Introduction
The John Day Formation of Oregon represents nearly ten million years (late Whitneyan to early Hemingfordian North American Land Mammal Ages [NALMA]: middle Oligocene to early Miocene: Rupelian to early Burdigalian) and has produced a mammalian fauna that is very diverse, including over 100 species (Hunt and Stepleton, Reference Hunt and Stepleton2004; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb and Whistler2004; Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008; Fremd, Reference Fremd2010; Korth and Samuels, Reference Korth and Samuels2015). However, only a single specimen of a marsupial, which was referred to a new species, Peratherium merriami by Stock and Furlong (Reference Stock and Furlong1922), has ever been described from it. At the time of its description, this was the latest-occurring Tertiary marsupial in North America. It differed from other described species mainly in its larger size. In the most recent survey of the middle Tertiary marsupials of North America (Korth, Reference Korth1994), this species was transferred to the genus Herpetotherium Cope, Reference Cope1873, but the holotype remained the only record of this species (Korth, Reference Korth2008). Recently discovered specimens referable to H. merriami from the John Day Formation has allowed for a better analysis of the species and a more complete comparison with other described species of the genus.
Herpetotherium has traditionally been considered in the order Marsupialia within the family Didelphidae, and generally in the subfamily Herpetotheriinae (see citations in Korth, Reference Korth2008, p. 39). However, in a recent systematic analysis based on cranial morphology, Herpetotherium was included in the Metatheria but viewed as a sister-group to the Marsupialia, and raised to the rank of family (Horovitz et al., Reference Horovitz, Martin, Bloch, Ladevèze, Kurz and Sánchez-Villagra2009, fig. 5).
Materials and methods
Dental terminology follows that of Crochet (Reference Crochet1980) with modification for stylar cusps (Fig. 1); upper teeth are indicated by capital letters, lower teeth by lower-case letters (e.g. M1 or m1). Measurements were taken to the nearest 0.01 mm on an optical micrometer using the orientation presented in Korth (Reference Korth1994, fig. 1). Terminology for cranial anatomy follows that of Wible (Reference Wible2003). Reference to the stratigraphy of the John Day Formation and its recognized horizons are taken from Albright et al. (Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008). Horizons within the John Day Formation are subdivided as Ar1 (early Arikareean) to Ar3 (early late Arikareean) following the terminology of Janis et al. (Reference Janis, Gunnell and Uhen2008). Radiometric dates for each of these horizons in the John Day Formation are presented elsewhere (Fremd et al., Reference Fremd, Bestland and Retallack1994; Hunt and Stepelton, Reference Hunt and Stepleton2004; Albright, et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008; Korth and Samuels, Reference Korth and Samuels2015).

Figure 1 Schematic diagram of upper left molar with stylar cusps labeled.
Repositories and institutional abbreviations
JODA, John Day Fossil Beds National Monument; UCMP, University of California Museum of Paleontology.
Systematic paleontology
Order Metatheria Huxley, Reference Huxley1880
Faimly Herpetotheriidae Trouessart, Reference Trouessart1879
Genus Herpetotherium Cope, Reference Cope1873
Type species
Herpetotherium fugax Cope, Reference Cope1873.
Referred species
Herpetotherium valens (Lambe, Reference Lambe1908); H. merriami (Stock and Furlong, Reference Stock and Furlong1922); H. marsupium (Troxell, Reference Troxell1923); H. youngi (McGrew, Reference McGrew1937); H. knighti (McGrew, Reference McGrew1959).
Occurrence
Middle Eocene (Uintan: Lutetian) to middle Miocene (Barstovian: Serravallian) of North America (39–18 Ma: Hunt and Stapleton, Reference Hunt and Stepleton2004; Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008).
Herpetotherium merriami (Stock and Furlong, Reference Stock and Furlong1922)
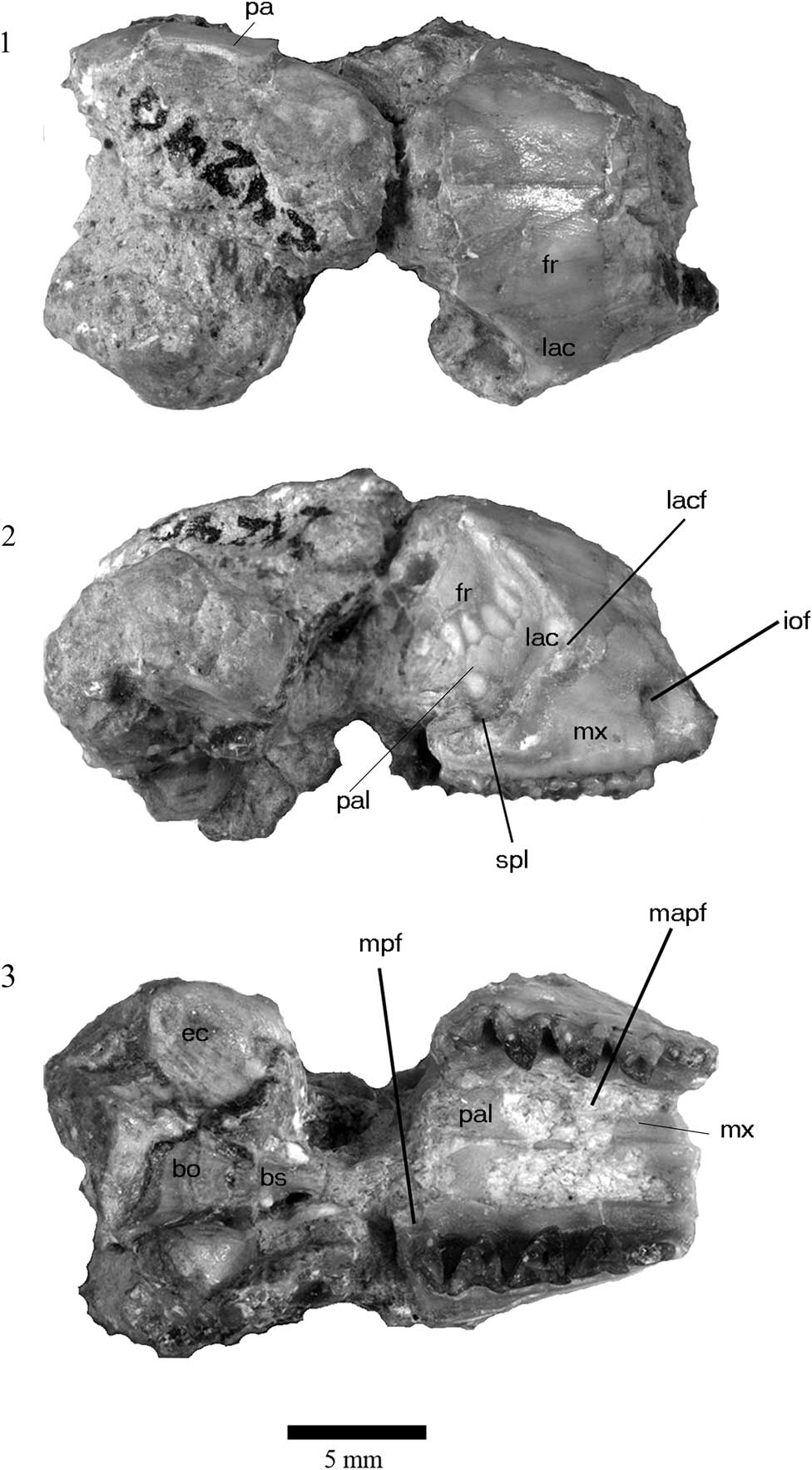
Figure 2 Cranium of holotype of Herpetotherium merriami, UCMP 24240, from the John Day Formation, Oregon. (1), dorsal view; (2) right lateral view; (3) ventral view (anterior to right). Abbreviations: fr, frontal bone; iof, infraorbital foramen; lac, lacrimal bone; lacf, lacrimal foramen; mapf, major palatine foramen; mpf, minor palatine foramen; mx, maxilla; pa, parietal; pal, palatine bone; spl, sphenopalatine foramen.
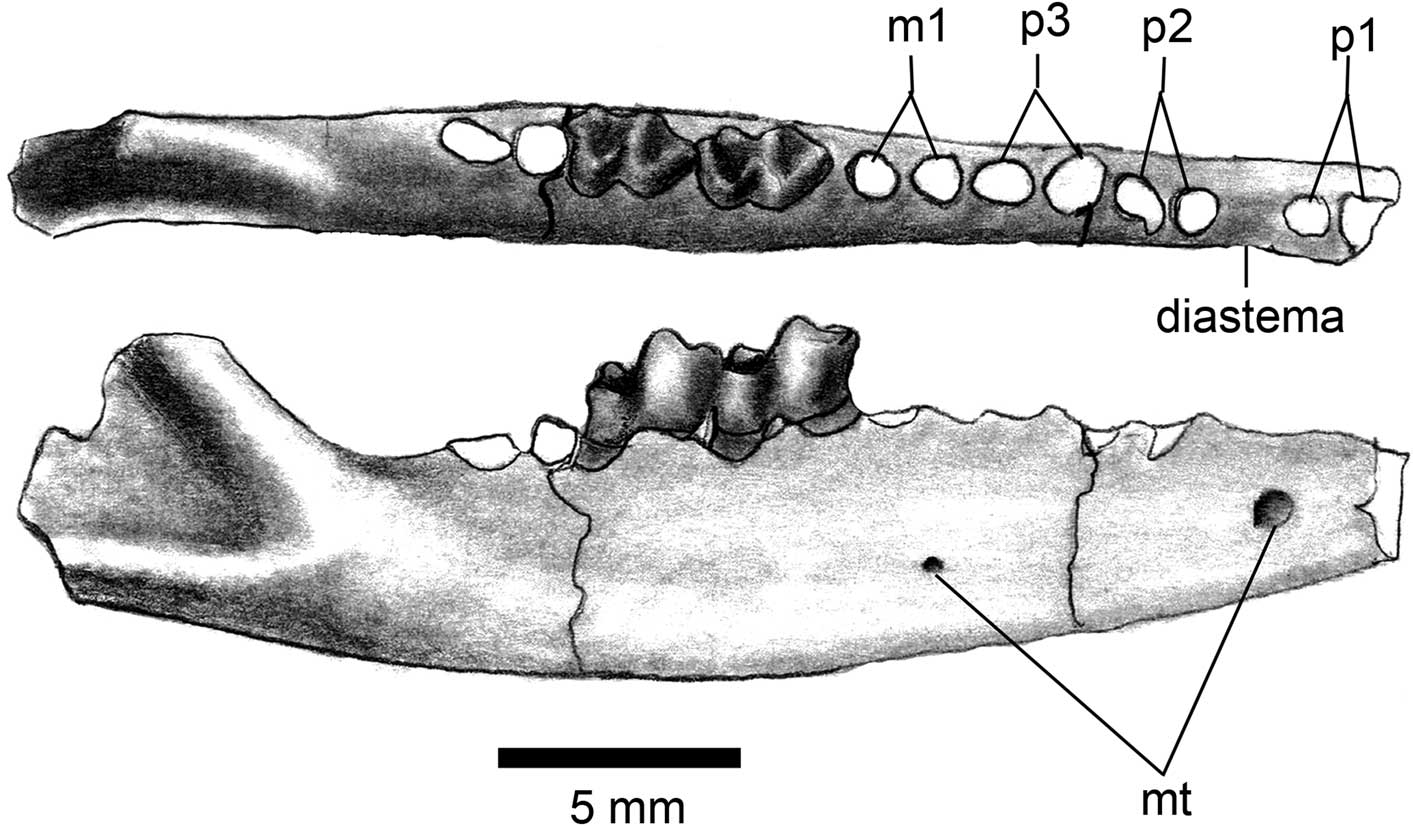
Figure 3 Dentary of Herpetotherium merriami, JODA 10348, right with m2-m3; anterior to right; occlusal view (upper); lateral view (lower); abbreviaton: mt, mental foramen.
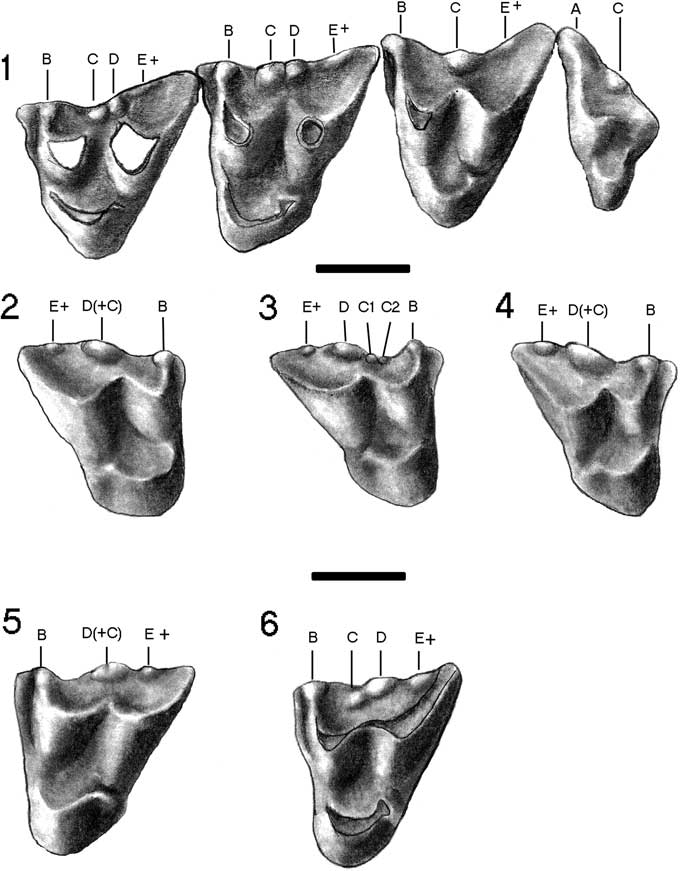
Figure 4 Upper dentition of Herpetotherium merriami from the John Day Formation, Oregon. (1) UCMP 24240 (holotype), left M1-M4. (2) JODA 12859, right M1. (3) JODA 12858, right M1. (4) JODA 12860, right M2. (5) JODA 12851, left M2. (6) 12852, left M2. Bar scales=1 mm.
1922 Peratherium merriami Reference Stock and FurlongStock and Furlong, p. 312, figs. 1 to 5.
1994 Herpetotherium merriami (Stock and Furlong); Korth, p. 381, table 5.
Holotype
UCMP 24240, rostrum and palate of skull (Fig. 2) with left P3-M4 and right M1-M4 and associated partial dentaries with left p3-m4 (in part) and right m1-m3.
Emended diagnosis
Large species, larger than all other species except H. valens and H. marsupium; stylar cusps C and D on M1 and M2 large and fused at their bases either with distinct apices or fused into a single elongated cusp; stylar cusp E always absent; an accessory stylar cusp is present between cusp D and posterobuccal corner of the tooth (designated as cusp E+); stylar cusps B and E+ are slightly smaller than C or D on M1-M3; large, central stylar cusp on M3-M4 (cusp C); short diastema separating p1 and p2 on dentary.
Type locality and horizon
Logan Butte, Lower Turtle Cove Member, John Day Formation, Crook County, Oregon (Arikareean [Ar1]: late Oligocene; see Korth and Samuels, Reference Korth and Samuels2015, fig. 26).
Additional referred specimens
JODA 16801, partial maxilla with M1-M3; JODA 12858, 12859, M1; JODA 12848, 12853, 12860, M2; JODA 12846, 12847, 12849–12852, 12854–12857, M3; JODA 12845, M4; JODA 10348, dentary with m2-m3; JODA 12862, dentary fragment with p3; JODA 12861, dentary fragment with m1.
Occurrence
JODA 12845–12862 from Lone Rock, Unit L, Kimberly Member (Ar3); JODA 10348 from Haystack Valley Member (Ar3); JODA 16801 from Unit K1, Turtle Cove Member (Ar2); all from John Day Formation, Oregon. (Arikareean: late Oligocene: late Rupelain to Aquitanian).
Description
The partial cranium (UCMP 24240) is badly damaged except for the majority of the palate. Dorsally, the frontal extends from the postorbital constriction to approximately 2.5 mm anterior of the zygomatic arch (Fig. 2.1). There are no recognizable sutures with the nasals on the specimen. There is no indication of parasagittal crests on the frontal. The lacrimal forms the anterior edge of the orbit and extends anteriorly onto the rostrum. The only other bones visible in dorsal view are a small fragment of the parietal on the left side of the skull and a posterodorsal part of the maxillaries on both sides.
In lateral view (Fig. 2.2), the infraorbital foramen on the maxilla opens anteriorly and is dorsal to the anterior root of M1. The lacrimal bone extends dorsally and anteriorly onto the rosturm. There is a single minute lacrimal foramen on the lacrimal bone, just posterior to the anterior ridge of the orbital margin. There is no evidence of a second foramen on the fossil skull, but it cannot be verified due to damage to the specimen. The lacrimal does not extend posteriorly onto the orbital wall. Similarly, the palatine bone extends slightly dorsally. The anterior margin of the orbit is above the posterior margin of M2. The sphenopalatine foramen is dorsal to the anterior margin of M4 in the palatine bone. Due to damage, no other observations can be made on the lateral side of the cranium other than the relative size of the neurocranium, which is slightly larger than the anterior portion of the skull.
Ventrally, two foramina are identifiable on the palatal surface (Fig. 2.3). Anteriorly, there is a large major palatine foramen that is oval in outline and extends from the anterior margin of P3 to the anterior margin of M3 (Table 1). The minor palatine foramen is present along the posterior margin of the palate lingual to M4.
Table 1 Cranial and dentary measurements of Herpetotherium merriami. Measurements in mm; *, indicates estimated measurement. Abbreviation: mapf, major palatine foramen.

The remainder of the skull is badly damaged. The narrow basisphenoid is along the center line of the cranium and its suture with the basioccipital is recognizable. The ventral part of the bulla is present on the right side, and only the anterior-most part is preserved on the right left side.
Both dentaries are preserved in the holotype, but the anterior portion is broken away from both rami (Stock and Furlong, Reference Stock and Furlong1922, fig. 3). One specimen (JODA 10348; Fig. 3) preserves the alveoli for all of the premolars. It is evident from these that all three premolars are two-rooted, similar in size, and that there is a small diastema between p1 and p2 (Fig. 3). There is a larger mental foramen on the dentary below the diastema between p1 and p2 and a second smaller foramen below m1.
The dentition of Herpetotherium has been described elsewhere in detail (Stock and Furlong, Reference Stock and Furlong1922; Green and Martin, Reference Green and Martin1976; Korth, Reference Korth1994; Eberle and Storer, Reference Eberle and Storer1995). Only the features distinctive to H. merriami will be described herein. On the upper molars there is a smaller cusp posterior to D, but anterior to the position of cusp E (posterobuccal corner of the tooth) that is designated here as cusp E+ that is also unique to H. merriami. The distinguishing features of the stylar cusps of H. merriami are mainly on M1 and M2 (Fig. 4). Stylar cusp D is always the largest cusp and positioned posterior to the center of the buccal border. Cusps B and E+ are slightly smaller; B anterior to the center of the buccal margin of the tooth and E+ posterior to cusp D, but not at the posterior edge of the tooth; cusp E is always lacking. Cusp C varies in occurrence. It is either nearly equal to cusp D and fused with it at the base (Fig. 4.1), or completely fused into a large elongated cusp (Fig. 4.2, 4.4). On a single specimen, JODA 12858, stylar cusp C is replaced by two minute cusps anterior to cups D (Fig. 4.3). Korth (Reference Korth1994) erroneously stated that cusp C on M1 of the holotype was much smaller than cusp D and on its anterior slope, similar to the condition in other species of the genus. However, on both M1 and M2 of the holotype (Fig. 4.1; Stock and Furlong, Reference Stock and Furlong1922, fig. 4), these cusps are subequal in size and do not totally fuse into a single cusp as in M3.
The only distinctive character of the lower dentition is the size of p1 (based on alveolus); nearly equal p2 (Fig. 3).
Remarks
Although the size range of the cheek teeth of Herpetotherium merriami overlaps with those of H. fugax (smaller measurements) and H. valens (larger measurements), the means of nearly all dental measurements are in between those of these other species (Table 2; Figs. 5, 6). It is markedly smaller than the Duchesnean to early Chadronian H. marsupium (Krishtalka and Stucky, Reference Krishtalka and Stucky1983; Rothecker and Storer, 1996; Kihm and Schumaker, Reference Kihm and Schumaker2015). Morphologically, H. merriami is distinguishable in the size and arrangement of the stylar cusps on the upper molars. In overall size, the stylar cusps of H. merriami are comparatively much larger than in any other species (see comparative figures in Green and Martin, Reference Green and Martin1976; Korth, Reference Korth1994; Eberle and Storer, Reference Eberle and Storer1995; Hayes, Reference Hayes2005; Kihm and Schumaker, Reference Kihm and Schumaker2015). On M1 and M2 of H. merriami stylar cusp D is not central and is the largest cusp; cusp C is of nearly equal size and twinned with D (Fig. 4.1) or fused together into a large continuous cusp (Fig. 4.2). The presence of an accessory stylar cusp, E+, is not known in any other species of the genus. Traditionally, stylar cusp E is in the posterobuccal corner of the tooth (= metastylid of eutherian mammals), whereas cusp E+ is midway between the traditional stylar cusps D and the posterobuccal corner of the tooth (Fig. 1; Crochet, Reference Crochet1980, fig. 2). Stylar cusps B and E+ are always present, but slightly smaller than C and D. In both H. fugax and H. valens, the arrangement of stylar cusps is variable and the cusps often vary in size. On M1 of these species cusp C, if present, is always small and on the anterior slope of cusp D, whereas in H. merriami cusps C and D nearly equal in size as distinct cusps (=twinned or double cusps of Hayes, Reference Hayes2005), or fused into a single elongated cusp (=blade of Hayes, Reference Hayes2005). This type of arrangement is present but rare in the other species (Eberle and Storer, Reference Eberle and Storer1995; Hayes, Reference Hayes2005, table 2) and always present on specimens of H. merriami. On M3 of H. merriami there is a main, large central cusp (cusp C) and slightly smaller cusps B and E+ (Fig. 4.1). On M3 of H. valens, cusp C is always doubled and the cusps are of different sizes (Eberle and Storer, Reference Eberle and Storer1995, fig. 3D–G) and in H. fugax the central cusp is frequently double or fused into an elongated cusp (Hayes, Reference Hayes2005, table 2). The Arikareean H. youngi differs from H. merriami in its much smaller size (Figs. 5, 6) and in the reduction in the number of stylar cusps (often only cusp C present on M1 or M2; Korth, Reference Korth1992, Reference Korth1994).

Figure 5 Comparison of lengths of upper molars of species of Herpetotherium from the late Paleogene of North America. Vertical axis does not represent actual time span of each provincial age. Numbers to the right of range bars are the number of specimens measured. Data for H. marsupium from Kihm and Schumaker (Reference Kihm and Schumaker2015); for H. valens from Eberle and Storer (Reference Eberle and Storer1995) and Kihm and Schumaker (Reference Kihm and Schumaker2015); for H. fugax from Setoguchi (Reference Setoguchi1978), Korth (Reference Korth1994, Reference Korth2015) and Hayes (Reference Hayes2005); for H. youngi from Korth (Reference Korth1992).

Figure 6 Comparison of lengths of lower molars of species of Herpetotherium from the late Paleogene of North America. Vertical axis does not represent actual time span of each provincial age. Numbers to the right of range bars is the number of specimens measured. Data for H. marsupium from Kihm and Schumaker (Reference Kihm and Schumaker2015); for H. valens from Eberle and Storer (Reference Eberle and Storer1995) and Kihm and Schumaker (Reference Kihm and Schumaker2015); for H. fugax from Setoguchi (Reference Setoguchi1978), Korth (Reference Korth1994, Reference Korth2015) and Hayes (Reference Hayes2005); for H. youngi from Korth (Reference Korth1992).
Table 2 Dental measurements of Herpetotherium merriami. Measurements in mm. Abbreviations: L, anteroposterior length; W, transverse width; N, number of specimens; M, mean; OR, range of measurements; SD, standard deviation; CV, coefficient of variation.

Hayes (Reference Hayes2005) noted that in M1 and M2 of larger specimens of H. fugax, cusps C and D were more likely separate, and on smaller specimens, they were either completely or partially fused. On H. merriami, the larger specimens have two distinct central cusps, but on smaller individuals these are fused. However, when this fusion occurs in H. merriami the result is a very elongated stylar cusp that is similar in length to the two individual stylar cusps, not a single smaller cusp C as in H. fugax or H. youngi.
The specimens from the lower horizons of the John Day Formation (Ar1–2), the holotype and JODA 16801, are the largest and have subequal stylar cusps C and D on M1 and M2. The remaining specimens (from higher in the section; Ar3) have the elongated and completely fused stylar cusps and are smaller in overall size of the tooth (Table 1). This follows Hayes’ (Reference Hayes2005) observation of the smaller individuals having fewer stylar cusps, and follows the trend in H. fugax that there is a slight reduction in size through time (Korth, Reference Korth1994, fig. 2; Hayes, Reference Hayes2005, fig. 5).
In the dentary of H. fugax, p1 is markedly smaller than p2 and p3, but in H. merriami p1 is nearly equal to the length of p2 (Fig. 3; Fox, Reference Fox1983, fig. 1b; Korth, Reference Korth1994, fig. 8.1). Both species have a small diastema between p1 and p2.
The skull of H. merriami differs from that of Recent didelphids such as Monodelphis (Fig. 2; also see Wible, Reference Wible2003). In Monodelphis, the lacrimal foramen is doubled and only single in H. merriami. The infraorbital foramen on H. merriami is dorsal to the anterior root of M1, just slightly more posterior than in Monodelphis where it is dorsal to the posterior root of P3 (Wible, Reference Wible2003, fig. 2), and the lacrimal bone extends more dorsally and anteriorly in H. merriami than in Monodelphis. The lacrimal in H. merriami does not extend posteriorly onto the orbital wall as in Monodelphis (Wible, Reference Wible2003, fig. 4). Similarly, the palatine bone does not extend as far dorsally as in Monodelphis. Stock and Furlong (Reference Stock and Furlong1922) noted that the anterior margin of the orbit was above the posterior margin of M2 in H. merriami and H. fugax, and that of the Recent Marmosa was above the anterior margin of M1. In Monodelphis, the anterior margin of the orbit is dorsal to the anterior edge of M2 (Wible, Reference Wible2003, fig. 2). In both instances, the position of the anterior margin of the orbit is more posterior in Herpetotherium than in Recent genera. The sphenopalatine foramen is dorsal to the anterior margin of M4 in the palatine bone in H. merriami, slightly more posterior than in Monodelphis where it is dorsal to M3. The major palatine foramen in H. merriami extends from the anterior margin of P3 to the anterior margin of M3; longer and more anterior than in Monodelphis where it extends from the anterior margin of M1 to the posterior margin of M2, and the minor palatine foramen is slightly more anterior than in Monodelphis (Wible, Reference Wible2003, fig. 5). In all of these features that differ from Monodelphis, it appears that the cranium of H. merriami has a slightly more elongated and less transverse rostrum.
Hayes (Reference Hayes2005, fig. 3A, B) referred two isolated M2s from the Arikareean of Florida to “Herpetotherium cf. merriami”. These specimens are similar in size to those of H. merriami from Oregon, but stylar cusp C is markedly smaller than cusp D and on its anterior slope, a characteristic of H. fugax. Due to the variability of these cusps and the small sample from Florida, these specimens cannot be definitely referred to H. merriami.
Discussion
Herpetotherium merriami, known through most of the Arikareean (latest Oligocene: Ar1–Ar3), is one of the last occurring marsupials in North America before the immigration of Recent didelphids from South America in the Pleistocene (McKenna and Bell, Reference McKenna and Bell1997; Korth, Reference Korth2008). The Miocene record of marsupials in North America is limited to several specimens of an indeterminate species of Herpetotherium from the Hemingfordian of South Dakota (Green and Martin, Reference Green and Martin1976) and a single isolated tooth from the Barstovian of Texas (Slaughter, Reference Slaughter1978). This also follows the trend in the reduction of marsupial species in North America beginning in the Chadronian, when as many as seven species and five genera are present (Kihm and Schumaker, Reference Kihm and Schumaker2015), to the early Arikareean, from which only four species and two genera are recognized (Korth, Reference Korth1994, Reference Korth2008; Hayes, Reference Hayes2005).
Within Herpetotherium, there is a general reduction in size through time, from the larger early Chadronian H. marsupium and H. valens, to the smaller H. fugax in the Orellan through the early Arikareean, to the smaller Miocene specimens (Figs. 5, 6; Korth, Reference Korth1994, fig. 2; Hayes, Reference Hayes2005, figs. 5, 6). However, H. merriami does not follow this pattern and is similar in size to the larger, earlier Chadronian species.
In terms of morphological change through time in Herpetotherium, with the reduction in overall size, there is also a reduction in the number of stylar cusps on the upper molars, especially in populations of H. fugax, which spans the longest time interval (Hayes, Reference Hayes2005), and in the Arikareean H. youngi (Korth, Reference Korth1992, Reference Korth1994). Again, H. merriami is the exception. It has stylar cusps that are not only maintained or increased in number (presence of cusp E+), but also in the size of the stylar cusps relative to the size of the molars. This unique nature of the size and morphology of the cheek teeth of H. merriami, separate from the trends in other species, may be attributed to its relative geologic isolation from the other known species of the genus that are predominantly from the Rocky Mountains, Great Plains, and Florida.
Conclusions
Herpetotherium merriami was previously known only from the holotype. With the addition of more than 20 specimens from the type area described here, its range of variation and consistency of morphology can be better examined. Herpetotherium merriami is distinct from other species of the genus by its large size, the consistent presence of stylar cusp E+ on M1–M3, and the enlargement and fusion of stylar cusps C and D on M1 and M2. Its large size and more complex pattern of stylar cusps on the upper molars is a reversal in the trend through the Tertiary of a reduction in size and simplification (reduction in number) of stylar cusps.
Acknowledgments
Photographs of the holotype of H. merriami were taken and generously provided by P. Holroyd (UCMP). Figures 1, 3, and 4 were by W. Korth. Access to the JODA collections was granted by J. Samuels of that institution. Earlier versions of this manuscript were reviewed by Dr. J. Storer and three anonymous reviewers.










