Introduction
The first specimens of the pterosaur Pteranodon, two isolated distal ends of wing metacarpals, were found in 1870 by O.C. Marsh and the Yale College Scientific Expedition in the Smoky Hill Chalk Member of the Niobrara Formation of western Kansas (Marsh, Reference Marsh1871). Over the next ten years, hundreds of additional specimens of Pteranodon plus ten or so specimens of the smaller Nyctosaurus were collected in the Smoky Hill Chalk Member by Marsh and various parties working for him. Less-intensive collecting has continued to the present day such that there are over 1000 specimens of Pteranodon in museum collections.
Bennett (Reference Bennett1992, Reference Bennett1994a, Reference Bennett2001) studied all available Pteranodon specimens from the Smoky Hill Chalk Member and found two species, P. longiceps Marsh, Reference Marsh1876 and P. sternbergi Harksen, Reference Harksen1966, which differed in jaw and cranial crest shape, but no evidence that more that one species was present at any time, so they seem to have formed a single anagenetically evolving lineage with P. sternbergi from the lower parts of the Smoky Hill Chalk Member evolving into P. longiceps from the upper parts of the Smoky Hill Chalk Member and the Sharon Springs Member of the Pierre Shale. Specimens were bimodally distributed with the small size-class with estimated wingspans in life of 3.0–4.8 m roughly twice as abundant as the large size-class with wingspans of 4.8–6.7 m. The ontogenetic ages of specimens were determined using size-independent criteria reflecting the extent of ossification and fusion of bones, and immature individuals were found to make up ~15% of the sample and to be present in both size-classes such that the size-classes could not be age-classes (Bennett, Reference Bennett1993). The size-classes differed only in cranial crest size and pelvic morphology, and were interpreted as dimorphic sexes: the small class consisting of females with small crests and large pelvic canals to allow the passage of relatively large eggs and the large class consisting of males with small pelvic canals and large crests to attract mates and/or intimidate rivals in a polygynous mating system (Bennett, Reference Bennett1992). The absence of specimens of Pteranodon with wingspans of <3 m from the Smoky Hill Chalk Member was not attributed to a bias against the preservation of small specimens because the Smoky Hill Chalk Member also preserves specimens of the pterosaur Nyctosaurus with wingspans of 1.6–3.3 m. Therefore, based on the absence of small individuals and the presence of large subadults with immature skeletons, it was argued that Pteranodon exhibited rapid growth to adult size while under parental care before flying and feeding independently (Bennett, Reference Bennett1993). Similarly, based on the absence of small juveniles from the collections of Nyctosaurus from the Smoky Hill Chalk Member and ornithocheiroids from the Cambridge Greensand of England and the Romualdo Formation of Brazil, it was argued that the pattern of rapid growth while under parental care was shared with other large pterodactyloids.
A new juvenile specimen of Pteranodon collected from the Smoky Hill Chalk Member is so small that it challenges the interpretation of rapid growth to large size before flying and feeding (Bennett, Reference Bennett2014a). This paper describes the specimen and discusses its implications for Pteranodon’s growth and behavior. The interpretation of rapid growth while under parental care is rejected and it is concluded that Pteranodon exhibited ontogenetic niches, flying and feeding independently from hatching and growing to adult size over several years while occupying different environments and ecological niches at different stages of its life history. Evidence is also presented that most other pterosaurs exhibited ontogenetic niches.
Repositories and institutional abbreviations
AMNH, American Museum of Natural History, New York, New York; BSP, Bayerische Staatssammlung für Paläontologie und Geologie, Munich, Germany; FHSM, Sternberg Museum of Natural History, Fort Hays State University, Hays, Kansas; FMNH, Field Museum of Natural History, Chicago, Illinois; GPIT, Paläontologische Forschungs-, Lehr- und Schausammlung, Institut für Geowissenschaften (formerly the Geologische-Paläontologische Institut), Universität Tübingen, Tübingen, Germany; JME-SOS, JuraMuseum (Solnhofen Sammlung), Eichstätt, Germany; KUVP, Natural History Museum, University of Kansas, Lawrence, Kansas; LACM, Los Angeles County Museum of Natural History, Los Angeles, California; MTM, Hungarian Natural History Museum, Budapest, Hungary; NHMW, Naturhistorisches Museum Wien, Vienna, Austria; NSM, National Science Museum, Tokyo, Japan; RAM, Raymond M. Alf Museum of Paleontology, Claremont, California; SM, Senckenberg Museum, Frankfurt, Germany; TM, Teyler Museum, Haarlem, Netherlands; and YPM, Peabody Museum of Natural History, Yale University, New Haven, Connecticut.
Material and methods
The new specimen, FHSM 17956, was examined with stereo microscope, measured, and photographed. A small piece of cortical bone that was removed from the posterior side of the neck of metacarpal (Mc) IV, just proximal to the distal condyles, was sectioned, ground, and polished, and the resulting thin sections were examined and photographed with optical and polarizing microscopes. Measurements of other specimens of Pteranodon and Nyctosaurus were taken from Bennett (Reference Bennett2001, and unpublished data). Wingspans in life were estimated using the method of Bennett (Reference Bennett2001), in which span equals two times the summed lengths of the humerus, antebrachium, McIV, and wing phalanges (WP) 1–4; the omission of the pectoral girdle and carpus compensates for flexures of the elbow, wrist, and metacarpophalangeal joints and the curvature of the wingfinger. In addition, where elements were missing or incomplete, their lengths were calculated from linear regression equations calculated from available samples (Bennett, Reference Bennett2001, Reference Bennett2007a, Reference Bennett2013a).
Ontogenetic ages of specimens were determined using size-independent criteria reflecting the extent of ossification and fusion of the skeleton (Bennett, Reference Bennett1993, Reference Bennett1995), and unless otherwise stated all discussion of immaturity and maturity refers to osteological rather than sexual maturity. The ontogeny of pterosaurs is divided into six stages: eggs; hatchlings and young-of-the-year; small juveniles; large juveniles that are significantly smaller than mature adults; subadults with immature skeletons, but not significantly smaller than mature adults; and adults with mature skeletons.
Kellner (Reference Kellner2010) revised Pteranodon and split it into four species in three genera, but that interpretation has not been generally accepted (e.g., Witton, Reference Witton2013; Martin-Silverstone et al., Reference Martin-Silverstone, Glasier, Acorn, Mohr and Currie2017). Several errors in the revision have been identified and will be addressed elsewhere, therefore Bennett’s (Reference Bennett1994a) taxonomic scheme is accepted here.
A privately held fragmentary pterosaur specimen from the Smoky Hill Chalk Member referred to as Tweety or Ptweety has been reconstructed and interpreted as a very small Pteranodon, even though it is within the size range of Nyctosaurus specimens. Based on my examinations of the specimen, the morphology of its notarium, humeri, and McIV is consistent with that of Nyctosaurus, but inconsistent with that of Pteranodon, so the specimen provides no evidence of a Pteranodon smaller than FHSM 17956. Unfortunately, the reconstruction so misused the specimen that it is of no scientific value whatsoever.
Description
The new specimen, FHSM 17956, is an incomplete right wing preserved in dorsal view on a roughly pentagonal slab of pale yellow chalk ~54 x 19 x 4 cm (Fig. 1). It was collected from the Smoky Hill Chalk Member of the Niobrara Formation in the SE¼, NE¼, SE¼ of Sec. 29, T11S, R23W of Trego County, Kansas by Mr. Glenn Rockers of Hays, Kansas, who prepared the specimen and donated it to the FHSM. Exposures in the E½, SE¼ of Sec. 29 have gray chalk at the base, ~2 m of pale yellow chalk with a series of five very fine-grained bentonite seams spaced 25, 32, 20, and 27 cm apart, and ~3.5 m of massive bioturbated chalk caprock at the top, and preserve abundant Platyceramus platinus (up to 1.2 m diameter) and Pseudoperna congesta. The caprock is Hattin’s (Reference Hattin1982) Marker Unit 10 and the sequence of bentonite seams beneath it compares well with what he described immediately below the caprock, as does the abundance of bivalve fossils. Based on the specific locality information, the specimen was collected from the fossiliferous chalk no more than 1.5 m below Marker Unit 10, and is of upper middle Santonian age.
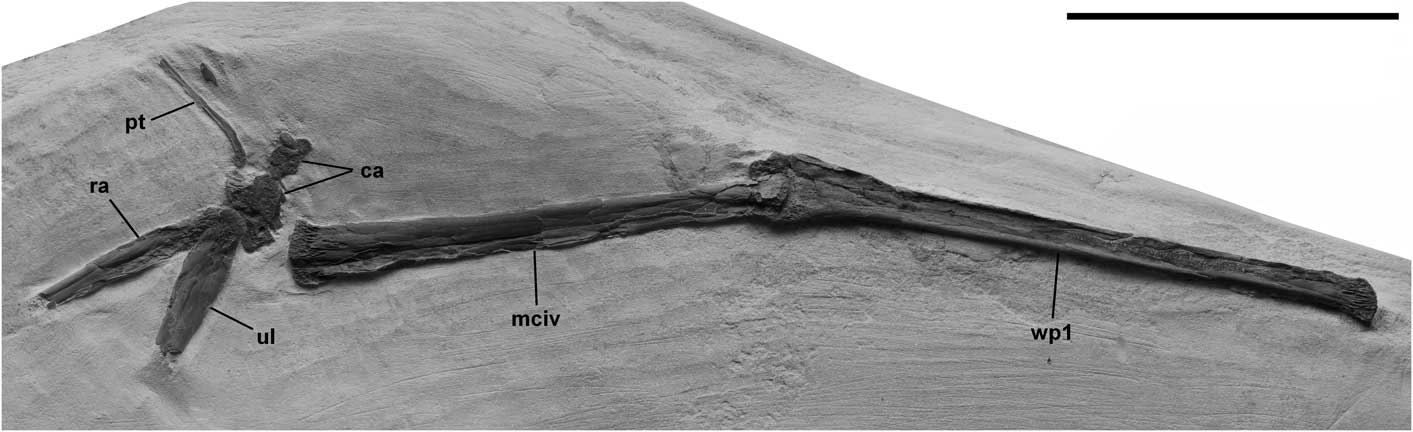
Figure 1 Pteranodon sp. indet. (FHSM 17956). Photograph of incomplete right wing in dorsal view. Abbreviations: ca, carpus; mciv, metacarpal IV; pt, pteroid; ra, radius; ul, ulna; and wp1, wing phalanx 1. Scale bar is 10 cm.
The specimen consists of the distal halves of the radius (61 mm) and ulna (50 mm), the carpus, and complete McIV (160 mm) and WP1 (194 mm). The bones were flattened by compression, as is typical of Smoky Hill Chalk Member pterosaur specimens, and some of the bone of WP1 in an area ~7.5 cm x ~5 mm near the distal end was lost and reconstructed with plaster painted to resemble the adjacent bone. The bones of the specimen are largely articulated and were presumably held in articulation by ligaments until arriving on the sea floor, but there are minor dislocations that probably resulted from gravity or currents after the ligaments decomposed. Thus, the radius, ulna, and carpus are preserved in close association, but are separated from McIV by ~6 mm, and the individual carpal elements exhibit dislocations. McIV and WP1 are articulated with the metacarpophalangeal joint extended as in flight.
The radius and ulna halves exhibit irregular breaks with no impressions of the missing portions in the chalk, and they are splayed apart proximally even though the distal ends are preserved nearly in contact with the proximal syncarpal. This indicates that the bones were broken or perhaps bitten off before preservation of the specimen.
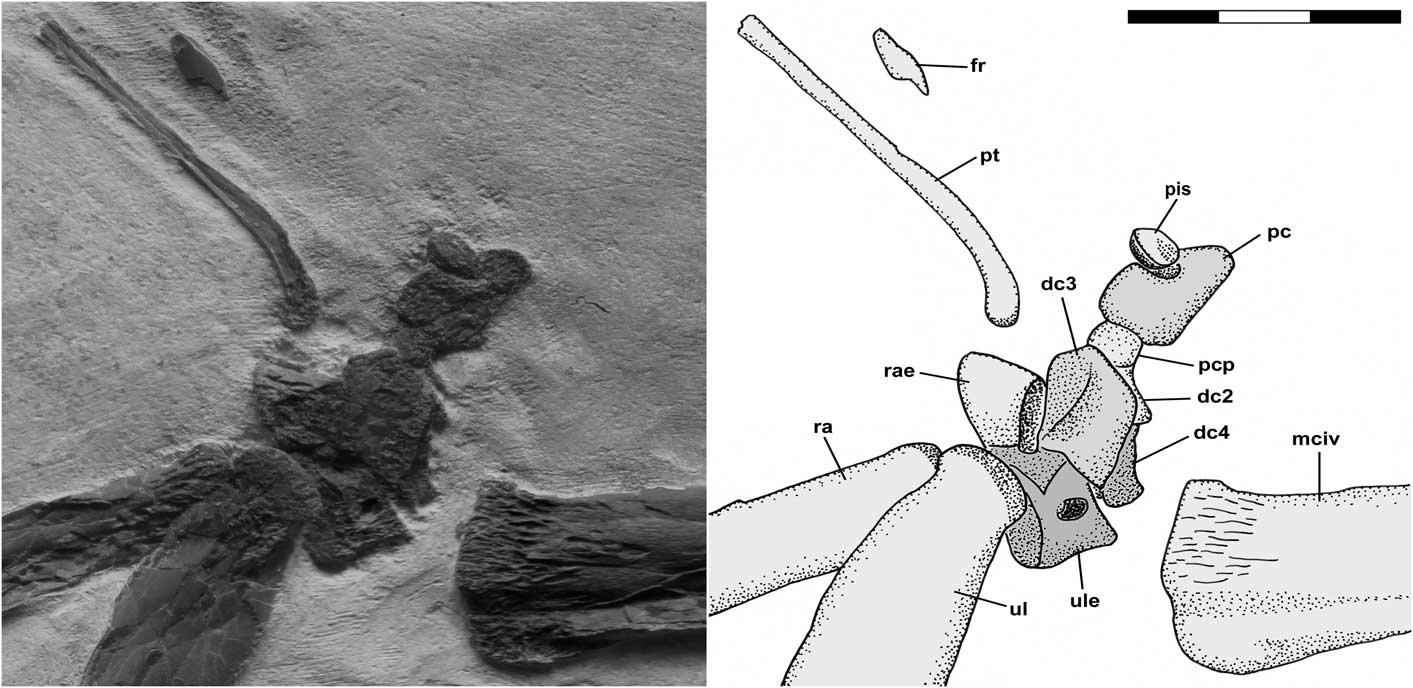
The carpus is shown in Figure 2. All carpal elements are severely deformed by compression such that they preserve little of their original morphology, and all exhibit pitted surfaces indicative of incomplete ossification. The radiale and ulnare that would have fused to form the proximal syncarpal are preserved slightly disarticulated from one another and close to the distal radius and ulna and the distal row of carpals. Few details of the radiale are discernable other than its wedge-shaped dorsal aspect and part of the intersyncarpal articular surface. The ulnare exhibits the articular surface for the dorsal articular facet of the ulna and its slender-waist dorsal aspect is pierced by an oval pneumatic foramen. Its intersyncarpal articular surface is not exposed. The three distal carpals that would have fused to form a distal syncarpal are preserved slightly disarticulated from one another and close to the proximal carpals, but well separated from the proximal end of McIV. Most prominent is the subrectangular dorsal aspect of distal carpal 3, which is pierced by an oval pneumatic foramen. Most of distal carpal 2 is hidden behind distal carpal 3, but its prominent preaxial carpal process is visible anterior to distal carpal 3, and distal carpal 4 is largely hidden behind distal carpal 3. The preaxial carpal is still articulated with the preaxial carpal process of distal carpal 2, and its fovea is directed upward with the pisiform (=Sesamoid A of Bennett, Reference Bennett2001, Reference Bennett2008) articulated in it. The pteroid is preserved in dorsal view a short distance away from the carpals. It is shaped like an ice hockey stick and is ~42 mm long as preserved, although its non-articular end is missing. The articular end of the pteroid has a pitted articular surface indicative of incomplete ossification. There is a more or less straight section ~11 mm long and ~4 mm wide, a gentle bend of ~25°, and then the bone narrows to 2 mm to form a long straight shaft extending proximally. A small bone fragment that does not seem to be related to the carpus lies ~8 mm from the shaft of the pteroid.
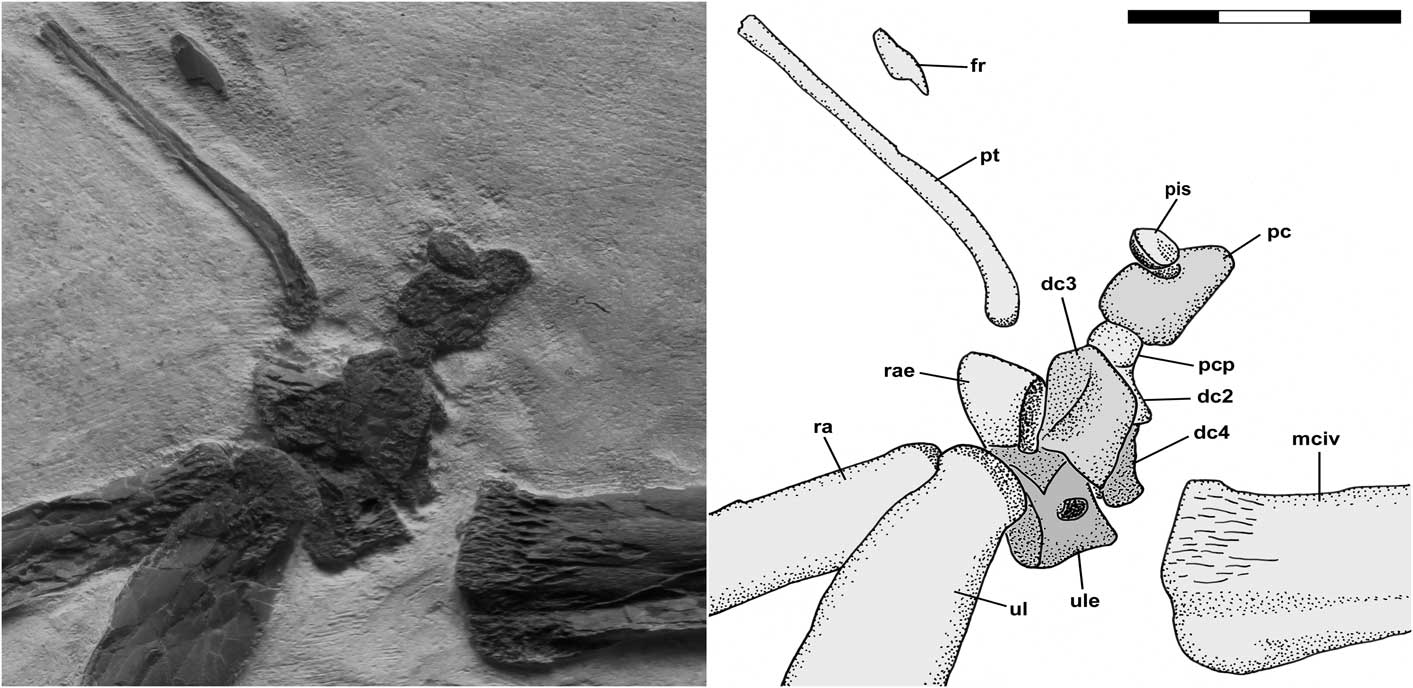
Figure 2 Pteranodon sp. indet. (FHSM 17956). Detailed photograph and interpretive drawing of right carpus in dorsal view. Individual carpal elements are indicated with different shades of gray. Abbreviations: dc, distal carpal; fr, indeterminate bone fragment; mciv, metacarpal IV; pc, preaxial carpal; pcp, preaxial carpal process of distal carpal 2; pis, pisiform; pt, pteroid; ra, radius; rae, radiale; ul, ulna; and ule, ulnare. Scale bar is 3 cm.
Metacarpal IV is crushed such that it seems to present a posterolateral view, whereas WP1 is crushed such that it seems to present a fully dorsal view. The posterior margin of McIV is broken and some fragments presumably were lost. The extensor tendon process is articulated with the rest of WP1, but is unfused. The articular ends of both bones were poorly ossified and their fragile cortical bone was lost when prepared with a rotating wire brush, revealing the internal septate bone. The bones are otherwise unremarkable.
Ontogenetic status
The articular surfaces of the radius, ulna, pteroid, McIV, and WP1 are pitted and rough, indicating incomplete ossification, and similar pitting is visible on the carpal elements as well. The diaphyses of the radius, ulna, pteroid, McIV, and WP1 exhibit immature grain with many vascular canals opening onto the external surface of the bone. The proximal and distal rows of carpals are not fused into proximal and distal syncarpals, and the extensor tendon process is not fused to WP1. Thus, the specimen exhibits immature states of all three size-independent criteria that Bennett (Reference Bennett1993) used to identify immature specimens of Pteranodon and does not exhibit any evidence of osteological maturity, so it is immature.
Thin section
Cortical bone was sampled from the posterior side of the neck of McIV, just proximal to the distal condyles where it was thought that the bone would be thickest and provide the best chance of finding a line of arrested growth (LAG), if present. The resulting thin section (Fig. 3.1) is 540 µm thick, and exhibits well-vascularized plexiform bone (sensu Francillon-Vieillot et al., Reference Francillon-Vieillot, de Buffrénil, Castanet, Géraudie, Meunier, Sire, Zylberberg and Ricqlès1990) with vascular canals opening onto the external surface. The internal surface is uneven and appears to be resorptive. No LAG is evident, but a second section ground much thinner exhibits multiple discontinuous narrow circumferential bands of blue against a background of magenta when examined under polarized light with a first-order retardation (=λ) plate (Fig. 3.2). The spacing of the bands (~80 µm) suggests some sort of regular variation in fiber orientation. The pattern of bone and vascularization is similar to that previously found in the immature bone of a subadult Pteranodon (Bennett, Reference Bennett1993, fig. 2C), although the bone is thinner and the spacing between vascular canals is somewhat less than in the subadult.
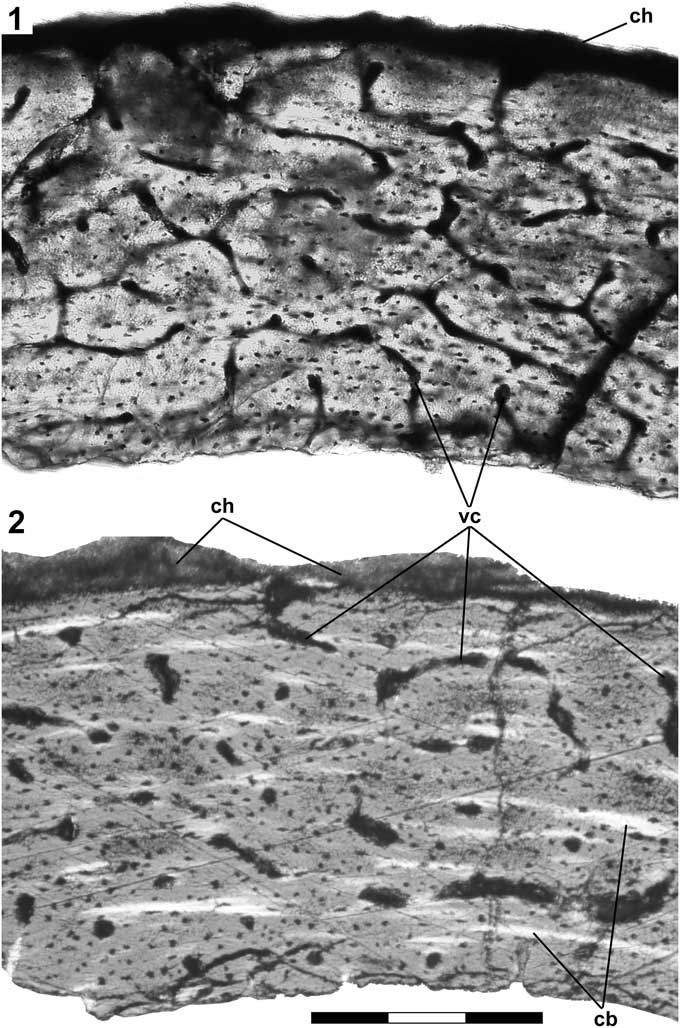
Figure 3 Pteranodon sp. indet. (FHSM 17956). (1) Thin section of cortical bone from the distal end of metacarpal IV photographed under visible light; (2) thinner section photographed under polarized light and first order retardation (=lambda) plate showing narrow discontinuous circumferential bands of blue (light gray) against a background of magenta (medium gray); dark band at top is chalk residue on external surface of bone. Abbreviations: cb, circumferential band; ch, chalk; and vc, vascular canal. Scale bar is 300 μm.
Discussion
Only two pterosaur genera, Pteranodon and Nyctosaurus, are known from the Smoky Hill Chalk Member (Bennett, Reference Bennett1994a). FHSM 17956 is referred to Pteranodon because the morphology of its distal syncarpal with prominent preaxial carpal process and ice hockey-stick-shaped pteroid (Bennett, Reference Bennett2001, and unpublished data) are as found in Pteranodon and unlike Nyctosaurus, in which the syncarpal has a short preaxial carpal process and the pteroid has a pointed lateral end extending distally beyond the articulation (Williston, Reference Williston1903; Bennett, Reference Bennett2003). In addition, the proportions of FHSM 17956 (i.e., ratio of lengths of McIV and WP1, ratios of proximal width and diameters of distal condyles to McIV length, and ratios of proximal, midshaft, and distal widths to WP1 length, Bennett, Reference Bennett2001, and unpublished data) are consistent with those of Pteranodon and inconsistent with those of Nyctosaurus (Fig. 4). There is no evidence that the specimen represents a new species, but it is not clear which Pteranodon species it does represent. Bennett (Reference Bennett1994a) thought that the transition between P. sternbergi and P. longiceps occurred in the middle of the Smoky Hill Chalk Member and arbitrarily chose the non-fossiliferous caprock (Marker Unit 10, Hattin, Reference Hattin1982) as the dividing line between the species. Because the specimen was collected from just below the caprock and does not exhibit any characters used to differentiate the species of Pteranodon, it is considered to be Pteranodon sp. indet.
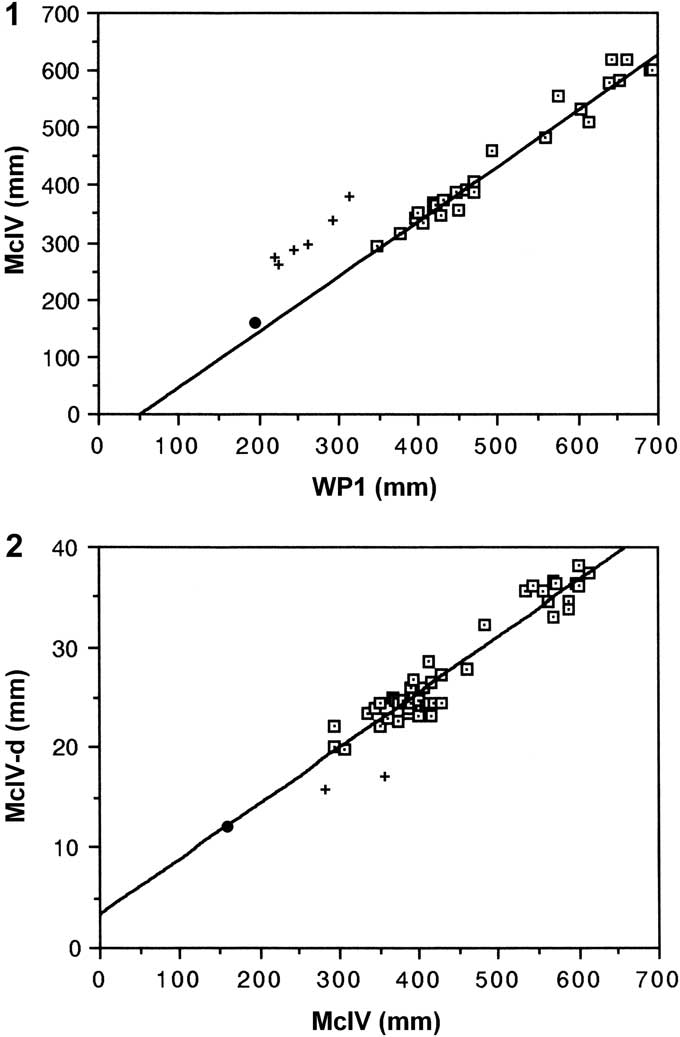
Figure 4 Scatter plots of limb element measurements of Pteranodon and Nyctosaurus from the Smoky Hill Chalk Member, Niobrara Formation. (1) Metacarpal (Mc) IV length plotted against wing phalanx (WP) 1 length. (2) Greatest diameter of dorsal condyle of distal wing metacarpal (McIV-d) against McIV length. Pteranodon data from Bennett (Reference Bennett2001, fig. 119D, I) indicated by dotted squares, Nyctosaurus data from Bennett (Reference Bennett2003, and unpublished data) indicated by plus signs, and FHSM 17956 indicated by the large dot. FHSM 17956 was not included in the samples from which the regression equations were calculated.
The lengths of the missing elements of FHSM 17956 were calculated using Bennett’s (Reference Bennett2001) regression equations, and the wingspan in life estimated as 1.76 m (Table 1). The smallest relatively complete previously known specimen of Pteranodon, AMNH 4908, consists of a partial trunk skeleton and tail, scapulocoracoid, humerus through WP2, both femora and tibiae, and a disarticulated foot (Bennett, Reference Bennett2001; Table 1), had an estimated wingspan in life of 3.33 m. Just slightly larger is YPM VP 2345, an incomplete wing of an immature individual, and YPM VP 2350, an isolated WP1 (352 mm long). The greatest diameters of the dorsal condyles of McIV of AMNH 4908 and YPM VP 2345 are 20 and 19.9 mm, respectively. There are eight fragmentary specimens with greatest dorsal condyle diameters of 18–20 mm (FMNH P27476, YPM VP 2454, KUVP 85401, YPM VP 2755, LACM 51136 “B”, YPM VP 2444, 2490, and 2710, arranged in order of increasing diameter), although it should be noted that abrasion of the poorly ossified condyles may have reduced their diameters somewhat. The smallest of the eight specimens is 10% smaller than AMNH 4908, so presumably would have had an estimated wingspan in life of 3.0 m. Thus, FHSM 17956 is much smaller than the smallest previously known specimens; only ~53% the size of AMNH 4908 and ~59% the size of FMNH P27476. There is no information as to the sex of FHSM 17956, but if it was female it would have been ~53% the size of the smallest previously known female, whereas if it was male it would have been only ~37% the size of the smallest previously known male.
Table 1 Length measurements (in mm except as noted) of limb elements and the greatest diameter of the dorsal distal condyle of the wing metacarpal of small specimens of Pteranodon from the Smoky Hill Chalk Member. Wingspans in life estimated by the method of Bennett (Reference Bennett2001).

* length of incomplete or missing limb bone calculated with linear regression equations (Bennett, Reference Bennett2001)
** wingspan estimated based on comparison to AMNH 4908
FHSM 17956 exhibits immature states of the size independent criteria Bennett (Reference Bennett1993) used to assess maturity of specimens, i.e., incomplete ossification of epiphyses, immature grain on limb bone shafts, and no fusion of bones, and so is immature. Though much smaller than the smallest previously known specimens, it is otherwise indistinguishable from the immature subadults present throughout the sample of Pteranodon; thus no more immature morphologically than the most immature previously known specimens. However, because it is much smaller than the subadults, it is considered to be a juvenile. As such, it was presumably significantly younger than all previously known specimens.
Although FHSM 17956 is much smaller than previously known specimens, its morphology provides no evidence to suggest that it was incapable of flying out into the Western Interior Seaway where it was found (~200 km from the nearest shore). It is unlikely that it died elsewhere and floated out to sea before sinking to the bottom and becoming preserved; large dinosaurs (e.g., Claosaurus, Niobrarasaurus) occasionally floated to sea, but the Smoky Hill Chalk Member preserves no evidence that small terrestrial vertebrates (e.g., lizards, immature crocodilians) did so. One reviewer argued that there is no evidence that FHSM 17956 flew to its resting place, an isolated wing ripped off a carcass onshore could conceivably have floated to sea. That is true, but following that line of reasoning there is no evidence that any of the >1000 specimens of Pteranodon flew out to sea and no evidence that any pterosaur actually could fly. Despite that, all pterosaur workers find it most parsimonious to interpret pterosaurs as having flown. It is also most parsimonious to interpret FHSM 17956, smaller than but otherwise indistinguishable from the ~150 immature subadults in the sample of Pteranodon, as having flown out to sea, though it may not have done so intentionally and might have been blown by a storm.
The thin section (Fig. 3) demonstrates that the bone histology is similar to that of immature subadult Pteranodon (Bennett, Reference Bennett1993, fig. 2C), so is consistent with the interpretation of the specimen as immature. No LAG was found in the thin section, just as none was found in the thin section of the subadult, although LAGs do occur in Pteranodon bones (L.E. Wilson, personal communcation, 2016). Chinsamy et al. (Reference Chinsamy, Codorniú and Chiappe2008, Reference Chinsamy, Codorniú and Chiappe2009) documented rapid growth without LAGs in Pterodaustro for the first 2–3 years until ~50% of full size was attained. Four LAGs were found in a tibia ~91% of full size, with the inter-LAG spacing decreasing outward demonstrating that the growth rate decreased progressively until full size was attained in ~7 years. If Pteranodon grew as Pterodaustro did, then LAGs would not be expected in FHSM 17956 because it is only ~46% the size of a fully grown modal female with ~3.8 m wingspan.
Bennett (Reference Bennett1992) proposed that Pteranodon’s marked sexual dimorphism and female biased sex ratio was indicative of a lek-type mating system with large males competing for access to more abundant females. In addition, the presence of immature specimens with poorly ossified epiphyses and limb bone shafts with virtually all bones unfused combined with the absence of specimens of <3 m wingspan was interpreted as evidence of rapid growth to adult size. I assumed that juveniles could not have sustained rapid growth while flying and feeding independently, and so thought that Pteranodon hatchlings would have required parental feeding before flying and feeding independently, much as many extant seabirds do (Bennett, Reference Bennett1993). The discovery of FHSM 17956 does not affect the interpretation of sexual dimorphism, but falsifies the assumption of rapid growth and parental feeding before flying and feeding independently.
If small juveniles were capable of flying out to sea, then why are they so rare in the Smoky Hill Chalk Member? It is conceivable that there was a bias against the preservation of small specimens. The sample of Nyctosaurus from the Smoky Hill Chalk Member consists of 30 specimens, most of which have estimated wingspans of 1.6–2.0 m. If one were to assume that Pteranodon and Nyctosaurus were equally abundant in the Western Interior Seaway, then the relative rarity of small Nyctosaurus specimens (30 Nyctosaurus versus >1000 Pteranodon) might suggest a bias against the preservation of small individuals, but if that were the case then small juveniles of Pteranodon would still be ~30 times rarer than similarly sized specimens of Nyctosaurus. However, there is no evidence that Nyctosaurus was as abundant as Pteranodon and no evidence of a bias against the preservation of small pterosaurs. Therefore, the extreme rarity of small juveniles of Pteranodon in the Smoky Hill Chalk Member is interpreted as reflecting an extreme rarity in life because they did not normally go to sea until their wingspans were ~3 m. Juveniles between ~1.5 and ~3 m wingspan must have been occupying different environments and ecological niches than adults did. Thus, Pteranodon exhibited ontogenetic niches.
Ontogenetic niches have been defined as varying patterns of resource use as individuals increase in size from hatching or birth to adulthood and beyond (Werner and Gilliam, Reference Werner and Gilliam1984), such that individuals in different stages of their life history occupy different environments and/or ecological niches in sequence. Ontogenetic niches are found in a wide variety of animals. Most fishes exhibit several shifts of food type and often habitat as they grow; most amphibians transition from aquatic, often herbivorous larvae to carnivorous terrestrial adults, and newts return to water after a terrestrial eft stage with associated shifts in food type (Tomašević Kolarov et al., Reference Tomašević Kolarov, Ivanović and Kalezić2011); and many reptiles switch from carnivory to herbivory with increasing size. There can also be morphological changes associated with habitat and dietary changes; for example, hatchling Alligator mississippiensis (Daudin, 1802) occupy well-vegetated swamps and marshes, have short blunt snouts and needle-like teeth suited to piercing, and feed primarily on insects; juvenile A. mississippiensis have longer snouts and transition to crustaceans and small vertebrates; and adults prefer deeper open waters of rivers and lakes, have long and broad snouts and robust blunt teeth suited to crushing, and feed on turtles and large mammals (Subalusky et al., Reference Subalusky, Fitzgerald and Smith2009; Gignac and Erickson, Reference Gignac and Erickson2015). However, many birds and mammals do not exhibit ontogenetic niches other than a period of parental feeding before transitioning to feeding independently in the same environment and niche as their parents.
Ontogenetic niches are often simply a necessary consequence of the great increases in body length and mass that some organisms exhibit, e.g., a few mm to 2.5 m body length in Atlantic Goliath Grouper Epinephalus itajara (Lichtenstein, Reference Lichtenstein1822) (Artero et al., Reference Artero, Koenig, Richard, Berzins, Guillou, Bouchon and Lampert2015) or a 7000-fold increase in body mass in Alligator (Gignac and Erickson, Reference Gignac and Erickson2015), with the animal switching to larger and larger foods and the environments where those foods can be obtained as it grows. However, selection will favor those environments and niches that provide an optimum balance of predation risk and growth rate (Dahlgren and Eggleston, Reference Dahlgren and Eggleston2000).
Ontogenetic niches have been recently described in dinosaurs (Codron et al., Reference Codron, Carbone, Müller and Clauss2012, Reference Codron, Carbone and Clauss2013; Fowler et al., Reference Fowler, Freedman Fowler, Scannella and Horner2013), which exhibited great increases in body length and mass as they grew to adulthood. Therefore, it should not be surprising that Pteranodon exhibited ontogenetic niches as hatchlings with wingspans of ≤1 m that grew into adults with wingspans up to ~6 m. With the exception of FHSM 17956, the sample of Pteranodon from the Smoky Hill Chalk Member consists of ~85% mature adults and ~15% immature subadults of similar size (Bennett, Reference Bennett1993). The fossils are so abundant in the Smoky Hill Chalk Member of the Niobrara Formation and the Sharon Springs Member of the Pierre Shale Formation, making up ~10% of tetrapod fossils in both the Smoky Hill Chalk Member (SCB, personal observation) and the Sharon Springs Member (Nicholls and Russell, Reference Nicholls and Russell1990), that adults and subadults must have spent much time in the Western Interior Seaway. Pteranodon may also have been long-lived because a thin section of wing bone of an adult (Bennett, Reference Bennett1993, fig. 2D; Fig. 5) exhibits three closely spaced circumferential bands ~22 μm apart, which compare well to the closely spaced LAGs in the peripheral cortex of the dinosaur Syntarsus (Chinsamy, Reference Chinsamy1990, fig. 1C). Spaced ~22 μm apart and ~480 μm below the external surface, if interpreted as LAGs and annual lines, the thickness of bone between the first LAG and the external surface would represent ~22 years if the growth rate was constant. Thus some individuals may have survived many years after reaching skeletal maturity, which would be similar to long-lived albatrosses that can live 40 years or more (Bird Banding Laboratory, 2016).
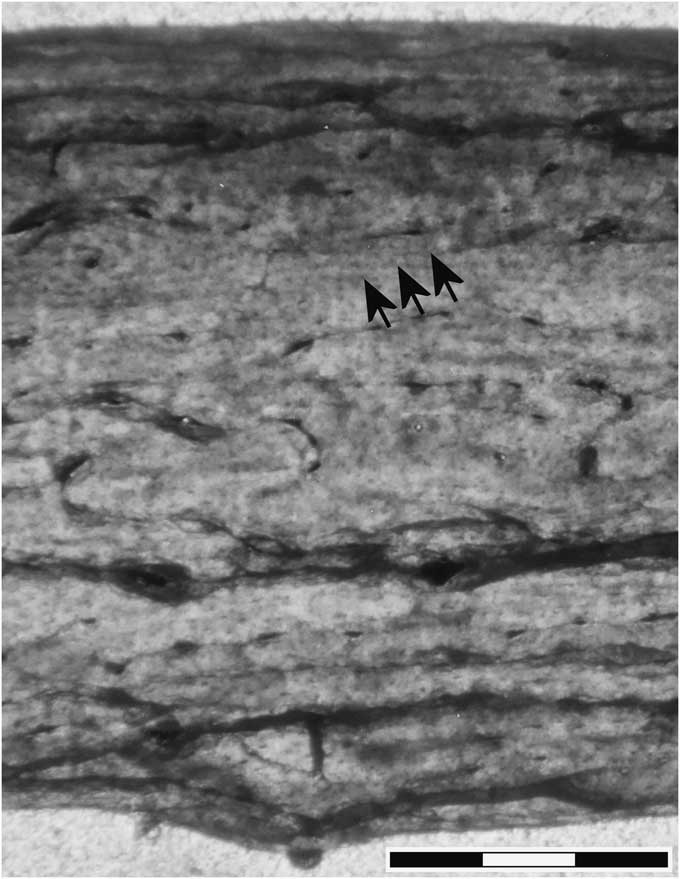
Figure 5 Pteranodon sp. indet. Thin section of cortical bone of limb bone shaft of a mature adult after Bennett (Reference Bennett1993, fig. 2C). Arrows point to closely spaced circumferential bands interpreted as lines of arrested growth of a very slow growing fully mature adult individual. Scale bar is 500 μm.
The Smoky Hill Chalk Member in western Kansas consists of ~180 m of impure chalk deposited in open sea 150–300 m deep in the middle of the Western Interior Seaway (Hattin, Reference Hattin1982), which supported a diverse and abundant planktonic and pelagic biota, but had a depauperate benthos because of poorly oxygenated bottom waters (Stewart, Reference Stewart1990; Everhart, Reference Everhart2005). Pteranodon at sea fed on fish and perhaps invertebrates as well. An isolated mandible of a female Pteranodon, AMNH 5098, is preserved in dorsal view with ~3 cm diameter mass of fish vertebrae just behind the posterior margin of the symphyseal shelf of the mandible (Fig. 6.1), and another female specimen consisting of an articulated cranium and mandible was preserved in left lateral view with a small mass of fish remains in a similar position immediately behind the posteroventral end of the symphysis (G. Rockers, personal communication, 2015). In both specimens, the masses were presumably ejected from the gut perimortem. Brown (Reference Brown1943, p. 106) commented on AMNH 5098 and stated that the mass included “the joint of a crustacean,” but my examinations failed to find any such evidence. Most of the vertebrae are 3.5–4.0 mm long, but the largest vertebra is 8.8 mm long. It is not clear what fish species the vertebrae pertain to, but they are consistent with those of Enchodus, which is common in the Smoky Hill Chalk Member. The smaller vertebrae are comparable to those of an Enchodus ~23–27 cm long, whereas the largest vertebra is comparable to those of an Enchodus ~53 cm long, a fish that was quite large relative to the head and body of Pteranodon (Fig. 6.2). Thus, the Smoky Hill Chalk Member represents a pelagic feeding habitat in the middle of the Western Interior Seaway where adult and subadult Pteranodon spent much of their time feeding on relatively large fishes, and FHSM 17956 is a one-in-a-thousand occurrence of a small juvenile that apparently strayed from its normal habitat and flew out to sea.

Figure 6 Pteranodon and prey. (1) AMNH 5098, incomplete mandible of Pteranodon in dorsal view with mass of fish vertebrae preserved just behind symphysis. (2) Reconstruction of Pteranodon axial skeleton in left lateral view modified from Bennett (Reference Bennett2001) and scaled to size of AMNH 5098 with skeletal reconstructions of fish (after Woodward, 1902–Reference Woodward1912, fig. 12) scaled to sizes of the typical and largest fish vertebrae in the mass. Abbreviations: fv, mass of fish vertebrae, rr, right ramus of mandible; lr, left ramus of mandible; lfv, largest fish vertebra; and pm, posterior margin of symphyseal shelf. Scale bars are 3 and 15 cm, respectively.
The Sharon Springs Member in western Kansas, South Dakota, and Wyoming consists of ~15 m of gray claystone and shale deposited in the Western Interior Seaway as the seaway regressed, narrowed, and received more terrigenous sediment (Gill et al., Reference Gill, Cobban and Schultz1972). Like the Smoky Hill Chalk Member, on which it often lies conformably, it supported a diverse and abundant pelagic biota that was simply the continuation of the Smoky Hill Chalk Member’s fauna (Carpenter, Reference Carpenter1990, Reference Carpenter2008). The sample of Pteranodon from the Sharon Springs Member is much smaller than that from the Smoky Hill Chalk Member, but like that of the Smoky Hill Chalk Member it consists primarily of mature adults with a small percentage of immature subadults of virtually adult size (Bennett, Reference Bennett2001). Thus the Sharon Springs Member also represents a pelagic feeding habitat in the middle of the Western Interior Seaway where adult and subadult Pteranodon fed on relatively large fishes.
The absence of hatchlings and juveniles in the Western Interior Seaway poses three questions. At what age did Pteranodon subadults go to sea? What were Pteranodon hatchlings and juveniles doing before they went to sea? Where were Pteranodon hatchlings and juveniles?
At what age did Pteranodon subadults go to sea?
Relatively little is known about pterosaur growth rates. Bennett (Reference Bennett1995; Fig. 7.1) studied the sample of Rhamphorhynchus muensteri Goldfuss, Reference Goldfuss1831 from the Solnhofen Limestone monographed by Wellnhofer (Reference Wellnhofer1975) and found it to consist of two distinct size-classes plus a few larger individuals that might represent more size-classes. Specimens in the small size-class were most immature, specimens in the medium size-class were older but still immature, and only the largest specimens could be considered fully mature adults. I argued that the size-classes represent year-classes resulting from seasonal sampling of a population of individuals growing at a more or less uniform rate, and that the growth rate was comparable to that of extant Alligator. If Rhamphorhynchus maintained a constant growth rate, it would have taken ~3 years for hatchlings to grow to the size of the largest specimen (~1.8 m wingspan); however, it is likely that the growth rate declined with increasing size and age and it took somewhat longer. Similarly, Bennett (Reference Bennett1996, Reference Bennett2013a; Fig. 7.2) studied the sample of Pterodactylus antiquus (Sömmerring, Reference Sömmerring1812) from the Solnhofen Limestone monographed by Wellnhofer (Reference Wellnhofer1970) and found it to consist of two distinct year-classes plus a few larger individuals that might represent more year-classes, which again suggested it would have taken at least three years to reach adult size (~1.1 m wingspan). The patterns of growth of Rhamphorhynchus and Pterodactylus compare well to that of Pterodaustro (Chinsamy et al., Reference Chinsamy, Codorniú and Chiappe2008, Reference Chinsamy, Codorniú and Chiappe2009), which grew rapidly for 2–3 years until half grown and then grew progressively more slowly for another ~4 years until reaching full size (~2.5–3 m wingspan, Witton, Reference Witton2013; L. Codorniú, personal communication, 2015), except that Pterodaustro took longer to reach its greater full size. There is no evidence that juvenile Pterodaustro grew faster than juvenile Rhamphorhynchus in order to reach the larger size of adults, so Pterodaustro presumably prolonged the period of rapid growth.
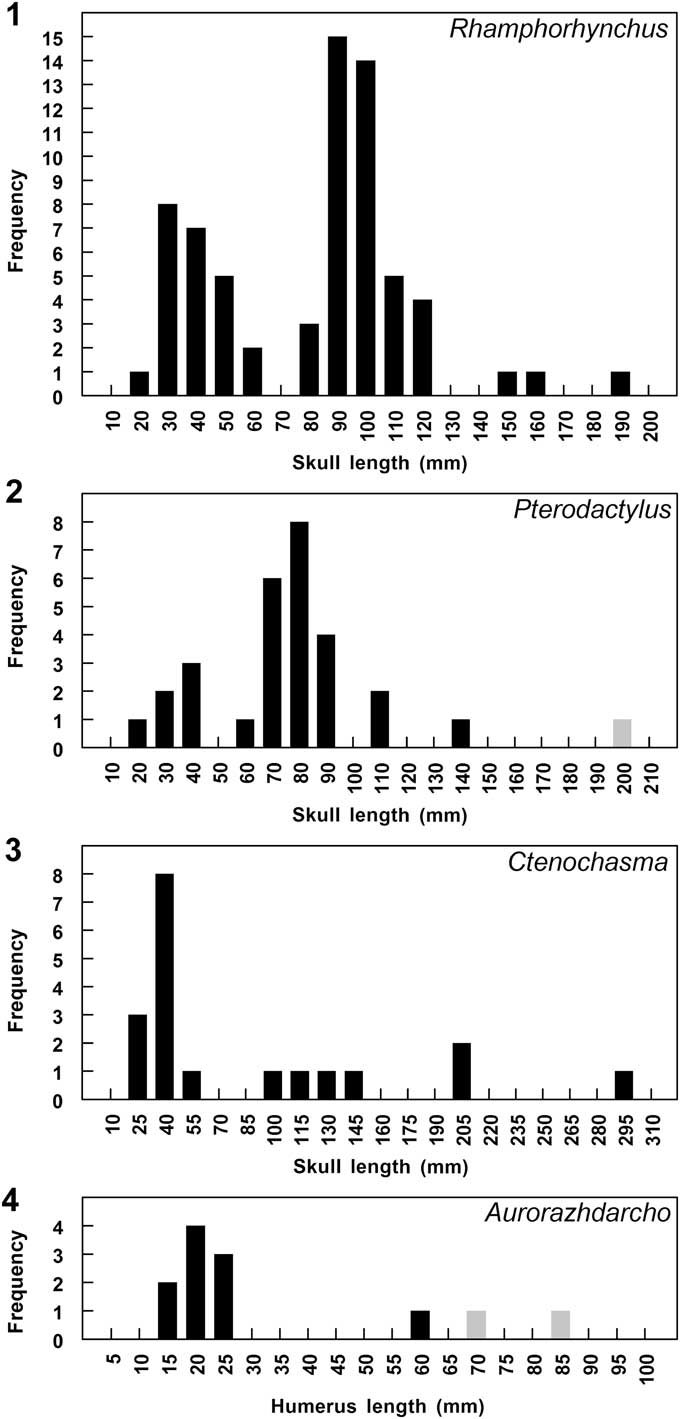
Figure 7 Size-frequency histograms of samples of pterosaurs from the Solnhofen Limestone. (1) Rhamphorhynchus muensteri skull length (data from Wellnhofer, Reference Wellnhofer1975; Bennett, Reference Bennett1995). (2) Pterodactylus antiquus skull length (data from Wellnhofer, Reference Wellnhofer1970; Bennett, Reference Bennett2006, 2103a), gray bar at right is the adult from the Hienheim Beds. (3) Ctenochasma elegans skull length (data from Wellnhofer, Reference Wellnhofer1970; Bennett, Reference Bennett2007a, Reference Bennett2013a). (4) Aurorazhdarcho micronyx humerus length (data from Wellnhofer, Reference Wellnhofer1970; Bennett, Reference Bennett2006, Reference Bennett2013a), gray bars at right represent approximate size of Gnathosaurus subulatus Meyer, Reference Meyer1834 specimens known only from skulls. See text for explanation.
Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) examined the bone histology of selected elements of five specimens of Rhamphorhynchus. Two specimens in the first year-class exhibited fibrolamellar bone with occasional laminar endosteal bone, and on that basis they were interpreted as early juveniles. Two specimens in the second year-class exhibited parallel-fibered bone and lamellar bone with LAGs. The smaller of the two exhibited two closely spaced LAGs in the inner half of the cortex and a thick layer of parallel-fibered bone in the outer half of the cortex with a few primary osteons but lacking LAGs, and was interpreted as a late juvenile. The larger specimen in the second year-class, MTM V 2008.33.1, exhibited both primary and secondary osteons and three LAGs in the outer half of the cortex of the ulna, but only one in the femur, and was interpreted as an adult exhibiting skeletal maturity. The closely spaced LAGs in these specimens make it unlikely that every LAG is an annual line, but they may record successive stops and starts during a season of slowed growth. The largest specimen, BSP 1929.I.69, which exhibited parallel-fibered bone with primary osteons and 5–6 LAGs, was also interpreted as an adult. Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) also discussed two specimens described by Padian et al. (Reference Padian, Horner and Ricqlès2004), one similar in size and histology to the specimens in the small year-class, and RAM V97017/258, similar in size to MTM V 2008.33.1, which exhibited two widely spaced LAGs and fibrolamellar bone transitioning to lamellar bone with reduced vascularity in its ulna, three LAGs in its femur, and four LAGs in its tibia. Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) interpreted RAM V97017/258 as an adult.
Based on their interpretation that MTM V 2008.33.1, RAM V97017/258, and the larger BSP 1929.I.69 were all mature adults, Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) concluded Bennett’s (Reference Bennett1995) medium year-class was not a real ontogenetic stage because some mature adults occurred in it. However, Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) did not use the terms juvenile and adult as Bennett (Reference Bennett1995) did. Whereas Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) interpreted MTM V 2008.33.1 as adult because of its histology and the fusion of bones, Bennett (Reference Bennett1995) applied that term only to the largest most mature specimens and would have viewed the presence of an external fundamental system as the definitive indicator of histological adulthood just as Woodward et al. (Reference Woodward, Horner and Farlow2011) did in Alligator. Despite exhibiting relatively mature histology, MTM V 2008.33.1 is less mature than BSP 1929.I.69 because its jaws and teeth are less robust than those of BSP 1929.I.69, just as BSP 1929.I.69 is less mature than GPIT RE 7321 because its jaws and teeth of are less robust than those of GPIT RE 7321. In the end, Prondvai et al.’s (2012) histological information is consistent with and supports Bennett’s (Reference Bennett1995, Reference Bennett1996) interpretation that the small and medium year-classes of Rhamphorhynchus consist of small immature and larger more-mature specimens, respectively, and the finding that the upper end of the medium year-class includes specimens with relatively mature histology and fused bones refines the interpretation.
Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) interpreted the transition to lamellar bone with primary and secondary osteons in Rhamphorhynchus as resulting from the onset of flight based on comparisons to enantiornithine birds, and it was stated that juveniles would have been restricted to the ground and/or trees and would have received parental feedings or somehow fed themselves. There are several problems with that interpretation. It is not clear that the histological transition necessarily reflects anything other than a decrease in growth rate with increasing size, and there is no reason to think that pterosaur ontogeny would be comparable to that of enantiornithines (assuming that the relationship between ontogeny and histology in enantiornithines is correctly understood) because pterosaurs were not closely related to birds and are as different from birds and bats as birds and bats are different from each other. More importantly, the smallest, most immature specimens of Rhamphorhynchus were no more than a few weeks old, yet had skeletal anatomy and proportions similar to those of adults (Unwin, Reference Unwin2006; Lü et al., Reference Lü, Unwin, Deeming, Jin, Liu and Ji2011). The long, well-ossified, although actively growing forelimb bones that would be subjected to bending loads in flight, extensive patagia (e.g., BSP 1938 I 503), and low wing loadings because of their small size indicate they were capable of powered flight. Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) mischaracterized that interpretation as superprecociality (i.e., active locomotion within minutes of hatching), and argued against it and presumably precociality (i.e., relatively mature and mobile from hatching in contrast to the relative helplessness at hatching of altriciality) as well on the grounds that pterosaur eggs represented only a small investment of nutrients relative to maternal body size. Pterosaur eggs were not particularly small relative to maternal trunk volume (Unwin and Deeming, Reference Unwin and Deeming2008; Lü et al., Reference Lü, Unwin, Deeming, Jin, Liu and Ji2011), but it is the absolute rather than relative size that is important for precociality and some diapsids with relatively much smaller eggs produce super-precocial hatchlings (e.g., baby sea turtles dig out of the nest and sprint for the safety of the sea immediately after hatching, and baby crocodilians swim well immediately). Pterosaur hatchlings seem to have been precocial, but it is unlikely that they were superprecocial because of the tight folding of the wingfinger within the egg (Unwin and Deeming, Reference Unwin and Deeming2008). In most pterosaurs, the wingfinger’s interphalangeal joints allowed only slight flexion and extension (Bennett, Reference Bennett2007b, Reference Bennett2013b), and so it probably took hours to days for the wingfinger and patagium to attain flight configuration. Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) also suggested that hatchlings’ flight muscles would not have been powerful enough for flight, but there is no evidence of that because hatchlings of the superprecocial Australian Brush-Turkey, Alectura lathami Gray, Reference Gray1831, can fly within 24 hours of hatching (Starck and Ricklefs, Reference Starck and Ricklefs1998; Dial and Jackson, Reference Dial and Jackson2011). Hatchling diapsids usually have residual yolk constituting ~10% of hatching mass that can sustain the individual for some weeks (Allsteadt and Lang, Reference Allsteadt and Lang1995; Tucker et al., Reference Tucker, Filoramo, Paukstis and Janzen1998; Radder et al., Reference Radder, Warner, Cuervo and Shine2007; Wolanski et al., Reference Wolanski, Renema, Robinson, Carney and Fancher2007; Van Dyke et al., Reference Van Dyke, Plummer and Beaupre2011), and it is conceivable that hatchling pterosaurs used such residual yolk for some days of grounded flapping so as to attain the wing’s flight configuration, to exercise the pectoral musculature, and to train the neural control mechanisms needed for flight.
The large fully formed wings of hatchling Rhamphorhynchus and Pterodactylus are in marked contrast to the wings of neonate bats and most hatchling birds. The wings of Indian Pygmy Bat, Pipistrellus mimus (Wroughton, Reference Wroughton1899), neonates are small and there is a linear increase in wing length and area and decrease in wing loading until sustained flight is attained at ~29 days (first flight ~22 days; Isaac and Marimuthu, Reference Isaac and Μarimuthu1997). Those of larger fruit bats Cynopterus sphinx (Vahl, Reference Vahl1797) and Rousettus leschenaulti (Desmarest, Reference Desmarest1820) similarly exhibit a linear increase in wing length and area until attaining wingspans of 70-80% that of adults and first flight at ~50 days (sustained flight ~60 days) whereas wing loading decreases linearly for ~35 and ~50 days, respectively, before beginning to increase with increasing body mass (Elangovan et al., Reference Elangovan, Raghuram, Yuvana Satya Priya and Marimuthu2004, Reference Elangovan, Yuvana Satya Priya, Raghuram and Marimuthu2007). Birds are more variable than bats in this regard, but many hatchling birds lack flight feathers and grow considerably before flying, increasing wing span and area (Starck and Ricklefs, Reference Starck and Ricklefs1998). In addition, the long bones of wings of hatchling California Gulls, Larus californicus (Lawrence, Reference Lawrence1854), remain relatively weak as they grow to flight size and only attain the necessary strength for flight when wing flapping begins shortly before fledging at ~35 days (Pugesek, Reference Pugesek1983; Carrier and Leon, Reference Carrier and Leon1990). If pterosaurs delayed the onset of flight until they were half grown, then they probably would have hatched with small wings that only reached the necessary length, strength, area, and wing loading for flight as the individual approached half size and the onset of flight, but that is not the case.
One last problem is that Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012, p. 2) did not discuss how long Rhamphorhynchus would have been restricted to the ground and/or trees, receiving parental feedings or feeding themselves, and stated only that the presence of fibrolamellar bone suggested that “growth did not protract over several years.” The fact that fibrolamellar bone is correlated with ‘rapid’ deposition in extant endotherms has been used to argue that the presence of fibrolamellar bone in extinct organisms indicates ‘rapid’ growth; however, just as with the argument that flying vertebrates must be endothermic because extant vertebrate fliers are endotherms, correlation does not imply causation, and ‘rapid’ in pterosaurs may not be the same as ‘rapid’ in extant birds. Fibrolamellar bone has been reported in free living Alligator (Tumarkin-Deratzian, Reference Tumarkin-Deratzian2007) and in temnospondyls (Woodward et al., Reference Woodward, Horner and Farlow2014), so the presence of fibrolamellar bone in Rhamphorhynchus is not necessarily inconsistent with an Alligator-like growth rate, and any bone tissue will be deposited more slowly in an animal that allows its metabolic rate and body temperature to decrease significantly at rest and at night resulting in a low mean body temperature (e.g., endotherms exhibiting torpor, ectotherms) than in an animal that maintains a constant high metabolic rate and body temperature. Without temporal information to support it, Prondvai et al.’s (2012) suggestion that fibrolamellar bone in Rhamphorhynchus indicates that growth did not take several years is unreliable. Bennett’s (Reference Bennett1995, Reference Bennett1996) interpretation that the discontinuous distribution of small, medium, and large size-classes in the samples of Rhamphorhynchus and other pterosaurs and fishes from the Solnhofen Limestone are year-classes resulting from seasonal mortality and/or preservation of specimens provides the needed temporal information to evaluate the growth rate of Rhamphorhynchus. The time represented by the gap between the small and medium size-classes is ~1 year, which contradicts Prondvai et al.’s (2012) suggestion that growth did not take several years. Prondvai et al. (Reference Prondvai, Stein, Ösi and Sander2012) made no attempt to counter the interpretation of year-classes or explain away the discontinuous distributions.
The histological transition to slower-growing bone in Rhamphorhynchus seems to have occurred in the larger individuals in the medium year-class, so if juveniles delayed the onset of flight until the histological transition, then they would have delayed flight for ~1 year. That would be a long time to be grounded and receiving parental feedings, and the abundance of fossils of hatchlings and juveniles in the Solnhofen Limestones, significantly greater than that of non-flying lizards (Barthel et al., Reference Barthel, Swinburne and Conway Morris1990), suggests that they spent much time in the lagoons, which would be unlikely if they received parental feeding. It is also unlikely that juveniles could have fed themselves adequately on the ground or in the trees with their fish-grab dentition and their very long wingfingers and very short hindlimbs hampering both walking and climbing. If the onset of flight was correlated with the histological transition to a slower-growing bone in other pterosaurs, then Pterodaustro would not have flown for 2–3 years until half grown. Similarly, Pteranodon subadults that were not significantly smaller than mature adults would not have attained flight until after they arrived in the Western Interior Seaway because they exhibit well-vascularized plexiform bone without evidence of lamellar bone or LAGs (Bennett, Reference Bennett1993). FHSM 17956 with 1.76 m of well-developed wings are no different structurally or histologically from those of subadults, and would not have been able to fly until it almost doubled its size. In short, there is no evidence that Rhamphorhynchus and Pterodaustro could not have flown and sustained growth to half-size over the course of ~1 and 3–4 years, respectively, and the well-developed wings of hatchling Rhamphorhynchus and Pterodactylus, plus the fact that FHSM 17956 must have flown to the middle of the Western Interior Seaway, demonstrate that hatchlings and juveniles with plexiform and fibrolamellar bone were capable of flight. Therefore, Prondvai et al.’s (2012) interpretation of delayed onset of flight must be rejected.
In the end, we cannot know how old Pteranodon subadults were when they went to sea. However, if their growth rate was comparable to that of Rhamphorhynchus, Pterodactylus, and Pterodaustro, then it would have taken at least three years to reach the size of FHSM 17956 and another 3–4 years to reach ~3 m wingspan. In growing to modal wingspans of 3.8 and 5.6 m for females and males (Bennett, Reference Bennett1992), respectively, Pteranodon presumably prolonged the period of rapid growth even further than Pterodaustro did by delaying the transition from plexiform to lamellar bone until their wingspan was 3.0 m or more. Indeed, based on the assumption that a 3 m wingspan subadult would grow into a modal 3.8 m mature adult female, the transition would occur at ~80% of maximum size rather than the ~50% seen in Pterodaustro. Pteranodon also seems to have delayed the development of large cranial crests by males until after they went to sea (Bennett and Penkalski, Reference Bennett and Penkalski2017), which suggests that sexual maturation in both sexes was roughly coincident with skeletal maturation, as indicated by well-ossified epiphyses, mature grain on limb bone shafts, and fusion of bones some time after going to sea. That would be consistent with the interpretation of Chinsamy et al. (Reference Chinsamy, Codorniú and Chiappe2008, Reference Chinsamy, Codorniú and Chiappe2009) that the histological transition from fast- to slower-growing bone reflects the onset of sexual maturation and the diversion of nutrients and energy from rapid growth to reproduction.
What were Pteranodon hatchlings and juveniles doing before they went to sea?
Pteranodon hatchlings and juveniles presumably were flying and feeding independently like those of Rhamphorhynchus, Pterodactylus, and Pterodaustro. They would have resembled females in having pointed edentulous, roughly equilength jaws comparable to those of herons, storks, and many other extant birds, which would have been well suited to feeding on all manner of small animals. The more conservative interpretation is that Pteranodon hatchlings and juveniles fed on suitably sized aquatic prey (e.g., crustaceans, small fishes) in shallow waters and progressed to larger and larger prey as they grew. However, they also could have fed on suitably sized prey in terrestrial environments (e.g., arthropods, small tetrapods), much as extant storks do.
Where were Pteranodon hatchlings and juveniles?
At present, there is no evidence to suggest where Pteranodon hatchlings and juveniles were, but if they fed on suitably sized aquatic prey, then they could have lived around lakes, rivers, wetlands, and estuaries, or along beaches and coastlines, wherever small aquatic prey was available, whereas if they fed on suitably sized terrestrial prey, then they could have lived in varied terrestrial environments. Bennett (Reference Bennett1994b) envisioned Pteranodon laying eggs and rearing young in crowded colonies on islands free of terrestrial predators, much as do many extant seabirds. However, whereas pterosaurs do seem to have aggregated to lay their eggs in colonies (Bell and Padian, Reference Bell and Padian1995; Wang et al., Reference Wang, Kellner, Jiang, Wang, Ma, Paidoula, Cheng, Rodrigues, Meng, Zhang, Ning and Zhonghe2014), there is no evidence that they did so on islands and they probably laid their eggs near those environments that provided suitable niches for hatchlings so that hatchlings would not need to travel far to find food and relative safety.
The sample of Nyctosaurus gracilis (Marsh, Reference Marsh1876) from the Smoky Hill Chalk Member, though numerically smaller than that of Pteranodon, is similar to it in most respects. It consists of 30 specimens with wingspans of 1.6–3.3 m (Bennett, Reference Bennett1994a, Reference Bennett2003, and unpublished data), 11 of which are immature, but do not differ significantly in size from mature individuals and are considered to be subadults, whereas the other 19 are considered to be adults. There is size variation in the sample, but bimodality reflecting sexual dimorphism has not been demonstrated. As with Pteranodon, the sample of Nyctosaurus consists of mature adults and immature subadults of similar size that were feeding in the middle of the Western Interior Seaway and there is no evidence of hatchlings or juveniles. Although the absence of hatchling and juvenile Nyctosaurus is less significant statistically than the one in 1000 occurrence of a juvenile Pteranodon, the absence indicates that hatchlings and juveniles occupied different environment(s) and ecological niche(s) than adults such that Nyctosaurus exhibited ontogenetic niches.
The pattern of ontogenetic niches exhibited by Pteranodon and Nyctosaurus would have permitted hatchlings and juveniles to occupy environments that provided suitably sized prey and an optimal balance between prey availability and predation risk, yet permitted large adults to access the abundant supply of large fishes in the Western Interior Seaway that would not have been available if adults stayed around their breeding grounds and continental or coastal environments with hatchlings and juveniles. At sea, the marked size dimorphism of Pteranodon with modal males of 5.6 m wingspan, ~50% larger than modal females of 3.8 m wingspan (Bennett, Reference Bennett1992), would have enabled males to feed on significantly larger fishes than females. This suggests that the available food resources of the Western Interior Seaway were partitioned between Pteranodon sexes by size, and Nyctosaurus with wingspans of 1.6–3.3 m and more lightly built jaws than Pteranodon presumably fed on even smaller prey that represented a third partition of the Seaway’s food resources. Thus, Pteranodon sexes and Nyctosaurus seem to have occupied three different niches at sea so as to reduce competition.
Other pterosaurs
Having found evidence of ontogenetic niches in the size and age distributions of samples of Pteranodon and Nyctosaurus from the Smoky Hill Chalk Member and Sharon Springs Member, the size and age distributions of samples of other pterosaurs from selected well-studied Konservat-Lagerstätten were surveyed for evidence of ontogenetic niches (Table 2). Whereas the complete absence of eggs, hatchlings, small juveniles, and large juveniles save FMNH 17956 provides evidence of ontogenetic niches in Pteranodon, in other pterosaurs the presence of eggs, hatchlings, and small juveniles or the absence of subadults and adults can provide evidence of ontogenetic niches. Eggs are unlikely to travel far, so their presence indicates proximity to nesting grounds. Similarly, hatchlings are unlikely to travel great distances, so it is reasonable to assume that localities where hatchlings have been found are relatively close to nesting grounds. Subadults and adults probably could travel great distances, so their absence or unexpected rarity will suggest that they were not normally present or abundant in the depositional environment.
Table 2 Ontogenetic composition of samples of selected taxa from selected Konservat-Lagerstätten and corresponding interpretations of the Lagerstätte’s habitat in stratigraphic order. Data include N, number of individuals in taxon sample, and percentages (rounded to the nearest non-zero integer) of: E, eggs; H, hatchlings; SJ, small juveniles; LJ, large juveniles; SA, subadults; and A, adults. See text for discussion.

1 Data based on proximal humeral fragments, from A. Averianov (personal communication, 2015). 2Data from L. Codorniú (personal communication, 2015).
Solnhofen Limestone
The Solnhofen Limestone consists of well-bedded lithographic limestones deposited in shallow hypersaline lagoons connected with open-marine environments (Barthel et al., Reference Barthel, Swinburne and Conway Morris1990). Anoxic bottom waters provided conditions favorable to the preservation of vertebrate skeletons, and Unwin (Reference Unwin2006) reported >1000 pterosaur specimens have been found. Small fishes (e.g., Leptolepides) were a significant component in the fauna, and demonstrate that the lagoons and/or nearby waters would have provided abundant food for piscivorous pterosaurs.
The pterosaurs of the Solnhofen Limestone of southern Germany were monographed by Wellnhofer (Reference Wellnhofer1970, Reference Wellnhofer1975) and restudied by Bennett (Reference Bennett1995, Reference Bennett1996, Reference Bennett2006, Reference Bennett2007a, Reference Bennett2007b, Reference Bennett2013a, Reference Bennett2013c, Reference Bennett2014b). Bennett (Reference Bennett1995) determined the ontogenetic ages of Rhamphorhynchus muensteri specimens, and noted that the sample of 109 specimens monographed by Wellnhofer (Reference Wellnhofer1975) consists of two distinct size-classes interpreted as year-classes resulting from seasonal sampling of a population of individuals growing at a more or less uniform rate over the course of several years, plus a few larger individuals that might represent additional year-classes (Fig. 7.1). The first year-class consists of 33 hatchlings and young-of-the-year with wingspans of 29–59 cm, the second year-class consists of 73 yearlings with wingspans of 86–132 cm, there are two larger more mature specimens with wingspans of ~1.5 m, and there is one large fully mature adult specimen with a wingspan of 1.8 m (Wellnhofer, Reference Wellnhofer1975; Bennett, Reference Bennett1995).
Bennett (Reference Bennett1996) noted year-classes in samples of the pterodactyloids monographed by Wellnhofer (Reference Wellnhofer1970). The sample of Pterodactylus antiquus consists of 28 specimens based on Wellnhofer’s data, Bennett’s (Reference Bennett1996) study, and Jouve’s (Reference Jouve2004, see also Bennett, Reference Bennett2013a) synonymization of P. kochi (Wagner, Reference Wagner1837) with P. antiquus after the addition of the Vienna specimen (NHMW 1975/1756/0000, Wellnhofer, Reference Wellnhofer1987) and the exclusion of JME-SOS 4593 that Bennett (Reference Bennett2006) referred to Germanodactylus cristatus (Wiman, Reference Wiman1925). Note that Vidovic and Martill (Reference Vidovic and Martill2014) erected the genus Aerodactylus for the species Pterodactylus scolopaciceps (Meyer, Reference Meyer1860), which Wellnhofer (Reference Wellnhofer1970) considered a junior synonym of P. kochi. They referred several Pterodactylus specimens to Aerodactylus, which they considered to differ from Pterodactylus in possessing a unique combination of features, but did not cite any features that did not fall within the range of variation of the hypodigm of P. antiquus. Five scatter plots of length measures were presented to support their interpretation, but: (1) all reflect only minor differences in skull depth, (2) slopes of regression lines of their Morphotype 1 (=Aerodactylus) in two plots (Vidovic and Martill, Reference Vidovic and Martill2014, figs. 9, 10) differed markedly from those of other Solnhofen pterodactyloids such that the morphotype probably is an unnatural assemblage, and (3) no evidence was presented to suggest that the differences do not merely reflect preservational (e.g., orientation, compression) or individual variation. In the end, despite arguing that the specimens differed, they did not present evidence or arguments that the differences are taxonomically significant, and therefore Aerodactylus scolopaciceps is considered a junior synonym of Pterodactylus antiquus.
The sample of P. antiquus consists of two distinct year-classes plus a few larger individuals that might represent more year-classes (Fig. 7.2). The first year-class consists of six hatchlings and young-of-the-year with wingspans of 17–23 cm, the second year-class consists of 20 yearlings with wingspans of 33–54 cm, and there are two larger more mature specimens with wingspans of 64 and 71 cm, respectively. Note that the largest known specimen of P. antiquus is a large isolated skull of an adult with a wingspan of 1.06 m (Bennett, Reference Bennett2013a) from the Hienheim Beds that are stratigraphically equivalent to the Mörnsheimer Limestone.
The sample of Ctenochasma elegans (Wagner, Reference Wagner1861) consists of 19 specimens based on the 15 listed in Bennett’s (Reference Bennett2007a) revision plus the four specimens that Wellnhofer (Reference Wellnhofer1970) had referred to Pterodactylus micronyx Meyer, Reference Meyer1856 (his Ex. 29 at Neuberg, Ex. 33 at Brno, Ex. 34 [TM 13104], and Ex. 37 [SM 405/NHMW 2012/0118/0001]), which Bennett (Reference Bennett2013a) referred to Ctenochasma on the basis of skeletal proportions. The sample consists of two distinct year-classes plus a few larger individuals that might represent more year-classes (Fig. 7.3). All specimens of >125 mm skull length and >83 cm wingspan consist of isolated skulls. The first year-class consists of 12 hatchlings and young-of-the-year with wingspans of 23–36 cm, the second year-class consists of four yearlings with wingspans of 75–97 cm, there are two larger more mature specimens with wingspans of ~1.3 m, and one large fully mature adult specimen with a wingspan of 1.9 m.
The sample of Aurorazhdarcho micronyx (Meyer, Reference Meyer1856) consists of 12 specimens after the holotype of A. primordius Frey et al., Reference Frey, Meyer and Tischlinger2011 was synonymized with Pterodactylus micronyx and the four specimens mentioned above that were referred to Ctenochasma elegans (Bennett, Reference Bennett2013a) are excluded, but only ten specimens after the two specimens that have different skeletal proportions and may represent a distinct taxon (Bennett, Reference Bennett2013a) are excluded. All of them, except the holotype of Aurorazhdarcho primordius, form what is interpreted as a single year-class of hatchlings and young-of-the-year with wingspans of 23–46 cm (Fig. 7.4). The single large specimen had a wingspan of 1.06 m.
The sample of Germanodactylus cristatus (Wiman, 1925) is too small to reveal the presence or absence of year-classes, but consists of two hatchlings with wingspans of 21 and 22 cm, one subadult, and one adult with a wingspan of 96 cm. Bennett (Reference Bennett2006) noted that the two hatchlings came from a single quarry, Workerszell near Eichstätt, that seems not to have produced any other pterosaur specimens, which suggests that that part of the basin was near the Germanodactylus nesting grounds and provided conditions preferred by the hatchlings, and that juveniles occupied other habitats.
Comparison of the ontogenetic compositions of the samples of Solnhofen pterosaurs reveals three different patterns of abundance. Rhamphorhynchus and Pterodactylus share a pattern with two year-classes, the second more than twice as abundant as the first, and subadults and adults rare to absent. It is surprising that yearlings are more abundant than hatchlings. The abundance of hatchlings suggests that the nesting grounds were close to the Solnhofen lagoons, and the abundance of juveniles indicates that they remained near the lagoons feeding and growing for up to two years. If one assumes that hatchlings and yearlings had equivalent mortality and preservation rates, this could have resulted if hatchlings were present near the lagoons for only part of the depositional season whereas yearlings were present for the entire season. Alternatively, yearlings might have had a higher mortality rate than hatchlings because they were transitioning to a different food source. If the histological transition that occurred in the larger individuals in the medium year-class correlates with the onset of sexual maturity, then Rhamphorhynchus probably would have first bred at two years old. Although specimens of ~1.8 m wingspan are expected to be rare, the relative rarity of specimens between the upper limit of the medium year-class and the largest specimens, which would represent two or more additional year-classes, suggests that sexually mature breeding individuals spent most of their time elsewhere. Comparable histological information is not available for Pterodactylus, but it is possible that Pterodactylus was similar to Rhamphorhynchus in age at first breeding and preferred habitat of adults.
Ctenochasma exhibits a second pattern with a single year-class of hatchlings and young-of-the-year and low numbers of older juveniles through adults. This suggests that Ctenochasma individuals remained near the lagoons feeding on small aquatic prey throughout their life, and is consistent with a high hatchling mortality followed by reduced mortality of juveniles through adults well adapted to their environment.
Aurorazhdarcho exhibits a third pattern with a single year-class of hatchlings and young-of-the-year and a few large adults, whereas yearlings and larger juveniles are absent. This suggests that the nesting grounds were close to the Solnhofen lagoons and that hatchlings spent only their first year near the lagoons, whereas older juveniles and adults left the lagoons and fed elsewhere, perhaps returning to the area only to lay their eggs. This might have been because the dentition of Aurorazhdarcho was suited to feeding on considerably larger prey than Ctenochasma, such that the preferred foods of older juveniles and adults were not available in the Solnhofen lagoons.
Bissekty Formation
The Bissekty Formation consists of ~67 m of medium- to coarse-grained sands with occasional cross-bedding and abundant fossils reflecting a fluvial environment with a very diverse vertebrate fauna, including the pterosaur Azhdarcho lancicollis Nesov, Reference Nesov1984 (Archibald et al., Reference Archibald, Sues, Averianov, King, Ward, Tsaruk, Danilov, Rezvyi, Veretennikov and Khodjaev1998; Averianov, Reference Averianov2010). The sample of Azhdarcho consists of one hatchling, five small juveniles, four large juveniles, two immature subadults, and one mature adult based on a count of proximal humeral fragments (A. Averianov, personal communication, 2015). That pattern of abundance is consistent with progressively decreasing mortality of small juveniles through adults occupying the same riverine habitat. If not attributable to a preservational bias, the relative rarity of hatchlings may suggest that the depositional area of the Bissekty Formation was some distance from the nesting grounds and protected habitats where hatchlings presumably spent their first months.
Lagarcito Formation
The “Loma del Pterodaustro” Lagerstätte within the Lagarcito Formation consists of ~8 m of laminated claystones and massive siltstones with a few sandstone beds deposited in the middle of a large, shallow perennial freshwater lake that supported abundant algae and invertebrates, a somewhat restricted fish fauna, and the pterosaur Pterodaustro guinazi Bonaparte, Reference Bonaparte1970 (Chiappe et al., Reference Chiappe, Rivarola, Cione, Frenegal-Martinez, Sozzi, Buatois, Gallego, Laza, Romero, Lopez-Arbarello, Buscalioni, Marsicano, Adamonis, Ortega, McGehee and Di Iorio1998; Prámparo et al., Reference Prámparo, Ballent, Gallego and Milana2005, Arcucci et al., Reference Arcucci, Prámparo, Codorniú, Giordano, Castillo-Elías, Puebla, Mego, Gómez and Bustos-Escalona2015). The vast majority of Pterodaustro specimens came from ~1 m of laminated claystones and the sample consists of one egg with embryo, an incomplete eggshell, two hatchlings little bigger than the embryo, no small juveniles, two catalogued and many other large juveniles, two immature subadults, and many mature adults (L. Codorniú, personal communication, 2015). The data in Table 2 are based on the arbitrary assumption that ‘many’ equates to 10. The pattern of abundance suggests that large juveniles through adults occupied the same lacustrine habitat, whereas the rarity of small juveniles suggests that they occupied other habitats.
Pterodaustro has been interpreted as feeding while standing in shallow waters, dipping its curving jaws into the water to sieve out small organisms (Wellnhofer, Reference Wellnhofer1991a; Chiappe et al., Reference Chiappe, Rivarola, Cione, Frenegal-Martinez, Sozzi, Buatois, Gallego, Laza, Romero, Lopez-Arbarello, Buscalioni, Marsicano, Adamonis, Ortega, McGehee and Di Iorio1998); however, it does not exhibit any wading adaptations such as elongate limbs. Whereas sieving on the wing is precluded because of the excessive drag the mandible would have produced, it is possible that Pterodaustro fed while floating, duck-like and sieving surface waters where prey might congregate to feed on phytoplankton. If that were the case, they could have fed on a much greater proportion of the lake’s surface area and the lake could have supported much larger populations of Pterodaustro than if they were restricted to shallow waters. In addition, if small juveniles remained in shallow protected waters near shore and large juveniles and adults ventured onto deeper waters, which would explain the absence of small juveniles in the sample.
One reviewer suggested that the large feet of ctenochasmatoids could be interpreted as a wading adaptation, and questioned whether Pterodaustro could have floated in a posture that would have permitted it to sieve given that computational analyses of floating postures have suggested that pterosaurs were nose heavy (Hone and Henderson, Reference Hone and Henderson2013). Those analyses and similar computational analyses of pterosaur body masses (Henderson, Reference Henderson2010), like the apocryphal mathematical proof that bumblebees (Bombus spp.) cannot fly, are based on questionable assumptions, in this case assumptions as to the volume and density of pterosaur body parts. In particular, the floating posture analyses ignored the probability that there were extraskeletal pneumatic spaces within the wings, such as the retrophalangeal wedge in Rhamphorhynchus (Bennett, Reference Bennett2016), which would have significantly increased buoyancy anteriorly and presumably resulted in a stable floating posture. As for feet, if Pterodaustro sieved while floating, then their enlargement could be interpreted as for paddling rather than wading.
The presence of eggs indicates that the area of deposition of the “Loma del Pterodaustro” was close to the nesting grounds of Pterodaustro. Grellet-Tinner et al. (Reference Grellet-Tinner, Thompson, Fiorelli, Argañaraz, Codorniú and Hechenleitner2014) compared Pterodaustro to grebes and flamingoes and suggested that it deposited and brooded its eggs in floating nests; however, there is no evidence of active incubation of eggs in pterosaurs (Unwin and Deeming, Reference Unwin and Deeming2008). The presence of the eggs in sediments deposited in the middle of the lake could be explained by flooding events washing eggs deposited on land into the lake. The presence of eggs and hatchlings in the middle of the lake might seem at variance with the absence of small juveniles in the same sediments, but floods that could have washed eggs into the lake probably would have overwhelmed non- and poorly flying hatchlings as well, but could have been escaped by better-flying small juveniles.
Romualdo Member of the Santana Formation
The Romualdo Member of the Santana Formation consists of shales to sandstones deposited in a large lake or inland sea (Maisey, Reference Maisey1991), and its vertebrate fossils preserved in abundant calcareous concretions represent a diverse fauna of fishes, turtles, crocodilians, and pterosaurs. The concretions seem to have formed in thin horizons (Saraiva et al., Reference Saraiva, Hessel, Guerra and Fara2007), such that the temporal range of the fauna is restricted.
Anhanguerids dominate the Romualdo Member pterosaur fauna, and are oversplit taxonomically because authors have considered minor morphological differences to be taxonomically significant such that many species are represented by single specimens. Authors have often not presented evidence or argumentation to suggest that the differences do not merely reflect ontogenetic or individual variation (but see Pinheiro and Rodrigues, Reference Pinheiro and Rodrigues2017), and I find most characters used to differentiate species of Anhanguera of doubtful significance. In my opinion, there are at least two distinct species of anhanguerid in that the holotypes of Tropeognathus mesembrinus Wellnhofer, Reference Wellnhofer1987 and T. robustus Wellnhofer, Reference Wellnhofer1987 exhibit multiple differences, but I concur with Rodrigues and Kellner (Reference Rodrigues and Kellner2013) in viewing T. robustus as referable to Anhanguera and I find no evidence to view any Anhanguera specimens represented by skull materials as not conspecific.
I assembled a broad sample of 31 anhanguerid specimens, including 21 referred to the most abundant anhanguerid genus, Anhanguera, or synonymous genera described and/or illustrated by Buisonjé (Reference Buisonjé1980), Campos and Kellner (Reference Campos and Kellner1985), Wellnhofer (Reference Wellnhofer1985, Reference Wellnhofer1991b), Kellner and Tomida (Reference Kellner and Tomida2000), and Veldmeijer (Reference Veldmeijer2002), four specimens assigned to Tropeognathus or Coloborhynchus (Wellnhofer, Reference Wellnhofer1987; Veldmeijer, Reference Veldmeijer2003a; Kellner et al., Reference Kellner, Campos, Sayão, Saraiva, Rodrigues, Oliveira, Cruz, Costa, Silva and Ferreira2013), the holotype of Maaradactylus kellneri (Bantim et al., Reference Bantim, Saraiva, Oliveira and Sayão2014), four specimens assigned to Brasileodactylus (Kellner, Reference Kellner1984; Veldmeijer, Reference Veldmeijer2003b; Veldmeijer et al., Reference Veldmeijer, Signore and Meijer2005, Reference Veldmeijer, Meijer and Signore2009), and the holotype of Araripesaurus castilhoi (Price, Reference Price1971). All specimens were evaluated for size and evidence of ontogenetic age using Bennett’s (Reference Bennett1993) size-independent criteria. Sixteen Anhanguera specimens are of a size corresponding to skull lengths of ~50 cm, whereas five specimens are of a size corresponding to skull lengths of ~62 cm. Four Anhanguera specimens exhibit evidence of immaturity, whereas the other 17 seem to be mature adults. All Anhanguera skulls exhibit low rostral crests, which is considered a diagnostic character of Anhanguera. The five specimens assigned to Tropeognathus, Coloborhynchus, and Maaradactylus are mature adults, larger and more robust than is typical of Anhanguera, but most only slightly larger than the largest specimens of Anhanguera, and all have large and tall rostral crests and/or deep symphyseal keels on their mandibles. The four specimens of Brasileodactylus resemble Anhanguera specimens, but lack rostral crests and symphyseal keels, and some are somewhat smaller than Anhanguera specimens. Two Brasileodactylus specimens are isolated incomplete jaws and their ontogenetic age cannot be assessed, but AMNH 24444 (skull length = 43 cm, Veldmeijer, Reference Veldmeijer2003b; thus ~15% smaller than the more abundant smaller specimens of Anhanguera with skull lengths of ~50 cm) and BSP 1991 I 27 (slightly larger than the smallest specimens of Anhanguera) exhibit size-independent indicators of skeletal immaturity. Lastly, the holotype of Araripesaurus castilhoi is an even smaller specimen, ~33% smaller than the more abundant smaller specimens of Anhanguera, consisting of a partial right wing that exhibits unfused carpal elements indicating immaturity, but is otherwise indistinguishable from Anhanguera specimens.
The anhanguerid sample exhibits three size-classes. The large size-class consists of 10 specimens, the Tropeognathus, Coloborhynchus, and Maaradactylus specimens and the five largest Anhanguera specimens, which have skull lengths of ~65 cm or more and, with the exception of NSM-PV 19892, all have large and tall rostral crests and/or deep symphyseal keels on their mandibles. NSM-PV 19892 was referred to Anhanguera because its rostral crest was small and low, but that probably is a result of its immaturity. Presumably, if the specimen had grown to maturity, the crest would have been larger and taller and the skull would have resembled that of specimens assigned to Tropeognathus. The medium size-class consists of the 16 smaller Anhanguera specimens with skull lengths of ~50 cm and low rostral crests. The smaller specimens assigned to Brasileodactylus and the even smaller the holotype of Araripesaurus castilhoi form a third, small size-class. All specimens in the small size-class that can be evaluated for ontogenetic age exhibit evidence of immaturity, whereas the medium and large size-classes consist primarily of mature individuals, but immature individuals are present in both size-classes. The presence of immatures in the medium and large size-classes indicates that those size-classes cannot be age-classes, and because those immature specimens seem not to be significantly smaller than mature adults, they are considered to be subadults. The medium and large size-classes are dimorphic in rostral crest size, and, based on comparison to the dimorphic size-classes of Pteranodon, they are tentatively interpreted as sexes, small females with small and low crests, and large males with large and tall crests.
The pelvis of AMNH 22555, a specimen in the medium size-class that has deep pubes and ischia that would have resulted in a relatively large pelvic canal like that seen in female Pteranodon, provides further support for the interpretation of sexual dimorphism. The Brasileodactylus and Araripesaurus specimens in the small size-class seem to be immature and differ from specimens assigned to Anhanguera only in the lack of rostral crests and symphyseal keels, and because they are smaller than mature adults in the medium and large size-classes they are considered to be large juveniles. Presumably if the specimens had grown to maturity they would have developed rostral crests and mandibular keels and their skulls would have resembled those of presumed females assigned to Anhanguera or presumed males assigned to Tropeognathus, Coloborhynchus, and Maaradactylus.
According to the above interpretation, the anhanguerid sample consists of large males with large and tall rostral crests and symphyseal keels, small females with small rostral crests, and immature large juveniles lacking crests. Females are ~60% more abundant than males, males are ~30% larger than females, and large immature juveniles make up ~16% of the sample. The sample is similar to that of Pteranodon from the Smoky Hill Chalk Member in exhibiting sexual dimorphism, although the sex ratio and size dimorphism are not as marked as in Pteranodon. If those differences are real and do not result from collection and publication biases, they might reflect the fact that the Romualdo Member anhanguerids are ~20 my older than Pteranodon. Based on that interpretation, the Romualdo Member would represent a littoral multi-age feeding habitat of large juveniles through adults, and hatchlings and small juveniles were absent because they occupied other habitats.
Patterns and implications of ontogenetic niches
The best evidence for ontogenetic niches in pterosaurs comes from the samples of pterosaurs in which one or more ontogenetic stages are absent or markedly rarer than expected: (1) Pteranodon and Nyctosaurus in which juveniles are absent or virtually so, (2) Rhamphorhynchus in which mature presumably breeding adults are much rarer than expected given the abundance of yearlings, and (3) Aurorazhdarcho in which yearlings and older juveniles are much rarer than expected given the abundance of hatchlings and young-of-the-year. One reviewer suggested that there are many possible explanations other than particular ontogenetic stages of those taxa being somewhere other than the Western Interior Seaway or the Solnhofen lagoons, but they offered no specific explanations, and none that I can devise seems as plausible. In addition, the fact that samples of other pterosaurs are consistent with the interpretations of ontogenetic niches and do not provide evidence of parental care and/or delayed onset of flight until half grown supports the view that pterosaurs exhibited ontogenetic niches.
There seems to be variation in the patterns of ontogenetic niches in different pterosaurs. Ctenochasma and Azhdarcho have the simplest patterns. Ctenochasma’s pattern of abundance is consistent with hatchlings through adults occupying the same habitat and feeding on the same items throughout their life with no change of niche other than that hatchlings presumably fed on individuals of prey species, whereas juveniles and adults sieved to catch multiples. Azhdarcho’s pattern of abundance suggests that juveniles through adults occupied the same riverine habitat, probably feeding on differently sized prey, although hatchlings may have spent some time elsewhere. Other pterosaurs exhibit more complex patterns of ontogenetic niches. Pterodaustro’s pattern of abundance is similar to that of Ctenochasma except that large juveniles through adults probably fed on open lake waters, whereas hatchlings and small juveniles were elsewhere, probably in protected nearshore habitats. Rhamphorhynchus and Pterodactylus hatchlings and small juveniles remained in or around the Solnhofen lagoons that were presumably close to the nesting grounds, and large juveniles through adults presumably were elsewhere except when nesting. Large juveniles through adults of Anhanguera and subadults and adults of Pteranodon and Nyctosaurus were at sea and hatchlings and small juveniles were elsewhere. Note that one reviewer suggested that the recently described Hamipterus (Wang et al., Reference Wang, Kellner, Jiang, Wang, Ma, Paidoula, Cheng, Rodrigues, Meng, Zhang, Ning and Zhonghe2014) and Caiuajara (Manzig et al., Reference Manzig, Kellner, Weinschütz, Fragoso, Vega, Guimarães, Godoy, Liccardo, Ricetti and Moura2014), both of which are represented by samples with broad size ranges including juveniles through adults, should have been included in the survey of pterosaurs. However, the ontogenetic compositions of the samples have not been documented sufficiently for them to be considered here.
The occurrence of ontogenetic niches in most pterosaurs probably had a significant impact on pterosaur diversity. The pattern of ontogenetic niches and food resources partitioned by size in Pteranodon, Nyctosaurus, and Anhanguera would have resulted in greater overall abundance of the species than if they had been limited to continental or coastal environments, but also resulted in a less-diverse pterosaur fauna than if each different ontogenetic niche occupied by Pteranodon, Nyctosaurus, and Anhanguera was occupied by a distinct species. In addition, pterosaur faunas would have been biased toward large species because hatchlings and juveniles of large species such as Pteranodon and Anhanguera would have competed with any small species that lived in the continental or coastal environments. Therefore, the presence of ontogenetic niches would have meant that pterosaur diversity was like that of crocodilians, with relatively few large species and each having broad ecological roles in which they preyed on a wide variety of foods of different sizes while growing to ~10 times their size at hatching, filling what might have been many different niches. Thus, pterosaurs were not like speciose passerines with many similar small species filling many narrow ecological roles.
Andres et al. (Reference Andres, Clark and Xing2010, Reference Andres, Clark and Xing2014) suggested that there was a dichotomy between terrestrial (e.g., Sericipterus and Kryptodrakon) and aquatic pterosaurs. Whereas it is reasonable to accept that a particular species of pterosaur lived in the depositional environment in which its fossils are found, there is often no reason to think that the species was limited to that environment. Ctenochasmatoids with jaws specialized as fish traps and sieves were probably limited to feeding in aquatic environments, and the same may have been true of Rhamphorhynchus with jaws and teeth suited to spearing fishes. But many other pterosaurs had skulls, dentitions, and other features that would not have prevented them from succeeding in terrestrial environments, particularly as juveniles with usually somewhat more generalized morphologies (e.g., Dimorphodon, Scaphognathus, Anhanguera, Pteranodon). Therefore, it is not unreasonable to think that some pterosaurs may have exhibited ontogenetic niches such that they lived in terrestrial and aquatic environments at different times in their lives. There is nothing in the morphology of Sericipterus and Kryptodrakon (Andres et al., Reference Andres, Clark and Xing2010, Reference Andres, Clark and Xing2014) to distinguish them from pterosaurs found in aquatic environments; indeed, the dentition of Sericipterus has long slender teeth like those of its relatives Scaphognathus and Rhamphorhynchus found in aquatic environments. Thus, there is insufficient evidence to conclude that there was a dichotomy between terrestrial and aquatic pterosaurs.
Acknowledgments
I thank G. Rockers for donating the juvenile specimen to the FHSM, and A. Averianov and L. Codorniú for providing me with information about the ontogenetic compositions of collections of Azhdarcho and Pterodaustro, respectively. A.T. Karlin assisted with thin sectioning. Constructive reviews from M. Witton and L. Codorniú led to improvements in the manuscript, and an anonymous reviewer disagreed with everything. A platform presentation by D. Fowler (Fowler et al., Reference Fowler, Freedman Fowler, Scannella and Horner2013) brought the idea of ontogenetic niches to my attention and led to the immediate realization that most pterosaurs exhibit them.