3) thickened and bearing a distinct nonseriated carina, or (4) thickened and bearing a seriated distinct carina.
In summary, gross morphological comparisons of the skeletons of olivooids, carinachitids, hexangulaconulariids, and Paleozoic conulariids support the previous hypothesis (He, Reference He1987) that these fossil taxa represent closely related lineages within the Conulata. Since the olivooid soft body exhibits a manubrium within a subumbrellar cavity, tentacles, apertural lappets, and frenula (Han et al., Reference Han, Kubota, Li, Ou and Wang2016a, Reference Han, Li, Kubota, Ou and Toshinob), olivooids and hence all conulatans probably were medusozoans (Van Iten et al., Reference Van Iten, de Moraes Leme, Simões, Marques and Collins2006) that were related either to extant cubozoans (Han et al., Reference Han, Kubota, Li, Yao and Yang2013; Han et al., Reference Han, Kubota, Li, Ou and Wang2016a,Reference Han, Li, Kubota, Ou and Toshinob) or to scyphozoans (Dong et al., Reference Dong, Cunningham, Bengtson, Thomas, Liu, Stampanoni and Donoghue2013; Liu et al., Reference Liu, Li, Shao, Zhang, Wang and Qiao2014b; Van Iten et al., Reference Van Iten, Marques, Leme, Pacheco and Simões2014). The proposal that Conulata constitutes an independent phylum (Babcock et al., Reference Babcock, Feldmann, Hoffmann and Nitecki1986; Brood, Reference Brood1995) or the internal rachis of sea pens (Conway Morris and Chen, Reference Conway Morris and Chen1992) appears unlikely. Carinachitids, originally interpreted as the most primitive taxa within Conulata (He, Reference He1987), are interpreted here as a stock of phylogenetically intermediate forms between olivooids and hexangulaconulariids. The presence of corner sulci and faces with a median line (midline) probably represent synapomorphies of carinachitids, hexangulaconulariids, and conulariids. The general similarities shared by olivooids, hexangulaconulariids, carinachitids, and conulariids (i.e., radial symmetry), probably represent primitive conditions. Finally, the bimerous tetraradial symmetry of hexangulaconulariids may have been independently acquired in this lineage. However, these interpretations await future phylogenetical analysis.
Orientation of the radial symmetry planes in carinachitids
Similarities in gross morphology between carinachitids, olivooids, and conulariids suggest that their peridermal apertural lobes are homologous structures. If this hypothesis is correct, then the orientation of the meridian planes of olivooids and Olivooides-like medusozoans (Han et al., Reference Han, Kubota, Li, Yao and Yang2013, Reference Han, Kubota, Li, Ou and Wang2016a, Reference Han, Li, Kubota, Ou and Toshinob) may shed new light on the orientation of these planes in carinachitids and conulariids. In the soft body of Olivooides, the perradial frenula and apertural lappets, which correspond in position to the perradial pockets (e.g., Han et al., Reference Han, Kubota, Li, Yao and Yang2013, fig. 3), probably were responsible for the formation and closure of the plicate lobes of the periderm (Han et al., Reference Han, Kubota, Li, Ou and Wang2016a, figs. 3–5). Apart from the adradial frenulae and apertural lappets, no frenulae or apertural lappets are present in the interradii, where the interradial septa connect the subumbrellar and exumbrellar walls, and there is no interradial apertural lobe on the peridermal tube (Fig. 5). Similarly in carinachitids, the bulging faces and corner sulci may directly reflect the configuration of the tube aperture, and they may correspond in position, respectively, to the perradial pockets and interradial septa of the gastric cavity. This means: (1) that the midline of the facial ribs and the corner sulci were most likely located at the perradii and interradii, respectively; and (2) that the corner sulci may correspond to the former interradial septa/mesenteries (Fig. 3). This orientation may also apply to conulariids if indeed their apertural lobes are homologous with those of carinachitids and olivooids. It should be noted, however, that our suggested orientation of the interradial symmetry planes in conulariids differs from the traditional hypothesis, which is based on similarities between the conulariid and coronate periderms and between the midline carinae of Eoconularia loculata Wiman and the gastric septa of stauromedusans (Van Iten et al., Reference Van Iten, de Moraes Leme, Simões, Marques and Collins2006). According to this hypothesis, the apertural lobes and facial midlines in conulariids were situated at the interradii (Chapman, Reference Chapman1966; Werner, Reference Werner1966; Van Iten, Reference Van Iten1992a; Jerre, Reference Jerre1994). Confirming or disproving this hypothesis will require the discovery of additional and better-preserved relic soft tissues in conulariids.
Conclusions
A single, exceptionally well-preserved specimen of Carinachites spinatus, documented for the first time in the present paper, reveals that the apertural end of the skeletal tube of tetraradial carinachitids exhibits four plicate lobes that are similar to those of co-occurring olivooids and younger conulariids. Similarities between the lateral tube spines and the apertural lobes of carinachitids indicate that all of the transverse ribs on the faces were released adorally and were eventually displaced toward the edges of the tube, a pattern of growth similar to that of co-occurring olivooids. The internal anatomy and symmetry of Olivooides suggest a perradial and interradii disposition, respectively, for the four faces and corner sulci of carinachitids. These findings corroborate the previously proposed hypothesis that early Cambrian carinachitids, hexangulaconulariids, olivooids, and conulariids are closed related taxa within the subphylum Medusozoa, although olivooids may have retained certain primitive features.
Acknowledgments
We thank Drs. H. Van Iten (Hanover College, USA), C.B. Skovsted (Swedish Museum of Natural History), and G.A. Brock (Macquarie University) for their suggestions and linguistic improvement of the manuscript. We also thank H.J. Gong, J. Sun, J. Luo, and M.R. Cheng (Northwest University, Xi’an, China) for their assistance in the field and with lab work. X. Han prepared the 3D drawings of the carinachitid specimens, and Y.H. Liu (Chang’an University) provided two carinachitid photos. This work was supported by the Natural Science Foundation of China (NSFC grant 41272019, 41621003, 41372021, 41472015), the ‘973 project’ of the Ministry of Science and Technology of China (grant 2013CB835002, 2013CB837100), the Chinese Academy of Sciences (XDB10010101), and the State Key Laboratory of Palaeobiology and Stratigraphy (No. 163107).








