Introduction
The Ordovician through early Silurian was a significant interval in the evolutionary history of the Crinoidea. In general, echinoderms, including crinoids, experienced an adaptive radiation during the Great Ordovician Biodiversification Event (GOBE) (Guensburg and Sprinkle Reference Guensburg, Sprinkle, Zhuravlev and Riding2000; Sprinkle and Guensburg, Reference Sprinkle, Guensburg, Webby, Paris, Droser and Percival2004; Webby et al., Reference Webby, Paris, Droser and Percival2004; Peters and Ausich, Reference Peters and Ausich2008; Lefebvre et al., Reference Lefebvre, Sumrall, Shroat-Lewis, Reich, Webster, Hunter, Nardin, Rozhnov, Guensburg and Touzeau2013). The most significant crinoid biodiversification began in earnest during the Sandbian and continued through the Katian, after which crinoids experienced a mass extinction event at the onset of the Hirnantian glaciation (Peters and Ausich, Reference Peters and Ausich2008). Profound turnover for all echinoderms occurred during the Late Ordovician, and this event also resulted in a macroevolutionary turnover for crinoid faunas (Baumiller, Reference Baumiller1993; Ausich et al., Reference Ausich, Kammer and Baumiller1994; Ausich and Deline, Reference Ausich and Deline2012; Deline et al., Reference Deline, Ausich and Brett2012). Late Ordovician extinctions ended the early Paleozoic Crinoid Evolutionary Fauna (CEF), and preferential radiation of different crinoid clades during the post-Ordovician radiation yielded the middle Paleozoic CEF. Ordovician faunas were typically dominated by diplobathrid camerate, disparid, and hybocrinid crinoids, which were replaced by faunas typically dominated by monobathrid camerate, cladid, and flexible crinoids.
This extinction occurred during the Late Ordovician glacial epoch, with global climate change and habitat destruction resulting from the global ocean regression during this glaciation. Various drivers for this climate change have been proposed, among others as a consequence of plate tectonic history (Kump et al., Reference Kump, Arthur, Patzkowsky, Gibbs, Pinkus and Sheehan1999; Herrmann et al., Reference Herrmann, Patkowsky and Pollard2004; Nardin et al., Reference Nardin, Goddéris, Donnadieu, Le Hir, Blakely, Pucéa and Artez2011), high cosmic ray flux (Shaviv and Veizer, Reference Shaviv and Veizer2003), extensive volcanic eruptions that resulted in a large igneous province (Buggisch et al., Reference Buggisch, Joachimski, Lehnert, Bergström, Repetski and Webers2010), massive weathering of volcanic rocks (Lefebvre et al., Reference Lefebvre, Servais, François and Averbuch2010), and ocean euxinia (Zou et al., Reference Zou, Qui, Poulton, Dong, Wang, Chen, Lu, Shi and Tao2018). Whatever the causes, the Late Ordovician mass extinction is generally regarded as the second-most significant Phanerozoic mass extinction (Sepkoski, Reference Sepkoski and Walliser1996).
Until recently, the general absence of echinoderm fossil occurrences from the latest Ordovician (Hirnantian)–earliest Silurian (Llandovery) interval has prevented a nuanced understanding of this critical interval of pelmatozoan evolution. Recent discovery of Hirnantian and Rhuddanian crinoids (e.g., Eckert, Reference Eckert1984, Reference Eckert1990; Ausich, Reference Ausich1984a, Reference Ausichb, Reference Ausich1985, Reference Ausich1986a, Reference Ausichb, Reference Ausichc, Reference Ausich1987; Donovan, Reference Donovan1993; Fearnhead and Donovan, Reference Fearnhead and Donovan2007; Ausich and Copper, Reference Ausich and Copper2010; Ausich et al., Reference Ausich, Peter and Ettensohn2015b; Ausich and Wilson, Reference Ausich and Wilson2016) has improved our understanding of crinoids through this interval. However, it was not until the description of Anticosti Island crinoids was completed that sufficient data were available to evaluate crinoid evolutionary dynamics more critically through this biodiversity crisis (Peters and Ausich, Reference Peters and Ausich2008; Ausich and Deline, Reference Ausich and Deline2012). The extreme importance of Anticosti Island crinoid faunas necessitates the present paper, which describes new material and updates the known stratigraphic distribution of Ordovician to Silurian crinoids from Anticosti Island.
Geography and stratigraphic occurrences of Anticosti Island crinoids
Anticosti Island (Fig. 1) is in the Gulf of St. Lawrence north of the Gaspé Peninsula in Québec, Canada. The strata on Anticosti Island include a nearly complete Katian (Ordovician) through Llandovery (Silurian) stratigraphic section (Fig. 2), which was described initially by Richardson (Reference Richardson1857). Perhaps because the section is so complete compared to other regions in North America, the position of Ordovician-Silurian boundary on Anticosti Island has not been settled (e.g., Schuchert and Twenhofel, Reference Schuchert and Twenhofel1910; Twenhofel, Reference Twenhofel1928; Bolton, Reference Bolton1961; Cocks and Copper, Reference Cocks and Copper1981; Petryk, Reference Petryk and Lespérance1981; Long and Copper, Reference Long and Copper1987; Barnes, Reference Barnes1988; Copper, Reference Copper1989, Reference Copper2001; Desrochers et al., Reference Desrochers, Farley, Achab, Asselin, Kröger and Servais2008, Reference Desrochers, Farley, Achab, Asselin and Riva2010; Jin and Copper, Reference Jin and Copper2008; Copper et al., Reference Copper, Jin and Desrochers2013; and others). Most recently, Copper et al. (Reference Copper, Jin and Desrochers2013) regarded the top of the Ordovician as the top of the grainstone facies immediately above the Laframboise reefs.

Figure 1. Geologic Map Anticosti Island, Québec, Canada.
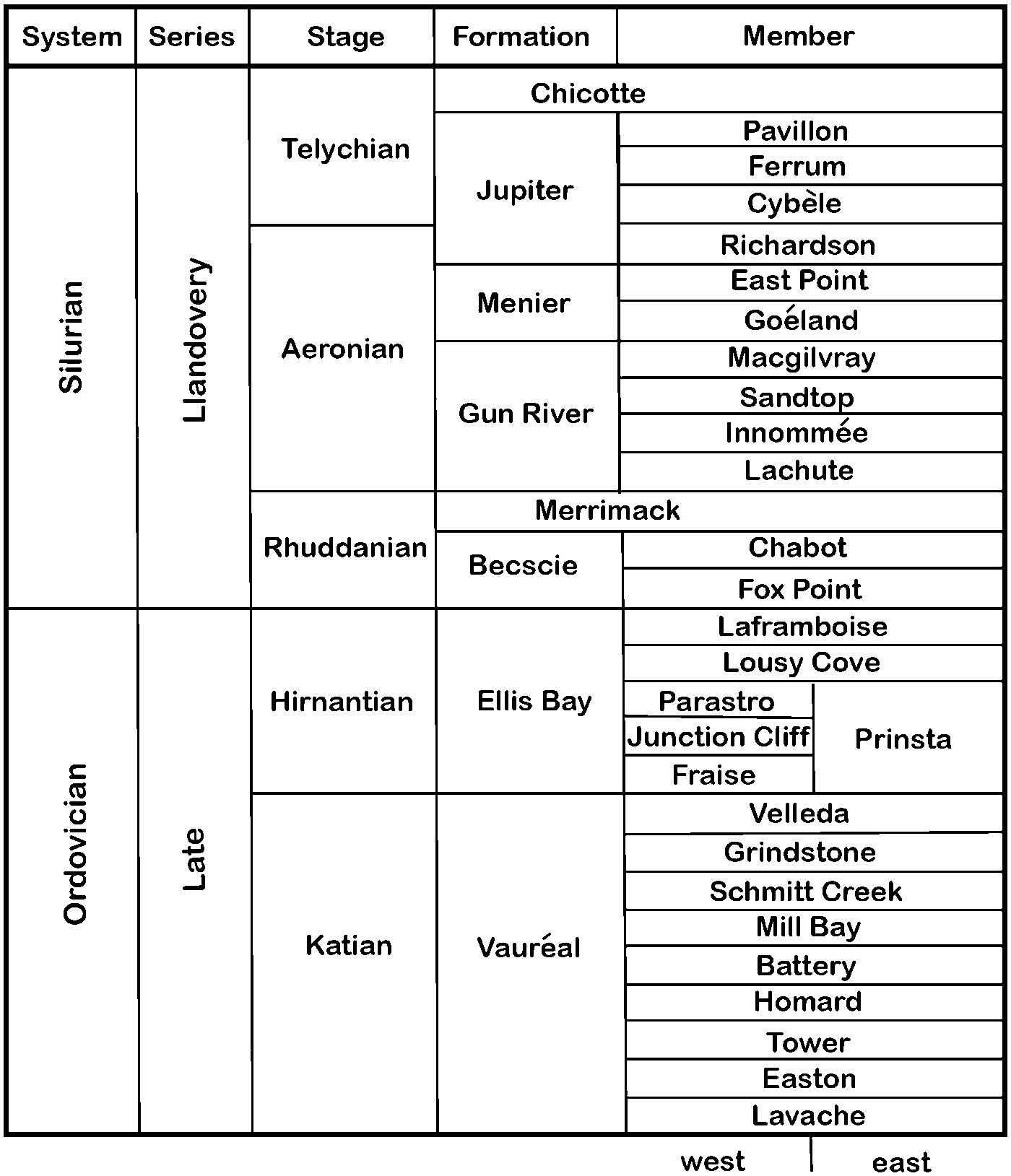
Figure 2. Ordovician to Silurian stratigraphic column for Anticosti Island (modified from Copper et al., Reference Copper, Jin and Desrochers2013).
Because of facies changes in the Ordovician-Silurian boundary interval from east to west across the island and the lack of many key, cosmopolitan, biostratigraphically important fossils, stratigraphic uncertainty has existed. A comprehensive synthesis of fossil stratigraphic distribution, facies, and sequence stratigraphy is the basis for the stratigraphic revisions proposed in Copper et al. (Reference Copper, Jin and Desrochers2013). For the present study, the significant changes include recognition that some Late Ordovician lithostratigraphic units do not extend across the island from east to west, new lithostratigraphic names have been applied for Hirnantian and Llandovery strata in western Anticosti Island, and the base of the Katian-Hirnantian boundary was changed.
Revised stratigraphic distribution of Anticosti crinoids
As noted above, revisions to understanding of Hirnantian and Llandovery chronostratigraphy (Fig. 2) of Anticosti Island necessitates reconsideration of the stratigraphic distribution of crinoids, which is listed in Supplementary Tables1–5. Several changes are noteworthy. One new genus and seven new species are described, including the second species of Becsciecrinus and Bucucrinus. Hirnantian taxa from the west end of the island previously described from the Grindstone and Velleda members of Ellis Bay are reassigned to new members (Copper et al., Reference Copper, Jin and Desrochers2013) (Supplementary Table 3); and with division of the former Jupiter Formation into the Menier and Jupiter (revised) formations, crinoid distributions are revised accordingly (Supplementary Tables 4 and 5).
Based on the inferred stratigraphic occurrence of Gaurocrinus fimbriatus (Billings, Reference Billings1859) and Plicodendrocrinus observationensis Ausich and Copper, Reference Ausich and Copper2010, the range of both of these taxa are extended higher in the Katian to either the Schmitt Creek or Grindstone member of the Vauréal Formaiton. The existence of Xenocrinus rubus Ausich and Copper, Reference Ausich and Copper2010 in Silurian strata along the Jupiter River confirms that this species crossed the Ordovician-Silurian boundary, which verifies three boundary-crossing species: Dendrocrinus leptos Ausich and Copper, Reference Ausich and Copper2010, Protaxocrinus paraios Ausich and Copper, Reference Ausich and Copper2010, and X. rubus. Boundary-crossing genera are Dendrocrinus, Eomyelodactylus, Plicodendrocrinus, Protaxocrinus, and Xenocrinus. With the exception of Eomyelodactylus, all of these boundary-crossing taxa belonged to major clades that would dominate the Middle Paleozoic CEF (Baumiller, Reference Baumiller1993; Ausich et al., Reference Ausich, Kammer and Baumiller1994; Ausich and Deline, Reference Ausich and Deline2012). Further, despite the fact that disparids in general were not a typically dominant clade after the Ordovician, the myelodactyid clade radiated during the Silurian.
Plicodendrocrinus petryki n. sp. is the first Silurian species of Plicodendrocrinus described from North America. Stratigraphic range extensions include Becsciecrinus, which is now known from the Fox Point Member of the Becscie Formation (Rhuddanian) to the Ferrum Member of the Jupiter Formation (Telychian); Dimerocrinites elegans Springer, Reference Springer1928, which is now known from the Cybèle through Pavillon members of the Jupiter Formation (Telychian); and Aetocrinus gracilis Ausich and Copper, Reference Ausich and Copper2010 and Jovacrinus, which are now known from the Ferrum and Pavillon members of the Jupiter Formation (Telychian).
Paleoecological associations
Supplementary Tables 1–5 list the crinoids in each stratigraphic unit; but in most cases, crinoids are typically preserved either individually or with multiple specimens of the same species. Multiple species are rarely preserved on the same bedding surface. Striking exceptions are the crinoid slabs deposited as MPEP718.1 and MPEP718.2 from the Pavillon Member of the Jupiter Formation (Telychian) at Brisants Jumpers (Locality 6) (Fig. 3). These slabs have innumerable specimens present with three dominant taxa: Dimerocrinites elegans; Aetocrinus gracilis; and Eomyelodactylus springeri Ausich and Copper, Reference Ausich and Copper2010. Poorly preserved specimens of additional taxa may also be present. Dimerocrinites elegans is the larger of the three dominant species and is most evident on initial inspection of the material (the large pluricolumnals also belong to Dimerocrinites elegans). However, A. gracilis is the most abundant species, with much of the smaller, articulated or partially articulated crinoidal material belonging to A. gracilis. Eomyelodactylus springeri is rare but present on both MPEP718.1 and MPEP718.2.

Figure 3. Multispecies bedding surface from Locality 6 (Pavillon Member of the Jupiter Formation); MPEP718.1. Three dominant taxa are present on this bedding surface: (1) Dimerocrinites elegans Springer, Reference Springer1928 (see Fig. 5.3); (2) Aetocrinus gracilis Ausich and Copper, Reference Ausich and Copper2010 (see Fig. 13.5); and (3) Eomyelodactylus springeri Ausich and Copper, Reference Ausich and Copper2010 (see Fig. 12.2). Scale bar 5 mm.
The presence of gastropods on the tegmen or on other locations on crinoids is a well-known association in Paleozoic faunas, although the exact ecological interaction between gastropods and crinoids has engendered much discussion (e.g., Bowsher, Reference Bowsher1955; Meyer and Ausich, Reference Meyer, Ausich, Tevesz and McCall1983; Baumiller, Reference Baumiller1990, Reference Baumiller2002; Baumiller and Gahn, Reference Baumiller, Gahn, Kelley, Kowalewski and Hanson2003; Gahn and Baumiller, Reference Gahn and Baumiller2003, Reference Gahn and Baumiller2006; Baumiller et al., Reference Baumiller, Gahn, Savill, Heinzeller and Nebelsick2004). MPEP1138.6 is a specimen of Fibrocrinus phragmos Ausich and Copper (Reference Ausich and Copper2010) from the East Point Member of the Menier Formation (Aeronian). It is a partially disarticulated crown that is broken longitudinally parallel to the oral-aboral axis of the specimen. Present on the tegmen and otherwise concealed within the arms is a gastropod (Fig. 4.1). This is the first such association recorded in crinoids from Anticosti Island.

Figure 4. Paleoecological associations with Anticosti Island crinoids. (1) Longitudinal section through a slightly disarticulated crown of Fibrocrinus phragmos Ausich and Copper, Reference Ausich and Copper2010; immediately above the tegmen and within the arms is the cross section through a gastropod; East Point Member, Menier Formation; MPEP1138.6; (2) crinoid pluricolumnal completely encased by favositid coral; MPEP308.11. All scale bars 5 mm.
A second biotic interaction not previously known on Anticosti Island (Locality 2) is a tabulate coral as an epizoan on a crinoid pluricolumnal (Fig. 4.2). Both examples of this interaction have the coral growing around the pluricolumnal. In MPEP308.11, the coral grew over one end of the pluricolumnal, but the other end of the column is exposed. In MPEP308.10, both ends of the column are exposed. Because the coral colony grew all around the column, it is probable that this association only occurred when the crinoid was still erect. It is possible that MPEP308.11 is an indication that the host crinoid had lost its crown, but the column remained erect (see Ausich and Baumiller, Reference Ausich and Baumiller1993; Oji and Amemija, Reference Oji and Amemiya1998).
Materials and methods
Localities and specimen preparation
New specimens reported here are from several new, small collections not available to Ausich and Copper (Reference Ausich and Copper2010). One new collection is housed in the Royal Ontario Museum (ROMIP), Toronto, Canada. Seven additional collections have been donated to the Musée de paléontologie et l’évolution, Montréal, Canada (MPEP), including those made by David Clark, Nathalie Daoust/Mario Cournoyer, Pierre Groulx, Phillip Isotalo, Markus Martin, Daniel Saint-Laurent, and the Geological Survey of Canada (collected by Allen Petryk). Unfortunately, the location and stratigraphic data for these new collections is variable from precise locations to “along the Jupiter River.” Most of the more recent of these collections have well-documented geographic locations, and the stratigraphic positions of collections are precise or can be inferred with reasonable certainty. Unfortunately, this is not the case for all collections.
The key to the Allen Petryk field numbers did not accompany his specimens. However, when acquired, his collections were still in field bags. As far as possible, the stratigraphic intervals of his collections were inferred by the associated fauna (Supplemental Appendix).
In many cases, insufficient locality and stratigraphic data render a specimen useless for scientific study. However, Anticosti Island is a relatively small island with known stratigraphy and a well-documented fossil record. So, in nearly all cases, stratigraphic occurrences can be established or interpreted with reasonable certainty to at least the stage level. The Supplemental Appendix lists known or inferred geographic and stratigraphic information of the collections studied herein.
All measurements are in mm; * indicates an incomplete measurement or a crushed specimen; and measurement abbreviations are as follows: ACH, aboral cup height; ACW, aboral cup width; dACW, distal aboral cup height; pACW, proximal aboral cup height; AH, arm height; ASH, anal sac height; BH, basal plate height; BW, basal plate width; CaH, calyx height; CaW, maximum calyx width; CoH, column height; CrH, crown height; IH, infrabasal plate height; iIH, infrabasal circlet height in interradial position; rIH, infrabasal circlet height in radial position; IW, infrabasal plate circlet width; RH, radial plate height; and RW, radial plate width. Specimens were photographed either after whitening with NH4Cl, immersed in alcohol, or with no treatment.
Additional discussion of new occurrence data on Anticosti Island crinoids is in the Supplementary Data.
Repositories and institutional abbreviations
New material is deposited in the Royal Ontario Museum (ROMIP), Toronto, Canada; and Musée de paléontologie et de l’évolution, Montréal, Canada (MPEP). Previously published specimens cited are from the Geological Survey of Canada, Ottawa (GSC); the University of Alberta (UA); and the Yale University Peabody Museum (YPM).
Systematic paleontology
The classification used herein follows the phylogeny-based revision of crinoid higher taxa by Wright et al. (Reference Wright, Ausich, Cole, Peter and Rhenberg2017). Recent phylogenetic analyses find disparids to be sister to the Cladida, with hybocrinids nested within the Cladida and sister to the porocrinids (Ausich et al., Reference Ausich, Kammer, Rhenberg and Wright2015a; Wright, Reference Wright2017). At higher taxonomic levels, both disparids and hybocrinids belong to the infraclass Inadunata, which is placed within the newly resurrected subclass Pentacrinoidea (Wright et al., Reference Wright, Ausich, Cole, Peter and Rhenberg2017). Morphological terminology follows Ubaghs (Reference Ubaghs, Moore and Teichert1978) and Ausich et al. (Reference Ausich, Brett, Hess, Simms, Hess, Ausich, Brett and Simms1999), with modifications from Ausich (Reference Ausich1998), Ausich et al. (Reference Ausich, Kammer, Rhenberg and Wright2015a), and Webster and Maples (Reference Webster, Maples, Ausich and Webster2008, fig. 10.2; for brachial plate terminology). Plating of interrays is given by the number of plates in each range from the proximal-most plate to the last range before the tegmen, if known. In the posterior interray, the primanal is indicated by “P,” and the first interradial plate in regular interrays is indicated by “1.” “?” indicates that more distal plating is unknown.
Class Crinoidea Miller, Reference Miller1821
Subclass Camerata Wachsmuth and Springer, Reference Wachsmuth and Springer1885
Infraclass Eucamerata Cole, Reference Cole2017
Order Diplobathrida Moore and Laudon, Reference Moore and Laudon1943
Family Reteocrinidae Wachsmuth and Springer, Reference Wachsmuth and Springer1885
Genus Gaurocrinus Miller, Reference Miller1883
Type species
Gaurocrinus nealli Hall, Reference Hall1866.
Gaurocrinus fimbriatus (Billings, Reference Billings1859)
Figure 5.1, 5.2, 5.4
Holotype
GSC 1994.

Figure 5. Anticosti Island diplobathrid camerates. (1, 2, 4) Gaurocrinus fimbriatus (Billings, Reference Billings1859), MPEP 476.9; (1) B ray lateral view of calyx with arms mostly missing; (2) oral view of tegmen, not small, multiplated tegmen and relatively short anal sac at CD-interray side of tegmen; (4) CD interray view of theca and proximal-most brachials. (3) Lateral view of Dimerocrinites elegans Springer, Reference Springer1928; see Figure 3.1; Locality 6 (Pavillon Member of the Jupiter Formation); MPEP718.1. (5, 6) Bucucrinus isotaloi n. sp.; holotype, ROMIP 54202; (5) oral view of cross section through proximal portion of free arms that compare to (6); at arrows, note webbing that connects proximal arms; (6) lateral view of calyx and proximal arms; arrow denotes level of webbing in (5). All scale bars 5.0 mm.
Occurrence
Gaurocrinus fimbriatus is known with certainty from the Easton Member, Vauréal Formation (Katian); from several localities on Anticosti Island, Québec, Canada (Ausich and Copper, Reference Ausich and Copper2010). New specimens are from Locality 16, which are inferred to be from either the Schmitt Creek or Grindstone members of the Vauréal Formation (Katian).
Materials
MPEP476.4a, MPEP476.4b, MPEP476.7, and MPEP476.9.
Remarks
Several specimens of Gaurocrinus fimbriatus (Billings, Reference Billings1859) were reported by Ausich and Copper (Reference Ausich and Copper2010) with all of the verifiable occurrences from the Easton Member of the Vauréal Formation. Four new, well-preserved specimens are reported herein from the Petryk Collection. MPEP476.9 (Fig. 5.1, 5.2, 5.4) is a particularly instructive specimen because, unlike most other specimens, this has not been flattened during preservation. MPEP476.9 has variability in the number of fixed primibrachials and corresponding differences in the number of fixed intrabrachial plates within half-rays. Further, this specimen confirms the morphology of the tegmen previously known only from one specimen (GSC 12661 h, Ausich and Copper, Reference Ausich and Copper2010, pl. 1, fig. 3). The relatively low tegmen is composed of innumerable small plates, and the anal tube is a relatively short, conical structure (Fig. 5.2, 5.4).
New specimens of G. fimbriatus co-occur with Plicodendrocrinus observationensis in the Petryk collection (Locality 16). The only information accompanying these specimens is “Ordovician.” Gaurocrinus fimbriatus was known only from the Easton Member of the Vauréal Formation, and P. observationensis was known from the Easton and Tower Members of the Vauréal Formation (Ausich and Copper, Reference Ausich and Copper2010). However, these new crinoids co-occur with aulacerid stromatoporoids, which suggest they are also from higher in the Katian in either the Schmitt Creek or Grindstone member of the Vauréal Formation.
Family Rhodocrinitidae Roemer, Reference Roemer, Bronn and Roemer1855
Genus Bucucrinus Ausich and Copper, Reference Ausich and Copper2010
Type species
Bucucrinus saccus Ausich and Copper, Reference Ausich and Copper2010.
Diagnosis
Rhodocrinitid with high bowl-shaped calyx, only distal corners or all of infrabasal plates visible in lateral view, 1-2 plating in proximal part of regular interrays, P-3 plating in proximal part of CD interray, median ray ridges defined by convexity of ray plates, no anitaxial ridge, primanal heptagonal, no fixed pinnules, 30–40 free arms, brachials cuneate (modified from Ausich and Copper, Reference Ausich and Copper2010).
Bucucrinus isotaloi new species
Figure 5.5, 5.6
Holotype
ROMIP 54202.
Diagnosis
Bucucrinus with full height of infrabasal plates visible in side view, basal plates approximately as high as wide, radial plates approximately as high as wide, interradial plates slightly convex, first interradial plate as high as wide and larger than radial plates, at least 12 ranges of interradial plates, 30 free arms, proximal brachials with typical cuneate uniserial appearance but connected by presumably flexible plating connecting adjacent arms, ~13 typically appearing cuneate uniserial brachial actually a free brachial, free brachials 2.0–4.0 times wider than high.
Occurrence
Llandovery (Aeronian or Telychian) from strata along the Jupiter River; Anticosti Island, Québec, Canada (Locality 31).
Description
Calyx, large; high bowl shaped; broad ray ridge defined by convex ray plates (Fig. 5.6); interradial plates smooth, slightly convex.
Infrabasal circlet very low, visible in side view; 4.0% of calyx height; infrabasal concavity. Basal circlet height estimated to be ~11.0% of calyx height; basal plates five, equal in size; approximately as wide as high. Radial circlet estimated to be ~14.0% of calyx height; radial plates five, hexagonal, approximately as high as wide. Radial circlet interrupted in all interrays by sutural contact of basal plates with first interradial plates.
Normal interrays in contact with tegmen, plate sculpturing as described above; first interradial plate heptagonal, as high as wide, larger than radial plates and first primibrachials; in sutural contact with basal plate in all interrays. Second range typically with two plates; plating 1-2-3-2-3-2-2-1-2-2-2-. Above what appears to be the distal edge of the calyx (brachials assume appearance of typical free uniserial brachials) interray plating forms what may to be a flexible plated membrane connecting adjacent proximal arms that extends through at least the 12th of the “apparent free brachials” (Fig. 5.5).
Primanal not preserved.
First primibrachial hexagonal, approximately as high as wide, approximately the same size as radial plates and primaxil; second primibrachial axillary, septagonal. Second secundibrachial axillary, second tertibrachial axillary, and fourth tertibrachial axillary on medial tertibrachitaxes. Fields of intrabrachial plates between secundibrachials, tertibrachials, and quartibrachials medially within brachitaxes. Apparently flexible plating in inter- and intrarays connecting adjacent proximal arms to approximately the fourth tertibrachial medially and the 12th secundibrachial brachial abmedially. Tegmen not preserved.
Free arms 30, atomous as known, pinnulate. Brachials cuneate, uniserial, aborally convex, ~2.0–4.0 times wider than high. Arms free above flexible plating at approximately the 13th cuneate uniserial brachial.
Column not preserved.
Etymology
The species name recognizes Phillip Isotalo, who donated specimens for this study.
Materials
The holotype (ROMIP 54202) is the only known specimen.
Measurements
CrH, 69.5*; CaH, 43.9; CaW, 40*.
Remarks
The flexible plating between the proximal cuneate brachials is diagnostic for B. isotaloi n. sp. This is an unusual feature that is similar to crinoids such as Scyphocrinites (Silurian–Devonian, monobathrid Eucamerata), and may be sufficient to define a new genus; however, this is not considered advisable until more is known about the complete morphology of both B. saccus and B. isotaloi n. sp. Supplemental Table 6 lists the diagnostic features of the two species of Bucucrinus.
Data with the specimen indicate that it is from Silurian strata along the Jupiter River, Anticosti Island, which indicates a Llandovery age. Bucucrinus saccus was collected from the Jupiter River Rock Pool cliff section down river from six-mile cabin. Based on the matrix, it is plausible that the holotype of B. isotaloi n. sp. is also from either the Menier or Jupiter formation, but we cannot speculate on the member or whether it is from Aeronian or Telychian strata
Family Dimerocrinitidae von Zittel, Reference Zittel1879
Genus Dimerocrinites Phillips, Reference Phillips1839
Type species
Dimerocrinites decadactylus Roemer, Reference Roemer, Bronn and Roemer1855.
Dimerocrinites elegans Springer, Reference Springer1928
Figures 3.1, 5.3
Holotype
YPM 20483.
Materials
As noted in Ausich and Copper (Reference Ausich and Copper2010), many specimens are known. Numerous additional specimens are present in the new material on MPEP718.1–MPEP718.4: multiple specimens are on MPEP718.1 and MPEP718.2; MPEP718.3 and MPEP718.4 are single specimens.
Occurrence
Cybèle, Ferrum, and Pavillon members, Jupiter Formation (Llandovery, Telychian); Anticosti Island, Québec, Canada.
Remarks
Numerous new specimens of Dimerocrinites elegans Springer, Reference Springer1928 are from Brisants Jumpers (Pavillon Member, Jupiter Formation, MPEP718) (Locality 6). As noted above, specimens MPEP718.1 and MPEP718.2 are remarkable slabs with numerous complete and partial specimens of D. elegans (Figs. 3.1, 5.3) as well as Aetocrinus gracilis and Eomyelodactylus springeri.
Genus Becsciecrinus Ausich and Copper, Reference Ausich and Copper2010
Type species
Becsciecrinus adonis Ausich and Copper, Reference Ausich and Copper2010.
Becsciecrinus groulxi new species
Figures 6, 7.1

Figure 6. Becsciecrinus groulxi n. sp. (1–6) (1) Holotype and paratype on a single bedding surface, ROMIP 54198; (2, 5, 6) CD interray, lateral view of calyx and proximal column; holotype, ROMIP 54198a; (2) whitened; (5) no whitening; (6) photographed under alcohol; (3, 4) crown with long length of column, paratype, MPEP719.1; (3) entire specimen; (4) enlargement, specimen silicified and coarsely preserved. Scale bars 5.0 mm, unless otherwise labeled.
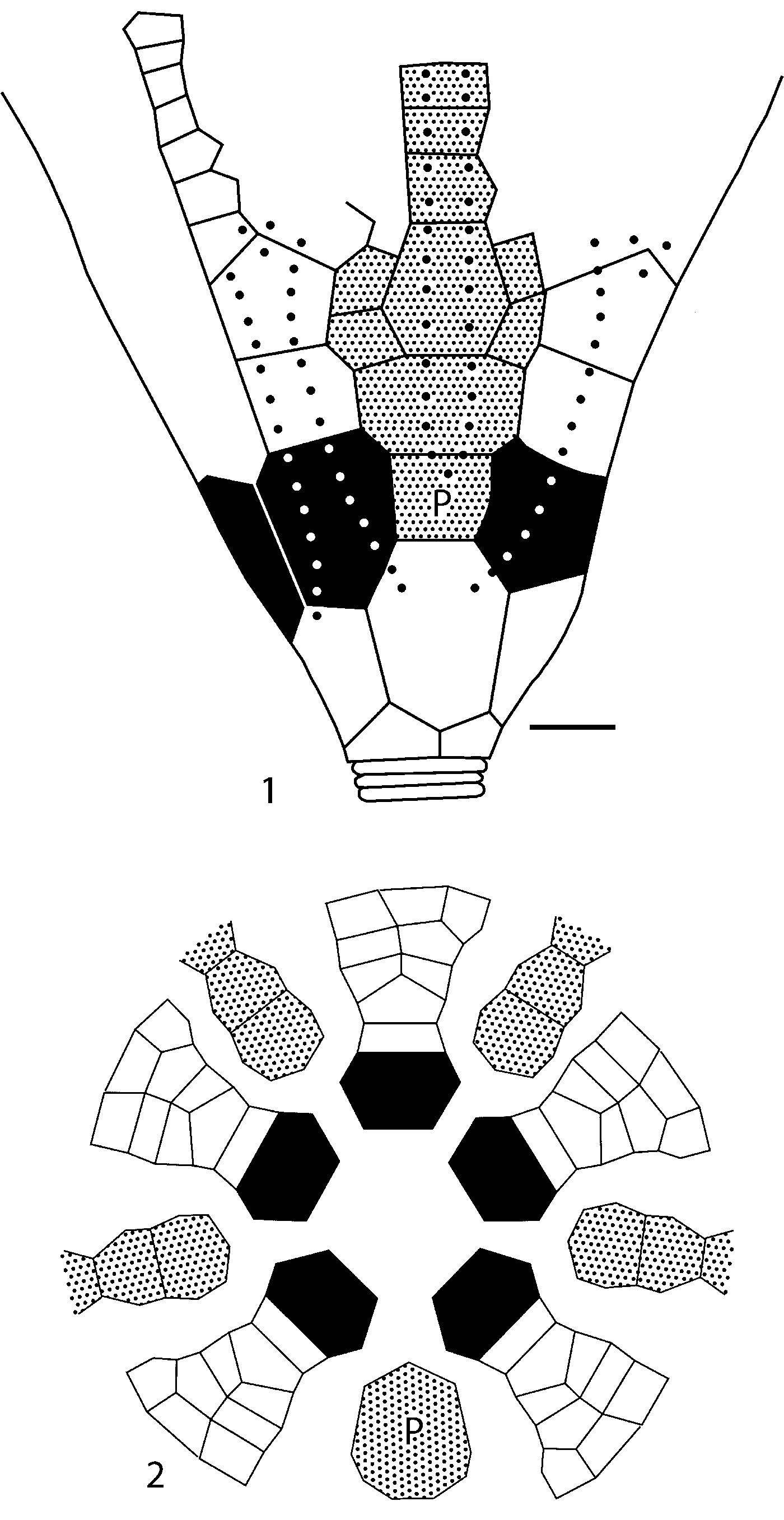
Figure 7. Camera lucida plate diagrams. (1) Becsciecrinus groulxi n. sp. (ROMIP 54198a) (scale bar 1.0 mm); (2) Lateranicrinus saintlaurenti n. gen. n. sp. Black shading, radial plates; stippled shading, interradial plates; P, primanal; and dotted lines, ray ridges along rays and anitaxial ridge in CD interray.
Holotype
ROMIP 54198a.
Diagnosis
Becsciecrinus with a medium cone-shaped calyx; plate sculpturing with radial ridges and irregular pitting; basal concavity absent; basal circlet ~30% of calyx height; radial circlet ~25% of calyx height, radial plates approximately as wide as high; first interradial plate higher than wide, approximately same size as radial plates, larger than first primibrachial; primanal higher than wide, larger than radial plates; column pattern N3231323.
Occurrence
Cybèle Member, Jupiter Formation (Llandovery, Telychian) at Jupiter-la-Mer, Anticosti Island, Québec, Canada (Locality 7); and from an unknown stratigraphic horizon along the Jupiter River (Locality 28).
Description
Calyx, small, medium cone-shaped (Fig. 7.1); wide, convex ridge (depressed sutures) from radials to basals and to median ray ridges defined by very convex fixed brachials (Fig. 6.2), basals with large proximal node, otherwise fine pitted plate sculpturing.
Infrabasal circlet either not visible in interradial position (covered by basal node) or visible radially, where visible ~8.0% of calyx height; without basal concavity. Basal circlet ~30% of calyx height; basal plates five, hexagonal, more than twice as high as wide, CD basal plate larger than other basal plates. Radial circlet ~25% of calyx height, interrupted in posterior; radial plates five, heptagonal, approximately as wide as high.
Normal interrays in contact with tegmen, depressed, first interradial hexagonal, higher than wide, approximately same size as radial plates, larger than first primibrachial. Second range with two plates; plating incompletely known.
Primanal hexagonal, higher than wide, anitaxial ridge from primanal onto tegmen (Fig. 6.2, 6.5), larger than radial plates, interrupts the radial circlet; plating in CD interray incompletely known, three plates above primanal; CD interray in contact with tegmen.
First primibrachial hexagonal, approximately same size as radial plates and primaxil; second primibrachial axillary. Small intrabrachial plates may be present between proximal secundibrachials. Fixed brachial plates ~37% of calyx height.
Tegmen unknown.
Free arms 10, high, branch once, pinnulate. Brachials rectangular uniserial, higher than wide; first secundibrachials in lateral contact medially with adjacent first secundibrachial; second secundibrachial with abmedial pinnule, third secundibrachial nonaxillary, fourth secundibrachial axillary. Distal tertibrachials with pinnules on all brachials (probably true for all tertibrachials) (Fig. 6.4). Secundibrachials and tertibrachials higher than wide; tertibrachials ~1.5 times higher than wide. Pinnules long (Fig. 6.3).
Column circular, heteromorphic (Fig. 6.5); column pattern N3231323. Proximally all columnals with convex latus with nodal the widest and highest and progressively decreasing in height and width in successive cycles.
Etymology
The species name recognizes Pierre Groulx, who donated specimens for this study.
Materials
Paratypes: ROMIP 54198b, ROMIP 54198c and MPEP719.1.
Measurements
ROMIP 54198a: CrH, 15*, CaH, 7.0; CaW, 7.3, CoH, 9.6*; ROMIP 54198b: CaH, 10.5*; CaW, 9.0, CoH, 32.0*; ROMIP 54198c: AH, 25.0*; and MPEP719.1: CrH, 46.0, CaH, 10.2; CaW, 8.1, CoH, 90.4*.
Remarks
The only previously recognized species of this genus was the type species, Becsiecrinus adonis, from the Fox Point and Chabot members of the Becscie Formation (Llandovery, Rhuddanian). The label on ROMIP 54198 indicates Silurian, Jupiter River (Locality 28), but MPEP719.1 from the Markus Martin collection is from the coast at the mouth of the Jupiter River, thus from the Cybèle Member of the Jupiter Formation (Locality 7). This Silurian occurrence extends the known range of Becsciecrinus to the Rhuddanian through Telychian. Becsiecrinus groulxi n. sp. is the second species of this genus and is compared to B. adonis in Supplemental Table 7.
MPEP719.1 is a pyritized specimen with very poorly preserved definition of plate boundaries (Fig. 6.4). Thus, ROMIP 54198a is designated as the holotype despite the imprecise locality data for this specimen. ROMIP 54198a is a partial crown with column attached that exposes the CD interray and adjacent rays (Fig. 6.2, 6.5, 6.6). ROMIP 54198b is a partial calyx with column attached (Fig. 6.1), and ROMIP 54198c is a broken specimen with a considerable amount of the arms preserved. The plating on the holotype is poorly defined, and a plate diagram is given in Figure 7.1.
Genus Cybelecrinus Ausich and Copper, Reference Ausich and Copper2010
Type species
Cybelecrinus ladas Ausich and Copper, Reference Ausich and Copper2010.
Cybelecrinus ladas Ausich and Copper, Reference Ausich and Copper2010
Figure 8

Figure 8. Cybelecrinus ladas Ausich and Copper, Reference Ausich and Copper2010; lateral view of two partial crowns and associated pluricolumnals; MPEP1148.1; scale bar, 5.0 mm.
Holotype
GSC 126819.
Occurrence
Cybelecrinus ladas is known from the Goéland Member, Menier Formation and Cybèle Member, Jupiter Formation (Llandovery, Aeronian to Telychian) of Anticosti Island, Québec, Canada. New material is from Localities 11 and 12.
Materials
Paratypes: GSC 126820–GSC 126823, UA13623a, UA13623b, UA13624a, and UA13624b. Several new specimens are on MPEP1148.1 (five specimens) (Fig. 8) and MPEP1149.1 (two specimens).
Remarks
Two small slabs (MPEP1148.1 and MPEP1148.2) contain excellently preserved specimens of C. ladas and preserve characters of the column (Fig. 8).
Order Monobathrida Moore and Laudon, Reference Moore and Laudon1943
Family Xenocrinidae Miller, Reference Miller1890
Genus Xenocrinus Miller, Reference Miller1881
Type species
Xenocrinus penicillus Miller, Reference Miller1881.
Xenocrinus rubus Ausich and Copper, Reference Ausich and Copper2010
Figure 9.1–9.3, 9.5

Figure 9. Anticosti Island monobathrid camerates. (1–3, 5) Xenocrinus rubus Ausich and Copper, Reference Ausich and Copper2010; (1) enlarged lateral view of specimen A in MPEP510.4; (2) enlarged lateral view of specimen B in MPEP510.4; (3) the two previous specimens on single bedding surface; MPEP510.4; (5) crinoid pluricolumnal “logjam” with three specimens of X. rubus; MPEP504.1; (4) Jovacrinus clarki n. sp., lateral view of crown, note distinctive pinnules MPEP1146.1. Scale bars, 5.0 mm.
Holotype
GSC 126697.
Occurrence
Crowns of X. rubus were originally known from the Juncliff (in today's lithostratigraphy), Lousy Cove, and Laframboise members of the Ellis Bay Formation, and only columnals were known from the Silurian (lower Chabot Member, Ellis Bay Formation) (Ausich and Copper, Reference Ausich and Copper2010). New specimens represented by crowns reported here expand the range of X. rubus to the Katian through at least the Rhuddanian (and perhaps the Aeronian), Anticosti Island, Québec, Canada.
Materials
Paratypes: GSC 126688a–d, GSC 126689a–f, GSC 126691, GSC 126692b, GSC 126693a–e, GSC 126696a–c, GSC 126698, and GSC 126699. New material includes MPEP504.1 (three specimens), MPEP510.4 (two specimens), and ROMIP 54197.
Remarks
Six new, well-preserved specimens of X. rubus Ausich and Copper, Reference Ausich and Copper2010 are in the collections reported on herein. MPEP504.1 is a slab composed primarily of aligned pluricolumnals, many exceeding 50 mm in length (Locality 19). Two of the X. rubus specimens are oriented parallel to the orientation of the pluricolumnals, and a third specimen is nearly perpendicular to this orientation (Fig. 9.5).
Ausich and Copper (Reference Ausich and Copper2010) reported X. rubus crowns only from the Hirnantian Ellis Bay Formation (Velleda [now Juncliff], Lousy Cove, and Laframboise members). They also reported the distinctive tetragonal Xenocrinus columnals from the Homard Member of the Vauréal Formation (Katian) to the lower Chabot Member of the Becscie Formation (Rhuddanian). Individual columnals were the only basis by which Ausich and Copper (Reference Ausich and Copper2010) extended the range of Xenocrinus across the Ordovician-Silurian boundary, thus making it one of the few species-level taxa that survived Late Ordovician extinctions. Three Xenocrinus specimens from the Petryk collection (MPEP504.1) are interpreted to be from the Prinsta Member of the Ellis Bay Formation (Hirnantian) (Locality 19). MPEP510.4 is from talus in the Becscie Formation along the Jupiter River, and ROMIP 54197 (Locality 27) is labeled as Silurian from along the Jupiter River, and supports the Silurian occurrence of X. rubus (Fig. 9.1–9.3).
Family Carpocrinidae de Koninck and LeHon, Reference Koninck and Le Hon1854
Genus Fibrocrinus Ausich and Copper, Reference Ausich and Copper2010
Type species
Fibrocrinus phragmos Ausich and Copper, Reference Ausich and Copper2010.
Fibrocrinus phragmos Ausich and Copper, Reference Ausich and Copper2010
Figures 4.1, 10

Figure 10. The tegmen of Fibrocrinus phragmos Ausich and Copper, Reference Ausich and Copper2010. (1) Exterior of well-preserved tegmen, note fixed ambulacral plates; holotype, GSC 126768 (from Ausich and Copper, Reference Ausich and Copper2010, pl. 5, fig. 8); the five primary peristomial cover plates are in the center of the tegmen (all slightly broken); the large central plate is the CD primary peristomial cover plate, and the other four primary peristomial cover plates are immediately above; (2) interior of tegmen, note opening of ambulacra that lead into mouth, central mouth, and fixed plates in ambulacra; MPEP1138.3. Scale bar 5.0 mm.
Holotype
GSC 126768.
Occurrence
East Point Member, Menier Formation (Aeronian), along the Trans-Anticosti Road, 1.9 km west of the Box-Bell Road junction (Locality 13) (Ausich and Copper, Reference Ausich and Copper2010).
Materials
Paratypes: GSC 126769–GSC 126780; other specimens reported in Ausich and Copper (Reference Ausich and Copper2010); and new material: MPEP1138.1–MPEP1138.8 and MPEP1139.1.
Remarks
Numerous new, variously preserved specimens of F. phragmos are available from Locality 13, from which this taxon was originally described. Particularly instructive is MPEP1138.3, which is a broken specimen exposing the interior surface of the tegmen (Fig. 10.2). The solid tegmen of F. phragmos is constructed of fixed primary peristomial cover plates, fixed ambulacral cover plates, and fixed interambulacral plates (Fig. 10.1). The interambulacral plates are very small; and based on preservation, it is possible that some of the abaxial-most cover plates are either loosely fixed or not fixed. On the underside of the tegmen (Fig. 10.2), the mouth and ambulacra (oral surface) are immediately beneath the cemented tegmen. A large, central, subelliptical opening is present for the mouth. A biseries of floor plates underlies the ambulacra. Fixed plates bifurcate two times in each ray, and fixed intra-ambulacral plates are present. A small circular pore is present between adjacent ambulacral floor plates. At the adaxial end of the fixed floor plates, two large pores are present in each ray between the oral frame plates and the adaxial-most fixed floor plates. In normal interrays, relatively few, large interradial plates unite with fixed floor plates to make a solid plated surface. Although not fully exposed, a large opening is present in the CD interray, which housed the hindgut that led to the anal opening on the tegmen surface.
Family Eucalyptocrinitidae Roemer, Reference Roemer, Bronn and Roemer1855
Genus Eucalyptocrinites Goldfuss, 1831
Type species
Eucalyptocrinites rosaceus Goldfuss, 1831.
Eucalyptocrinites archaios Ausich and Copper, Reference Ausich and Copper2010
Figure 11.3

Figure 11. Anticosti Island monobathrid camerates. (1, 2) Lateranicrinus saintlaurenti n. gen., n. sp.: (1) bedding surface with several specimens, including holotype and paratypes; ROMIP 54203; (2) enlarged lateral view of holotype, in (11.1) ROMIP 54203a. (3) Lateral view of partially crushed calyx with proximal column of Eucalyptocrinites archaios Ausich and Copper, Reference Ausich and Copper2010; MPEP495.3. (4) Camerate Indet. B, well-preserved arms and column, but details of calyx not preserved, specimen uncoated, ROMIP 54196. (5) Partial calyx and proximal arms of Camerata indet. A, MPEP311.86. Scale bars 5.0 mm, unless otherwise labeled.
Holotype
UA 13633.
Occurrence
Ferrum Member, Jupiter Formation (Llandovery, Telychian), Fire Tower Road, Anticosti Island, Québec, Canada (Ausich and Copper, Reference Ausich and Copper2010).
Materials
A single new specimen MPEP495.3 is present in the new collections.
Remarks
The holotype of Eucalyptocrinites archaios Ausich and Copper, Reference Ausich and Copper2010 is an excellently preserved specimen with only one or two columnals preserved (Ausich and Copper, Reference Ausich and Copper2010, pl. 6, figs. 2 and 3). The paratypes are all incomplete crowns. Therefore, the new specimen from the Petryk collection that has a portion of the column preserved significantly expands our knowledge of this species. The column of E. archaios is heteromorphic and holomeric. The nodal-internodal pattern is N121, with each cycle narrower and lower (Fig. 11.3). The nodals are 3.0 times wider than high, the priminternodals are 4.0 times wider than high, and the secundinternodals are 3.6 times wider than high. The latus on all columnals is strongly convex.
The new specimen is from the Petryk collection and is inferred to be from the Ferrum Member of the Jupiter Formation (Telychian) (Locality 18), which is the stratigraphic horizon from which this species was originally described (Ausich and Copper, Reference Ausich and Copper2010).
Family Patelliocrinidae Angelin, Reference Angelin1878
Genus Jovacrinus Ausich and Copper, Reference Ausich and Copper2010
Type species
Jovacrinus spinosus; by original designation.
Occurrence
Jovacrinus is only known from the Jupiter Formation on Anticosti, but with recognition of J. clarki n. sp., this genus now ranges from the Ferrum to the Pavillon members of the Jupiter Formation (Telychian). As listed below, several features distinguish species within Jovacrinus.
Jovacrinus clarki new species
Figure 9.4
Holotype
MPEP1146.1.
Diagnosis
Jovacrinus with sculpturing on aboral cup plates scattered nodes, first primibrachials tetragonal, one fixed secundibrachial, at least three ranges of fixed interradial plates, and uniserial to biserial arms.
Occurrence
Pavillon Member of the Jupiter Formation (Telychian) at the mouth of the Martin River, Anticosti Island, Québec, Canada (Locality 14).
Description
Crown with conical shape due to arm posture (Fig. 9.4). Calyx small; probably low bowl shaped; thin plated; aboral plate sculpturing scattered nodes, radials and fixed brachials without median ray ridge; calyx plate spines absent or not preserved (Fig. 9.4).
Basal circlet poorly preserved, probably visible in side view. Radial plates heptagonal, ~1.5 times wider than high, sculpturing as noted above.
Normal interrays in contact with tegmen (Fig. 9.4); first interradial hexagonal, approximately as high as wide, smaller than radials and larger than first primibrachial. Second range typically with two plates; plating 1-2-?.
Primanal probably heptagonal, 2.1 times wider than high, significantly smaller than radial plates, does not interrupt the radial circlet; plating in CD interray P-3-6(?)-? (additional plating unknown); posterior interray slightly wider than regular interrays; CD interray in contact with tegmen.
First primibrachial tetragonal, ~1.1 times wider than high, much smaller than radial plates, but larger than primaxil; second primibrachial axillary, pentagonal. First secundibrachial distal-most fixed brachial, second secundibrachial with an abaxial pinnule somewhat larger than other pinnules. Intrabrachial plates absent.
Tegmen not known.
Free arms 10, atomous. Brachials cuneate uniserial proximally, but biserial in at least distal half of arm. Brachials aborally convex without spines or nodes. Pinnules long, composed of at least six pinnulars.
Column circular; lumen unknown. Priminternodals and secundinternodals each separated by three tertinternodals. Long rhizoids along column (as known) borne on nodals, rhizoids up to 14 mm long. Details of column facets not known; facets of rhizoid segments as wide as nodals are high.
Etymology
The name recognizes David Clark, who collected this specimen and made it available for study.
Measurements
MPEP1146.1: CrH, 21.0*; CaH, 4.0*; CaW, 5.0*; RH, 1.8; RW, 2.4.
Remarks
The relatively small calyx, 10 arms with distinctive stout, long pinnules, and a column with long rhizoids through the column aligns this species with Jovacrinus. There is only one specimen of J. clarki n. sp., but it is distinctly different from the two other species in this genus, which are compared in Supplemental Table 8. The J. clarki n. sp. holotype is slightly crushed or broken because of the thin-plated calyx, so only approximate measurements are given.
Family Marsupiocrinidae Bronn in Bronn and Roemer, Reference Bronn and Roemer1855
Genus Lateranicrinus new genus
Type species
Lateranicrinus saintlaurenti n. gen. n. sp.; by monotypy.
Diagnosis
Marsupiocrinid with a very low bowl-shaped calyx, convex base with a basal concavity, basal plates confined to the basal concavity, ridge around basal concavity absent; radial plates project distally, in regular interrays three plates with one each in three ranges fixed in calyx, one plate above first interradial plate; two primibrachials, primaxil full width of brachitaxis, 10–15 free arms.
Occurrence
Silurian (Llandovery), Québec, Canada.
Description
See species description below.
Etymology
Lateranus (L., m.) is the god of the hearth, which is a reference to the provenance of this crinoid from along the Brick River.
Remarks
This new, relatively small marsupiocrinid is different based primarily on basal plates confined to the basal concavity, ridge around basal concavity absent; in regular interrays three plates with one each in three ranges fixed in calyx, one plate above first interradial plate; two primibrachials, primaxil full width of brachitaxis. These and other diagnostic characters for the Marsupiocrinidae are listed in Supplemental Table 9.
Lateranicrinus saintlaurenti n. gen. n. sp. is the second Llandovery-aged marsupiocrinid, with the other being Manticrinus from the Brassfield Limestone (Aeronian) of Ohio (U.S.A.). We suggest that Lateranicrinus n. gen. with more interradial plates in regular interrays and a primaxil that extends the full height of the primibrachitaxis is a more stemward marsupiocrinid than other known genera. However, determination of evolutionary relationships awaits thorough phylogenetic analyses.
Lateranicrinus saintlaurenti new species
Figures 7.2, 11.1, 11.2
Holotype
ROMIP 54203a.
Diagnosis
See generic diagnosis above.
Occurrence
As noted in the Supplemental Appendix, data accompanying this specimen only indicates Silurian Brick River (Locality 32). It is interpreted most likely to be from the Jupiter Formation (Llandovery, Telychian) Anticosti Island, Québec, Canada.
Description
Crown small, ovate. Calyx small, very low bowl-shaped (Fig. 11.2); arms not grouped; calyx plates convex (plate sculpturing not known).
Basal circlet small, beneath proximal columnal and confined to basal concavity. Radial circlet wraps around from edge of basal concavity to being visible in lateral view, <10% of calyx height, not interrupted in CD interray; radial plates five, heptagonal, ~1.2 times wider than high.
Regular interrays in narrow contact with tegmen; first interradial plate on upper shoulders of two radial plates, heptagonal, slightly wider than high, approximately same size as radials and much larger than first primibrachials; plating 1-1-1 (Fig. 7.2).
Primanal nonagonal, approximately equal in height and width, approximately same size as radial plates, does not interrupt radial circlet; plating in CD interray P-3-?.
First primibrachial tetragonal with arcuate suture with radial plate, ~1.6 times wider than high, much smaller than radial plates and approximately same size as primaxil; all fixed brachials full width of ray, half-ray, or quarter-ray; second primibrachial axillary, pentagonal, ~1.6 times wider than high. Either secundibrachitaxis unbranched or second secundibrachial axillary; fixed third secundibrachial or first tertibrachial distal-most fixed brachial. Intrabrachial plates absent. Tegmen not known.
Either two or three free arms per ray (two most common), atomous. In undivided half-rays, first free brachial cuneate uniserial, approximately as high as wide; in half-ray divided, first free brachial cuneate uniserial much wider than high; remainder of free brachials chisel biserial, aborally flattened through most of arm height, but in distal portion aborally convex and incurved toward oral-aboral axis. Arms pinnulate.
Column circular, holomeric (proximally), heteromorphic with pattern of N3231323, nodals and priminternodals much pronounced rounded epifacets. Other internodals with less pronounced convex epifacets.
Etymology
This species name recognizes Daniel Saint-Laurent, who donated specimens for this study.
Materials
Paratypes: ROMIP 54203b–ROMIP 54203i.
Measurements
Holotype: ROMIP 54203a**: CrH, 25.0*; CaH, 6.3; CaW, 11.0; CoH, 15.0*; Paratypes: ROMIP 54203b: CrH, 28.1; CaH, 6.4; ROMIP 54203c: CrH, 27.3; CaH, 6.6; CaW, 10.5; CoH, 4.0*.
Remarks
Lateranicrinus saintlaurenti n. gen n. sp. is represented by one small slab with nine specimens (five of which have lateral views of at least part of the calyx) (Fig. 11.1). The slab was over-prepared so that sculpturing on plate surfaces cannot be determined. No single specimen is preserved so that the entire calyx plating is revealed. Thus, Figure 7.2 is a composite diagram illustrating the calyx plating of Lateranicrinus saintlaurenti n. gen. n. sp.
Parvclass Cladida Moore and Laudon, Reference Moore and Laudon1943
Magnaorder Eucladida Wright, Reference Wright2017
Clade Articuliformes Wright, Reference Wright2017
Family Thalamocrinidae Miller and Gurley, Reference Miller and Gurley1895
Genus Thalamocrinus Miller and Gurley, Reference Miller and Gurley1895
Type species
Thalamocrinus ovatus Miller and Gurley, Reference Miller and Gurley1895; by original designation.
Occurrence
Silurian (Llandovery to Pridoli) to Devonian (Lochkovian), Canada, United States, and South Africa.
Remarks
McIntosh and Brett (Reference McIntosh and Brett1988) reviewed Thalamocrinus, concluding that seven valid species were present, including: T. cylindricus (Hall, Reference Hall1852) (non Miller and Gurley, Reference Miller and Gurley1895); T. elongatus Springer, Reference Springer1926; T. globosus Springer, Reference Springer1926; T. ovalus (Rowley, Reference Rowley1904); T. ovatus Miller and Gurley, Reference Miller and Gurley1895; T. robustus McIntosh and Brett, Reference McIntosh and Brett1988; and T. strimplei McIntosh and Brett, Reference McIntosh and Brett1988. Note that T. cylindricus Miller and Gurley, Reference Miller and Gurley1895 (non Hall, Reference Hall1852) was regarded a junior objective homonym, and McIntosh and Brett (Reference McIntosh and Brett1988) designated T. strimplei as the replacement name. The senior objective homonym, T. cylindricus (Hall, Reference Hall1852) (non Miller, and Gurley, Reference Miller and Gurley1895), was considered valid, but this is not recognized, as it should be, in Webster and Webster (Reference Webster and Webster2013).
Thalamocrinus species known in 1988 ranged in age from Silurian (Wenlock) to Devonian (Lochkovian) and were confined to the Laurentia paleocontinent. Jell and Theron (Reference Jell and Theron1999) described T. arenaceous from the Devonian (Emsian) of Australia; however, this generic assignment should be questioned because T. arenaceous has a pentagonal radianal plate rather than a tetragonal radianal plate, which is a diagnostic characteristic of Thalamocrinus (Lane and Moore, Reference Lane, Moore, Moore and Teichert1978; McIntosh and Brett, Reference McIntosh and Brett1988). Thalamocrinus daoustae n. sp. is the oldest known member of Thalamocrinus, extending its range to the Llandovery. Species-diagnostic characters for Thalamocrinus are relative size, aboral cup shape, nature of aboral cup plate sutures, height to width ratio of infrabasal plates, relative convexity of infrabasal plates, relative convexity of basal plates, relative convexity of radial plates, and proximal width of the infrabasal circlet compared to the width of the proximal columnals. Although not preserved in most species, the nodals and infranodals of T. daoustae n. sp. have a flat latus, whereas those of T. cylindricus have a very convex latus; and T. daoustae n. sp. has a robust anal sac with strongly sculpted plates and T. robustus has a much more gracile anal sac.
Thalamocrinus daoustae new species
Figure 13.1, 13.2

Figure 12. Anticosti Island myelodactylids and flexibles. (1) Partial crown and proxistele of Eomyelodactylus springeri, details obscured by heavy preparation MPEP718.6; (2) partially disarticulated crown with proxistele and mesistele of Eomyelodactylus springeri Ausich and Copper, Reference Ausich and Copper2010, see on Fig. 3.3; MPEP718.2; (3) disarticulated crown with proxistele and mesistele of Eomyelodactylus springeri Ausich and Copper, Reference Ausich and Copper2010, MPEP304.42. (4) Partially disarticulated crown with proxistele and short portion of the mesistele of Eomyelodactylus sp., MPEP1147.1. (5, 6) Hormocrinus quebecensis? Ausich and Copper, Reference Ausich and Copper2010, note infrabasal circlet missing, MPEP324.1: (5) CD interray view of crown; (6) A ray view of crown. Scale bars 5.0 mm.

Figure 13. Eucladid crinoids from Anticosti Island: (1, 2) Thalamocrinus daoustae n. sp. lateral view of crown; holotype, ROMIP 54199; (1) partial crown and column through mesistele; note change in morphology on the column from the proxistele through the mesistele; (2) enlargement of (1); note high infrabasal plates and sculpturing on anal sac plates. (3, 4) Plicodendrocrinus observationensis Ausich and Copper, Reference Ausich and Copper2010; MPEP476.5: (3) note pentalobate column and a trilobite hypostome covering part of aboral cup; (4) enlargement of (3). (5) Aetocrinus gracilis crown; see Fig. 3.2; MPEP718.1. (6) Plicodendrocrinus petryki n. sp.; holotype, ROMIP 54201. (7, 8) Plicodendrocrinus martini n. sp., holotype, MPEP1142.1: (7) enlargement of (8); note very low infrabasal circlet height in interradial positions; (8) note long anal sac and plate sculpturing of anal sac plates. All scale bars 5.0 mm.
Holotype
ROMIP 54199.
Diagnosis
Thalamocrinus with relatively small high cone-shaped aboral cup, aboral cup sutures flush, infrabasal plate height:width ratio 1.2, gently convex infrabasal plates, basal plate height:width ratio 1.8, gently convex basal plates, radial plate height:width ratio 1.2, and the width of the proximal aboral cup the same as the width of the proximal columnals.
Occurrence
The only data accompanying this specimen are Silurian, along the Jupiter River, Anticosti Island, Québec, Canada, which verifies a Llandovery age (either Aeronian or Telychian) (Locality 29).
Description
Crown small size, conical. Aboral cup high cone shape, height to maximum width ratio ~1.2, plates gently convex, smooth.
Infrabasal plates five, equal in size, infrabasal plate height:width ratio 1.2; infrabasal circlet ~30% of aboral cup height; evenly tapered proximal aboral cup, width equals width of proximal columnals; infrabasal concavity absent. Basal plates five, hexagonal, except for CD which is heptagonal, height:width ratio 1.8; basal plates approximately same size as radial plates, ~37% of aboral cup height; basal plate height:width ratio ~1.8. Radials five, heptagonal in all rays, height:width ratio 1.2; radial circlet ~33% of aboral cup height. Radial facets angustary or peneplenary, occupy ~70% of distal radial plate width; radial facet declivate, rounded abaxially, details of facet topography not known.
Total number of anal plates in cup not preserved, two visible, radianal extends below left part of C radial plate, tetragonal, as high as wide. Presumed anal X extends to distal height of aboral cup. Other aspects of CD interray unknown. Anal sac straight sided, ~3.0 times higher than aboral cup height, shorter than arm height; plates arranged in vertical columns, each plate with a central node and radiating ridges connecting to like ridges on adjoining plates.
Arms branch at least two times isotomously. First primibrachial slightly wider than high; fifth primibrachial axillary, fifth secundibrachial axillary. Brachials rectangular uniserial, narrow, aborally rounded, brachials distal to first primibrachial higher than wide.
Column circular, holomeric, latus flat. Proxistele heteromorphic, pattern N1, nodals 3.0 times wider than high, internodals 5.0 times wider than high; taper slightly distally to a homeomorphic mesistele with mesistele columnals slightly higher than wide; outer surface of epifacet rounded; lumen unknown. Columnal facets unknown.
Etymology
The species name recognizes Nathalie Daoust, who donated specimens for this study.
Measurements
Holotype: ROMIP 54199: CH, 18.2*; ACH, 5.0; pACW, 1.6; dACW, 4.1; IH, 2.0; BH, 2.4; BW, 1.6; RH, 2.0; RW, 1.8; CoH, 43.0*.
Remarks
The high, cone-shaped aboral cup of T. daoustae n. sp. is unique for Thalamocrinus. The depressed aboral cup plate sutures, strongly convex infrabasal and basal plates, and the proximal aboral cup wider than the proximal columnals are unique characters for T. robustus. Thalamocrinus daoustae n. sp. is most similar to T. strimplei McIntosh and Brett, Reference McIntosh and Brett1988 from the Wenlock and Ludlow of the United States and Canada. These and other diagnostic characters of Thalamocrinus species are listed in Supplemental Table 10.
Family Plicodendrocrinidae Jell, Reference Jell and Theron1999
Genus Plicodendrocrinus Brower, Reference Brower1995
Type species
Dendrocrinus casei Meek, Reference Meek1871.
Occurrence
Ordovician (Katian) to Silurian (Llandovery), United States, Canada; Ordovician (Katian) to Silurian (Wenlock or Ludlow), United Kingdom; Devonian (Lochkovian), Australia.
Remarks
Ausich and Copper (Reference Ausich and Copper2010) described two new Ordovician species of Plicodendrocrinus, P. epinettensis and P. observationensis. Both of these species had broadly plicate/convex plates, and both were from the Vauréal Formation (Katian). Two additional new species of Plicodendrocrinus are present in the new material studied here, P. petryki n. sp. and P. martini n. sp. Both of these taxa have prominent stellate ridges on aboral cup plates, which among other characters, determine these as distinct Anticosti Island taxa. Plicodendrocrinus petryki n. sp. is also from the Vauréal Formation. When originally described, Plicodendrocrinus was known exclusively from Ordovician taxa. Since, Donovan et al. (Reference Donovan, Widdison, Lewis and Fearnhead2010) described P. brevis from the Silurian of the United Kingdom, and Jell (Reference Jell1999) described P. australis from the Early Devonian of Australia. A third post-Ordovician species is described herein from Anticosti Island. This specimen lacks locality information beyond Silurian from the Jupiter River. Thus, it can only be considered Llandovery, but this is the youngest known species in North America. Species-diagnostic characters for Plicodendrocrinus are listed in Supplemental Table 3.
Donovan et al. (Reference Donovan, Widdison, Lewis and Fearnhead2010) described a species previously known only from an unpublished manuscript from 1963 by W.H.C. Ramsbottom. However, the description is clearly noted as having been written by S.K. Donovan. Thus, this taxon should be regarded as Plicodendrocrinus brevis Donovan in Donovan et al., Reference Donovan, Widdison, Lewis and Fearnhead2010. Donovan et al. (Reference Donovan, Widdison, Lewis and Fearnhead2010) correctly designated Plicodendrocrinus brevis Ramsbottom, 1963 as an unpublished nomen nudum (see International Commission of Zoological Nomenclature, 1999, Article, 50.1.1, p. 52). Authorship of this species is not Ramsbottom, as indicated in Webster and Webster (Reference Webster and Webster2013).
Plicodendrocrinus observationensis Ausich and Copper, Reference Ausich and Copper2010
Figure 13.3, 13.4
Holotype
GSC 126675.
Occurrence
Easton, Tower, and Schmitt Creek or Grindstone members, Vauréal Formation (Katian), Anticosti Island, Québec, Canada.
Materials
Paratype: GSC 126675, new specimen: MPEP476.5.
Remarks
Plicodendrocrinus observationensis was originally described from the Easton and Tower members of the Vauréal Formation. As noted in the Supplemental Appendix and the discussion of Gaurocrinus fimbriatus, the occurrence of P. observationensis at Locality 16 is inferred to be from either the Schmitt Creek or Grindstone Member of the Vauréal Formation.
Ausich and Copper (Reference Ausich and Copper2010) described Plicodendrocrinus epinettensis and P. observationensis, both from the Vauréal Formation (Katian) of Anticosti Island. The primary differences between these taxa are that P. observationensis has a more equidimensional aboral cup (H:W ~1.0), wider radial facets, and higher brachial plates (Ausich and Copper, Reference Ausich and Copper2010, table 24). MPEP476.5 is another specimen of P. observationensis, and it adds to our understanding of this species. The arms on this specimen are nearly complete (Fig. 13.3) and reveal that free arms have two isotomous divisions rather than a single division, which was the extent of the preservation on the holotype (Fig. 13.4). Also, MPEP476.5 has more brachials in the primibrachitaxis than the holotype, and the brachials are much higher than wide rather than having the height and width approximately equal. This species is now understood to have 5–8 primibrachials and 8–10 secundibrachials. Although only partially exposed, the plating of the anal sac is much better preserved on MPEP476.5 than on specimens reported in Ausich and Copper (Reference Ausich and Copper2010). The anal sac extends through most of the height of the arms. The plicate anal sac plates are typical for Plicodendrocrinus, and six columns of plates are present across the preserved anal sac. This new specimen is quite small, and it is possible that the very high brachial plates may represent a juvenile characteristic rather than an attribute of the adult members of the species.
Plicodendrocrinus petryki new species
Figure 13.6
Holotype
ROMIP 54201.
Diagnosis
Plicodendrocrinus with aboral cup plates with well-defined, single stellate ridges and plate triple junctions depressed; other plate sculpturing absent; width:height ratio of aboral cup 1.5; infrabasal plate radial height/infrabasal plate interradial height ~1.5; anal sac straight sided, approximately five plate columns per side, radial facet ~50% of radial plate width; brachials as wide as high; broadly convex aborally; column pentalobate to strongly pentalobate.
Occurrence
The single specimen is known only from the Silurian along the Jupiter River (Supplemental Appendix, Locality 30), Anticosti Island, Québec, Canada.
Description
Crown medium-sized, conical. Aboral cup high cone-shaped; height:width ratio ~1.5; plates smooth, with single stellate ridges connecting to those on adjoining plates; broadly convex to plicate; aboral cup plate triple junctions depressed (Fig. 13.6).
Infrabasals five, equal in size; infrabasal circlet ~31% of aboral cup height. Infrabasal plate radial height/infrabasal plate interradial height ~1.5. Basals probably five, hexagonal, higher than wide, larger than radials; basal circlet ~41% of aboral cup height. Radials probably five, pentagonal in all rays, ~1.5 times wider than high; radial circlet ~28% of aboral cup height. Radial facets angustary, horseshoe shaped, declivate, occupy ~50% of distal radial plate width; radial facet topography not known.
Posterior interray and oral surface not known. Anal sac approximately three times higher than aboral cup, straight sided; composed of medium-sized, hexagonal, gently convex, wider than high, plicate plates arranged in vertical columns; at least five columns per side; summit of anal sac unknown.
Arms branch at least two times in an isotomous pattern. Fourth primibrachial axillary; nonaxillary primibrachials ~1.5 times wider than high; brachials as wide as high; broadly convex aborally; fourth or fifth secundibrachial axillary; brachials rectangular uniserial.
Proximal column pentalobate to strongly pentalobate, holomeric, homeomorphic outer surface of epifacet rounded; lumen wide, pentalobate, ~45% of column diameter. Columnal facets unknown.
Etymology
The species name recognizes Allen Petryk, whose collection is included in this study.
Measurements
Holotype: ROMIP 54201: CrH, 26.9; ACH, 8.1; pACW, 3.2; dACW, 10.4; rIH, 3.1; iIH, 2.0; ICW, 2.7; BH, 4.3; BW, 3.4; RH, 3.0; RW, 4.1; ASH, 19.5*.
Remarks
Supplemental Table 11 is a listing of species-diagnostic characters for Plicodendrocrinus.
Plicodendrocrinus martini new species
Figure 13.7, 13.8
Holotype
MPEP1142.1.
Diagnosis
Plicodendrocrinus with aboral cup plates with well-defined, single stellate ridges and plate triple junctions depressed; other plate sculpturing absent; width:height ratio of aboral cup 1.25; infrabasal plate radial height/infrabasal plate interradial height ~6.0; anal sac straight sided, approximately eight plate columns per side, radial facet ~40% of radial plate width; primibrachials as wide as high and broadly convex aborally with a keel, secundibrachials higher than wide and keeled aborally; column pentalobate to strongly pentalobate.
Occurrence
Top of falls along Ruisseau Blanc, from the Vauréal Formation and interpreted to be from the Easton Member (Katian) (Locality 10), Anticosti Island, Québec, Canada.
Description
Crown medium-sized. Aboral cup high cone-shaped; height:width ratio ~1.5; plates smooth, with single dominant stellate ridge connecting to like ridges on adjoining plates; aboral cup plate triple junctions depressed.
Infrabasals five, equal in size; infrabasal circlet ~37% of aboral cup height. Infrabasal plate radial height/interradial plate height ~6.0 (Fig. 13.7). Infrabasal plates concave. Basals probably five, hexagonal, higher than wide, approximately same size as radial plates, extend proximally nearly to proximal columnal; basal circlet ~34% of aboral cup height. Radials probably five, pentagonal or hexagonal; radial circlet ~28% of aboral cup height. Radial facets angustary, horseshoe shaped, declivate, occupy ~40% of distal radial plate width; radial facet topography not known.
Parts of five anal plates in aboral cup; radianal same size as C radial plate and directly beneath C radial plate, equidimensional, in lateral sutural contact with anal X. Anal X between D radial plate and radianal/C radial plate. Two anal plates above anal X; both partially in aboral cup and sutured to D radial or C radial plates. The fifth anal plate is the second plate above and to the right of the anal X and is sutured to the uppermost shoulder of the C radial plate. Anal sac ~10 times higher than aboral cup height, straight sided; composed of small-sized, hexagonal, gently convex, wider than high, plicate plates arranged in vertical columns (Fig. 13.8); at least eight columns per side; summit of anal sac poorly preserved.
Arms branch at least two times in an isotomous pattern. Fourth or fifth primibrachial axillary; nonaxillary primibrachials as high as wide and broadly convex. Sixth or seventh secundibrachial axillary; nonaxillary secundibrachials higher than wide and aborally keeled.
Proximal column strongly pentalobate, holomeric; other aspects of column not preserved.
Etymology
The species name recognizes Markus Martin, who collected and prepared this specimen.
Measurements
MPEP1142.1: ACH, 3.5; pACW, 2.0; dACW, 5.3*; rIH, 1.2; iIH, 0.4; ICW, 1.4; BH, 1.9; BW, 1.9; RH, 1.9; RW, 2.7; ASH, 31.0.
Remarks
See discussion above and Supplemental Table 11 for comparison to other species of Plicodendrocrinus.
Family Botryocrinidae Wachsmuth and Springer, Reference Wachsmuth and Springer1886
Genus Fragucrinus Ausich and Copper, Reference Ausich and Copper2010
Type species
Fragucrinus bothros Ausich and Copper, Reference Ausich and Copper2010.
Fragucrinus bothros Ausich and Copper, Reference Ausich and Copper2010
Figure 14

Figure 14. Fragucrinus bothros Ausich and Copper, Reference Ausich and Copper2010. Note variable number of primibrachials and characteristics of the mesistele; MPEP518.2, photographed under alcohol. Scale bar 2.5 mm.
Holotype
GSC 126881.
Occurrence
Richardson Member of the Jupiter Formation; along Sandtop Main Road (Ausich and Copper, Reference Ausich and Copper2010); Anticosti Island, Québec, Canada. The specimen is only noted as from the Silurian (Locality 23).
Materials
The paratype is GSC 126675, and the new specimens are MPEP518.2a and MPEP518.2b.
Remarks
One complete crown with column attached and one partial crown with a longer column attached constitute the new specimens of F. bothros (Fig. 14). Previously only known from the holotype, these new specimens demonstrate that the number of primibrachials is variable (three to five) and the mesistele has a N121 pattern of nodals and internodals.
Acknowledgments
This paper is a continuation of fieldwork supported by the National Geographic Society (grant 6789-00) and the National Science Foundation (EAR-0205968). P. Copper, J. Jin, and A. Desrochers were stratigraphic and geographic guides during most Anticosti field seasons. We thank J. Iellamo for preparing specimens from the Petryk and Cournoyer/Daoust collections. We also thank the work and generosity of D. Clark, N. Daoust, the late P. Groulx, P. Isotalo, M. Martin, the late A. Petryk, and D. Saint-Laurent, who collected these specimens and made them available for scientific study. J. Dougherty and M. Coyne (Geological Survey of Canada) donated the Petryk collection to the MPEP. The authors also thank T.E. Guensburg, D.L. Meyer, and R. Mooi, for thoughtful reviews.
Accessibility of supplemental data
Additional information on new occurrences and further discussion of diagnostic characters are given the the Online Supplement on the Dryad Digital Repository: https://doi.org/10.5061/dryad.2j0b93b.
Taxa discussed in the Online Supplement but not in the manuscript above are Camerata indet. A (Fig. 11.5), Camerata indet. B (Fig. 11.4), Eomyelodactylus foerstei Ausich and Copper, Reference Ausich and Copper2010, E. springeri Ausich and Copper, Reference Ausich and Copper2010 (Figs. 3.3, 12.1–12.3), Eomyelodactylus sp. (Fig. 12.4), Ladacrinus? sp., Hormocrinus quebecensis? Ausich and Copper, Reference Ausich and Copper2010 (Fig. 12.5, 12.6), and Aetocrinus gracilis Ausich and Copper, Reference Ausich and Copper2010 (Figs. 3.2, 13.5).
















