Introduction
Baltic amber is one of the most well-known and diverse sources of fossil insect deposits for the late Eocene period (33.9 ± 0.1 to 37.2 ± 0.1 Ma) (Rasnitsyn and Quicke, Reference Rasnitsyn and Quicke2002). The original source of resin for this amber deposit probably was the pine forests that covered the Scandinavian territory during the Eocene, most of which was deposited along the eastern part of the Baltic Sea, particularly along its southern coast (Rasnitsyn and Quicke, Reference Rasnitsyn and Quicke2002). The number of insects recovered from Baltic amber is considerably large, with >3,000 fossil insect species described so far, many of which belong to the order Hymenoptera. Rovno amber (Priabonian stage, 33.9–37.8 Ma) from Ukraine is the southern coeval of Baltic amber (Perkovsky et al., Reference Perkovsky, Rasnitsyn, Vlaskin and Taraschuk2007; Simutnik et al., Reference Simutnik, Perkovsky and Vasilenko2020).
The parasitoid wasps of the family Braconidae found in Baltic amber were primarily studied and described by Brues (Reference Brues1923, Reference Brues1933, Reference Brues1939) almost a century ago. Additional descriptions and taxonomic changes of braconid taxa from Baltic amber were subsequently made by Tobias (Reference Tobias1987). Currently, there are >100 braconid species and 18 extinct braconid genera described from Baltic amber. In Rovno amber, however, there are far fewer records of fossil hymenopterans, which include taxa mainly belonging to the families Encyrtidae, Proctotrupidae, Formicidae, and Chrysididae (Kolyada and Perkovsky, Reference Kolyada and Perkovsky2011; Radchenko and Perkovsky, Reference Radchenko and Perkovsky2018; Martynova et al., Reference Martynova, Perkovsky, Olmi and Vasilenko2019; Simutnik and Perkovsky, Reference Simutnik and Perkovsky2020). To date, there are no descriptions of braconid taxa from this amber deposit, despite their numerous records (E.E. Perkovsky, personal communication, 2020).
Here we describe and illustrate one new braconid genus from Baltic amber belonging to the braconid subfamily Exothecinae, Palaeocolastes new genus, with its type species P. bruesi new species, as well as two additional species from the subfamilies Cheloninae (Ascogaster [Syntaphus] latitibialis new species) and Euphorinae (Meteorus arasnitsyni new species). We also describe a new species from the subfamily Microtypinae (Microtypus eocenus new species) from Rovno amber. In addition, we record another female of Diospilites brevicornis Brues, Reference Brues1933 (Diospilitinae) from Baltic amber and provide details of the variation found in some of the species’ diagnostic features.
Materials and methods
The four Baltic amber specimens discussed in this paper come from a quarry near Yantarny in Russia's Kaliningrad Province. The remaining amber specimen studied here was found in the Rovno region in Ukraine. Each of the specimens was purchased from a seller (www.amberinclusions.eu) specializing in amber. The seller provided the locality information for the specimens, but had no additional information about the Rovno locality.
Some of the digital photographs were taken at the Instituto de Biología, Universidad Nacional Autónoma de México (IB-UNAM), in Mexico City, with a Leica IC 3D digital camera mounted on a Leica MZ16 microscope and using the Leica Application Suite imaging system. Other photographs were taken with a Canon EOS 70D digital camera mounted on an Olympus SZX10 microscope at the Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia. Image stacking was performed using Helicon Focus 5.0. Figures were created with the program Adobe Photoshop CS6.
The terminology employed for morphological features, sculpture, and body measurements follows Belokobylskij and Maetô (Reference Belokobylskij and Maetô2009). Wing venation nomenclature follows Belokobylskij and Maetô (Reference Belokobylskij and Maetô2009), with the terminology of Sharkey and Wharton (Reference Sharkey, Wharton, Wharton, Marsh and Sharkey1997) shown in parentheses.
Repository and institutional abbreviation
All specimens examined in this study are deposited in the Palaeontological Institute of the Russian Academy of Sciences, Moscow, Russia (PIN).
Systematic paleontology
Class Insecta Linnaeus, Reference Linnaeus1758
Order Hymenoptera Linnaeus, Reference Linnaeus1758
Family Braconidae Nees von Esenbeck, Reference Nees von Esenbeck1811
Subfamily Cheloninae Foerster, Reference Foerster1863
Remarks
Members of the subfamily Cheloninae are relatively common in fossil deposits, particularly in Eocene Baltic amber (Brues, Reference Brues1933; Tobias, Reference Tobias1987; Belokobylskij, Reference Belokobylskij2014). Various species of the chelonine genera Ascogaster Wesmael, Reference Wesmael1835, Phanerotoma Wesmael, Reference Wesmael1838, Chelonohelcon Brues, Reference Brues1933, and perhaps Chelonus Panzer, Reference Panzer1806 were described previously from amber deposits or imprints in sedimentary rocks (Belokobylskij, Reference Belokobylskij2014).
Genus Ascogaster Wesmael, Reference Wesmael1835
Type species
Ascogaster instabilis Wesmael, Reference Wesmael1835 (= Chelonus abdominator Dahlbom, Reference Dahlbom1833), by subsequent designation (Foerster, Reference Foerster1863).
Remarks
The less-derived genus Ascogaster is one of the most common genera of fossil chelonines. The following species of Ascogaster have been described from Baltic amber (Brues, Reference Brues1933; Tobias, Reference Tobias1987): A. adentata Tobias, Reference Tobias1987; A. dilatata Brues, Reference Brues1933; A. gracilicornis Brues, Reference Brues1933; A. longicauda Tobias, Reference Tobias1987; A. pentagona Brues, Reference Brues1933; A. pinicola Brues, Reference Brues1933; A. praevolans Brues, Reference Brues1933; A. robusta Brues, Reference Brues1933; A. rutilipes Tobias, Reference Tobias1987; A. submersa Brues, Reference Brues1933; A. sylvestris Brues, Reference Brues1933; and A. thoracica Tobias, Reference Tobias1987. Three Ascogaster species were described from Bembridge Marls imprints (Belokobylskij, Reference Belokobylskij2014): A. brodiei Belokobylskij, Reference Belokobylskij2014; A. pygmaea Belokobylskij, Reference Belokobylskij2014; and A. yulei Belokobylskij, Reference Belokobylskij2014. Additionally, Syntaphus wheeleri Donisthorpe, Reference Donisthorpe1920, originally described as an ant from the same locality, also belongs to Ascogaster, although with a separate subgenus status (Belokobylskij, Reference Belokobylskij2014). Three additional species of Chelonus Panzer, Reference Panzer1806 described from the Florissant Formation in Colorado, USA (Chelonus depressus Brues, Reference Brues1910; C. muratus Brues, Reference Brues1910; and C. solidus Brues, Reference Brues1910) (Brues, Reference Brues1910) perhaps also belong to Ascogaster because they do not show reliable features that help to distinguish the former genus from Ascogaster.
Ascogaster (Syntaphus) latitibialis Belokobylskij and Zaldívar-Riverón, new species
Figure 1
Holotype
Female, Baltic amber, # JDC 8315 (PIN collection No. 964/1327). Eastern coast of the Baltic Sea, late Eocene period (33.9 ± 0.1 to 37.2 ± 0.1 Ma).

Figure 1. Ascogaster latitibialis n. sp., holotype, female. (1) Habitus, left side of amber; (2) habitus, right side of amber. Scale bars = 1 mm.
Diagnosis
This new Ascogaster species distinctly differs from the type species of the subgenus Syntaphus, A. (S.) wheeleri Donisthorpe, Reference Donisthorpe1920, described from Bembridge Marls (latest Eocene or earliest Oligocene; Belokobylskij, Reference Belokobylskij2014), by having: (1) the recurrent vein (1m-cu) of fore wing subinterstitial (versus distinctly postfurcal); (2) strongly widened hind tibia, which is ~3.5 times longer than its maximum width (versus less strongly widened, only 4.6 times longer than its maximum width); (3) mesosoma longer, 1.6 times longer than its maximum height (versus shorter, only 1.3–1.4 times longer than its maximum height); (4) precoxal sulcus (“sternaulus”) distinct (versus absent); (5) propodeum with wide and short lateral corner (versus with rather long lateral processes); and (6) vertex transverse striate in posterior half (versus entirely reticulate-areolate).
Description
Female. Body length 2.9 mm; fore wing length 2.4 mm.
Head
Head height without mandible (lateral view) 1.3 times its maximum length. Vertex in posterior half and upper part of temple in transverse striation. Temple (lateral view) ~0.8 times as long as transverse diameter of eye. Maximum diameter of eye (lateral view) 1.25 times its transverse diameter. Face weakly convex, 1.5 times higher than maximum diameter of eye. Malar space long, ~0.8 times as long as maximum diameter of eye, about equal to basal width of mandible. Clypeus weakly convex. Mandible medium length, weakly twisted. Occipital carina dorsally and laterally distinct. Head mainly rugose-reticulate, vertex in its posterior half with coarse transverse dense carinae prolonged laterally until the middle of its temple.
Antenna
Antenna setiform, weakly thickened before middle, 28-segmented, ~0.7 times as long as body. Scape almost 2.0 times longer than maximum width. First flagellar segment 2.5 times longer than apical width, 1.4 times longer than second segment. Penultimate segment 1.3 times longer than its width, almost as long as acuminate apical segment.
Mesosoma
Mesosoma 1.6 times longer than its maximum height. Precoxal sulcus (“sternaulus”) rather deep, wide, coarsely and sparsely crenulate. Pronotum laterally and mesopleuron entirely distinctly and densely areolate-reticulate. Posterior mesosternal furrow deep, crenulate, widened posteriorly. Posterior mesopleural oblique furrow with dense, wide, and coarse crenulate. Propodeum entirely areolate-reticulate, with distinct subpointed break in basal one-third, after which its posterior part subvertical declivous; without any lateral tooth, but with a wide and short corner. Metapleuron with rather wide areolae (larger than on mesopleuron).
Wings
Fore wing ~3.0 times longer than its maximum width. Radial (marginal) cell strongly shortened, rather wide, 2.8 times longer than its maximum width. Metacarpus (R1a) about as long as pterostigma, 1.4 times longer than distance from apex of radial (marginal) cell to apex of wing. Pterostigma wide, almost 3.0 times longer than maximum width. Radial vein (r) arising from 0.7 of pterostigma (distinctly behind middle). First radial abscissa (r) ~0.5 times as long as maximum width of pterostigma. Second radial abscissa (3RSa) 1.75 times longer than first radial abscissa (r), 0.2 times as long as the weakly curved third radial abscissa (3RSb), ~0.4 times as long as first radiomedial vein (2RS). Second radiomedial (submarginal) cell 1.8 times longer than maximum width. First medial abscissa ((RS+M)a) almost straight. Recurrent vein (m-cu) perhaps subinterstitial, 0.6 times as long as first radiomedial vein (2RS). Hind wing 4.8 times longer than its maximum width.
Legs
Hind tibia strongly widened toward posterior third, ~3.5 times longer than its maximum width, finely and densely punctate, about as long as hind femur, 1.1 times longer than hind tarsus. Longest hind tibial spur 0.6 times as long as maximum width of hind tibia. Hind basitarsus 0.55 times as long as second to fifth segments combined; second segment 0.5 times as long as basitarsus, about as long as fifth segment (without pretarsus).
Metasoma
Carapace (lateral view) with rather distinct, but not deep, first and second complete transverse sutures, almost no bend posteriorly on basal side, weakly widened toward posterior margin, the highest in posterior quarter, 3.5 times longer than its maximum height, about as long as mesosoma, 0.7 times as long as the mesosoma and head combined. First part of carapace (along upper part) 1.2 times longer than second part, 1.5 times longer than third part. Ovipositor short, its sheath about as long as the maximum width of metasomal carapace.
Color
Body black. Palps dark brown. Antenna black. Legs mainly black, hind tibia and tarsus dark brown. Wings distinctly infuscate. Pterostigma entirely black.
Male
Unknown.
Etymology
This species is named after its strongly widened hind tibia.
Remarks
The descriptions of most known fossil species of Ascogaster are not comprehensive and often lack distinct diagnostic characters, in many cases because of the condition or quality of the amber and especially imprints. A complete key for determination of all described fossil species of Ascogaster has not been created, except for a reduced key suggested by Brues (Reference Brues1933). However, our examined specimen has distinctly developed anterior metasomal sutures on its carapace and a very wide hind tibia, which are the main diagnostic characters of the subgenus Syntaphus Donisthorpe. We therefore consider this specimen as the second species of the former monotypic subgenus Ascogaster (Syntaphus).
Subfamily Diospilitinae Tobias, Reference Tobias1987
Diagnosis
Hypoclypeal depression perhaps not developed. Occipital carina present, but fine, fused below with hypostomal carina. Ocelli small. Palps short, maxillary palp perhaps 5-segmented, labial palp with two or three segments. Antenna distinctly thickened and 11-segmented. First flagellar segment elongated, slender basally, and distinctly widened towards apex; second to fifth segments inverted subconical and gradually shortened; flagellar segments almost entirely with distinct and dense elongate sensillae. Mesosoma short and high, 1.5–1.6 times longer than high. Notauli, precoxal sulcus, and prepectal carina of mesosoma absent. In fore wing, pterostigma wide and subtriangular. Radial (r) vein originating almost from middle of pterostigma. Radial (marginal) cell not shortened. First abscissa of medial ((RS+M)a) and second radiomedial (r-m) veins present; first and second transverse anal veins (1a and 2a) absent. Recurrent vein (1m-cu) strongly postfurcal to first radiomedial vein (2SR). Brachial (first subdiscal) cell closed distally by brachial (2cu-a) vein, which is shorter than vein 2CUa. In hind wing, recurrent vein (m-cu) absent. First submedial (subbasal) cell large; first abscissa of mediocubital vein (M+CU) much longer than second abscissa (1M). Legs slender; all femora narrow. Trochanters and trochantelli present and distinctly separated. Hind tibia weakly thickened distally; tibial spurs short. Claw simple, but distinctly thickened basally. Metasoma sessile, without delineated laterotergites on second and following tergites. First metasomal tergite relatively short. Suture between second and third tergites present but narrow. Sternites of metasoma strongly sclerotized. Hypopygium strongly developed and distinctly protruding posteriorly. Ovipositor short, apically slender, and simple (unarmed). Visible ovipositor sheath very short, subtriangular shape.
Remarks
This monotypic, extinct subfamily was erected by Tobias (Reference Tobias1987) for the genus Diospilites Brues, Reference Brues1933, which was originally described by Brues (Reference Brues1933) from Baltic amber.
Genus Diospilites Brues, Reference Brues1933
Type species
Diospilites brevicornis Brues, Reference Brues1933, by monotypy and original designation (Brues, Reference Brues1933, p. 98; Tobias, Reference Tobias1987, p. 845 [type genus of subfamily]; Yu et al., Reference Yu, van Achterberg and Horstmann2016 [as members of Aphidiinae: Ephedrini]).
Diagnosis
Besides the above diagnostic features mentioned for the subfamily Diospilitinae, Diospilites can be distinguished by having the following features: body robust, not depressed dorso-ventrally. Head transverse and rather short. Eyes large and bare; temple slightly shorter than eye. Antennae not longer than head and mesosoma combined. First flagellar segments ~1.2 times longer than second segment. Apical segment obtuse distally and without “spine.” Neck of prothorax short. Mesoscutum highly and curvedly elevated above pronotum, almost entirely covered by dense and rather short setae. Scutellum and propodeum slightly convex. Fore wing almost 2.0 times longer than its maximal width. Metacarp (R1a) 1.1 times longer than pterostigma. Second radial abscissae (3RSa) about as long as first radiomedial vein (r-m). Recurrent vein (1m-cu) short, distinctly convergent posteriorly with basal vein (1M). Discoidal (discal) cell petiolate, petiole (1RS) short. Nervulus (cu-a) distinctly postfurcal and subperpendicular. Brachial (subdiscal) cell rather short and narrow. Longitudinal anal vein (1-1A) slightly curved. In hind wing, radial vein (RS) perhaps completely absent. First abscissa of mediocubital vein (M+CU) almost 3.0 times longer than second abscissa (1M). Coxa of hind leg relatively large. All tarsi with elongated segments. Metasoma almost entirely smooth (invisibly on first tergite). Second tergite slightly longer than third one.
Remarks
This monotypic fossil genus was described from Baltic amber by Brues (Reference Brues1933). A redescription of this genus and its type species for both sexes was provided by Tobias (Reference Tobias1987).
Diospilites brevicornis Brues, Reference Brues1933
Figure 2
Holotype
Male, Baltic amber, “No. 9020, IV, No, 263” (lost).

Figure 2. Diospilites brevicornis Brues, Reference Brues1933, female. Habitus, lateral view. Scale bar = 0.5 mm.
Material
One female, Baltic amber, # 5584 (PIN collection No. 964/1328). Specimen found on the eastern coast of the Baltic Sea, late Eocene period (33.9 ± 0.1 to 37.2 ± 0.1 Ma).
Variation of female
Body length of female 2.8–3.1 mm, of fore wing 2.0 mm; in male, length of body 2.0 mm. Antenna 0.8 times as long as head and mesosoma combined. Only flagellar segments fifth to sixth narrow basally and distinctly straight or weakly widely curved toward middle of apex. Penultimate segment as long as ninth and eleven (apical). Mesosoma 1.4–1.5 times longer than maximum height. Propodeum perhaps with delineated areas. Fore wing equal to or weakly shorter than mesosoma and metasoma combined. Radial (marginal cell) not or weakly shortened. Third abscissa of radial vein (3RSb) 2.0–2.5 times longer than second abscissa (3RSa). Recurrent vein (1m-cu) almost 2.0 times longer than second abscissa of medial vein ((RS+M)b). Hind femur 1.4 times longer than fore femur; its length 4.0–4.8 times the maximum width in posterior half. Hind basitarsus 2.0–2.3 times longer than fifth segment (without pretarsus). Second and following metasomal tergites smooth.
Remarks
The examined female of D. brevicornis undoubtedly belongs to the only described species of this genus. The female of this species was originally described by Brues (Reference Brues1933), and its male was recorded later by Tobias (Reference Tobias1987), also from Baltic amber.
Subfamily Euphorinae Foerster, Reference Foerster1863
Remarks
The subfamily Euphorinae is a diverse group of koinobiont braconid wasps with a wide variety of hosts and stages of infestation (Tobias, Reference Tobias1965, Reference Tobias1966; Shaw, Reference Shaw1985). Members of this group are relatively common and almost cosmopolitan. Fossil species of the tribe Meteorini have been mainly recorded from the Eocene in Baltic amber and the Bembridge Marls imprints of the Isle of Wight (United Kingdom) (Brues, Reference Brues1933; Belokobylskij, Reference Belokobylskij2014).
Genus Meteorus Haliday, Reference Haliday1835
Type species
Ichneumon pendulator Latreille, Reference Latreille1799 (= Ichneumon pendulus Müller, Reference Müller1776), by subsequent designation (Haliday, Reference Haliday and Westwood1840). Ichneumon Linnaeus, Reference Linnaeus1758 is the type genus of the family Ichneumonidae, in which most braconid species were described during the 18th century.
Remarks
Four species of the genus Meteorus Haliday, Reference Haliday1835—M. brevis Brues, Reference Brues1933; M. crassicornis Brues, Reference Brues1933; M. elongatus Brues, Reference Brues1933; and M. interstitialis Brues, Reference Brues1933—were described from Eocene Baltic amber, whereas “Meteorus” longicornis Statz, Reference Statz1938 was described from an imprint in the Rott deposit in Germany (near the Oligocene/Miocene boundary). The taxonomic position of the species described from Baltic amber is clear, although the status of M. longicornis needs confirmation. Two additional species of this genus, M. applanatus Belokobylskij, Reference Belokobylskij2014 and M. crassitergum Belokobylskij, Reference Belokobylskij2014, were recently described from the Bembridge Marls imprints (Belokobylskij, Reference Belokobylskij2014).
Meteorus arasnitsyni Belokobylskij and Zaldívar-Riverón, new species
Figure 3
Holotype
Female, Baltic amber, # 4641 (PIN collection No. 964/1329). Specimen found on the eastern coast of the Baltic Sea, late Eocene period (33.9 ± 0.1 to 37.2 ± 0.1 Ma).
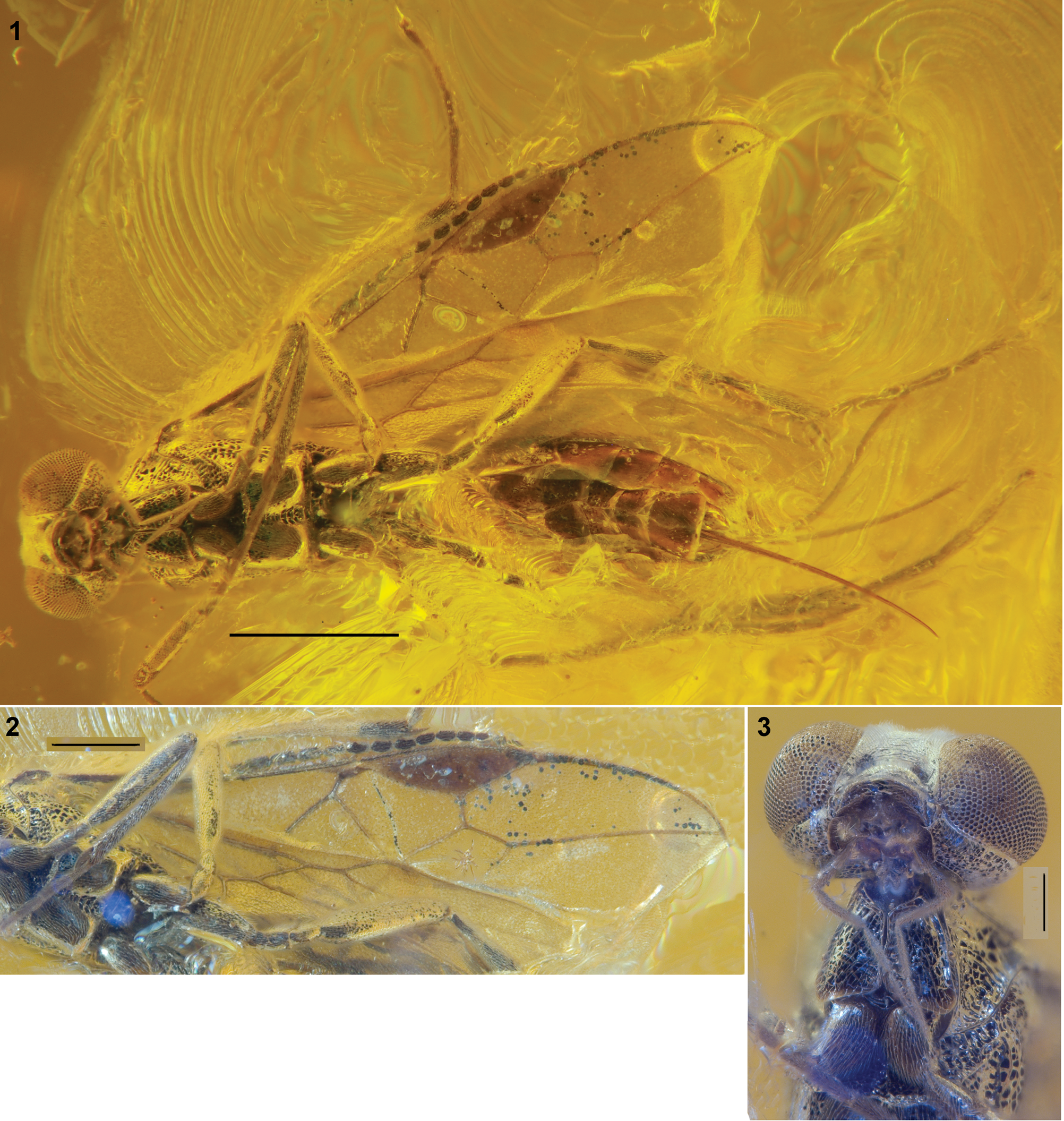
Figure 3. Meteorus arasnitsyni n. sp., holotype, female. (1) Habitus, ventral view; scale bar = 1 mm; (2) wings; scale bar = 0.5 mm; (3) head and anterior half of mesosoma, ventral view; scale bar = 0.2 mm.
Diagnosis
This new species is very similar to the fossil species M. applanatus Belokobylskij, though it differs from the latter by the face having very dense erect setae (versus such setae indistinct) laterally, the anterior abscissa of basal vein (1RS) long (versus short), and the second radial abscissa (3RSa) longer than the first abscissa (r) (versus almost equal in length to it). Comparison of this taxon with recent Meteorus species is considerably complicated by the lack of visibility for most of the important diagnostic features that have been proposed for the genus.
Description
Female. Body length 4.2 mm; fore wing length 4.0 mm.
Head
Width (dorsal view) 1.8 times its median length, 1.2 times wider than mesosoma. Temple behind eye (dorsal view) strongly and weakly roundly narrowed. Transverse diameter of eye (dorsal view) ~2.5 times length of temple. Face narrow, weakly convex, densely and almost entirely transverse striate with rugosity, with very dense, short, and erect white setae, 0.55 times as broad as transverse diameter of eye. Malar space very short. Clypeus distinctly convex, with dense and erect setae, its width 2.8 times larger than maximum height, almost equal to minimum width of face. Tentorial pits distinct and deep, distance between pits 3.5 times distance from pit to eye. Mandible rather small, not twisted. Occipital carina fused with hypostomal carina slightly higher than base of mandible.
Antenna
Antenna perhaps filiform, ~0.8 times as long as body. Penultimate segment ~2.5 times longer than its width, 1.3 times longer than acuminate apical segment.
Mesosoma
Mesosoma 1.8 times longer than its maximum breadth. Propleuron and side of pronotum distinctly and rather densely punctate with rugosity. Mesopleuron distinctly and rather sparsely punctate. Mesosternal furrow deep, crenulate, widened posteriorly.
Wings
Fore wing ~3.0 times longer than its maximum width. Radial (marginal) cell not shortened, 3.2 times longer than maximum width. Metacarpus (R1a) 1.2 times longer than pterostigma. Radial vein (r) arising from middle of pterostigma. First radial abscissa (r) ~0.7 times as long as maximum width of pterostigma. Second radial abscissa (3RSa) 1.5 times longer than first radial abscissa (r), 0.2 times as long as the straight third radial abscissa (3RSb), 0.65 times as long as first radiomedial vein (2RS). First medial abscissa ((RS+M)a) straight. Recurrent vein (1m-cu) antefurcal, 0.8 times as long as first radiomedial vein (2RS), ~6.0 times longer than second medial abscissa ((RS+M)b), subparallel to basal vein (1M). Discoidal (first discal) cell ~1.5 times longer than its maximum width. Nervulus (1cu-a) distinctly postfurcal. In the hind wing, basal vein (1r-m) 1.4 times longer than second abscissa of mediocubital vein (1M), 1.1 times longer than third abscissa of costal vein (R). Radial (marginal) cell weakly narrowed distally.
Legs
Fore femur 6.0 times longer than its maximum width. Fore tibia 1.2 times longer than fore femur, about as long as fore tarsus. Hind tibia thickened, 1.7 times longer than hind femur, almost as long as hind tarsus. Hind basitarsus 0.6 times as long as second to fifth segments combined. Second segment of hind tibia 0.5 times as long as hind basitarsus, 1.3 times longer than third segment.
Metasoma
Metasoma 1.2 times longer than head and mesosoma combined. First tergite invisible, acrosternite relatively short and perhaps basally not fused with tergite lateral borders. Hypopygium straight along posterior margin, without emargination. Ovipositor almost straight; its sheath 0.65 times as long as metasoma, 1.1 times longer than hind tibia, 1.2 times longer than mesosoma, 0.4 times as long as fore wing.
Color
Body mainly black. Palps brown. Antenna black apically. Legs entirely black. Wings hyaline, very faintly infuscate. Pterostigma entirely brown.
Male
Unknown.
Etymology
This new species is named in honor of Professor Alexandr Pavlovich Rasnitsyn, the prominent Russian hymenopterist and paleoentomologist, dedicated in celebration of his upcoming 85th birthday this year.
Remarks
Baltic and Rott fossil species of Meteorus (Brues, Reference Brues1933; Statz, Reference Statz1938) have the recurrent (1m-cu) vein distinctly postfurcal (entering the second submarginal cell) or at least interstitial, which contrasts with the Bembridge Meteorus species, which have the vein 1m-cu far antefurcal (entering the first submarginal cell well before its apex). We describe here a new species of Meteorus from Baltic amber that also has the vein m–cu distinctly antefurcal, thus resembling the Bembridge described taxa.
Subfamily Exothecinae Foerster, Reference Foerster1863
Remarks
Reliable fossil members of the subfamily Exothecinae s.s. were unknown previous to this study. Brues (Reference Brues1910) described the questionable exothecine Colastes abrogatus (Brues, Reference Brues1910) from the Florissant (early Oligocene) from an imprint originally belonging to the genus Exothecus Wesmael, Reference Wesmael1838. The latter species showed features that are unusual for members of Colastes Haliday, Reference Haliday1833, namely an almost petiolate metasoma, interstitial position of both nervulus (1cu-a) and recurrent vein (1m-cu), and large second submarginal cell distinctly widened toward its apex.
Genus Palaeocolastes Belokobylskij and Zaldívar-Riverón, new genus
Type species
Palaeocolastes bruesi Belokobylskij and Zaldívar-Riverón, n. gen n. sp., by present designation and monotypy.
Diagnosis
As for type species, by monotypy.
Etymology
Named after “palaeo” (Greek for “ancient”) and the generic name of its most similar living genus, Colastes, which belongs to the subfamily Exothecinae. Gender: masculine.
Remarks
The fossil Colastes abrogatus (Brues, Reference Brues1910), from the early Oligocene Florissant, is very similar to this new genus based on its interstitial position of nervulus (1cu-a), and perhaps by having its parallel vein (2Cub) arising from the middle of vein 2CUa. The latter species thus probably belongs to the new genus described here. Examination of the type material of this species is therefore needed to confirm its actual generic status.
Palaeocolastes bruesi Belokobylskij and Zaldívar-Riverón, new species
Figures 4–6
Holotype
Female, Baltic amber, # 5018 (PIN collection No. 964/1330). Specimen found on eastern coast of the Baltic Sea, late Eocene period (33.9 ± 0.1 to 37.2 ± 0.1 Ma).

Figure 4. Palaeocolastes bruesi n. gen. n. sp., holotype, female. (1) Habitus, lateral view; scale bar = 0.5 mm; (2) head and mesosoma, lateral view; scale bar = 0.2 mm.

Figure 5. Palaeocolastes bruesi n. gen. n. sp., holotype, female. (1) Head and anterior half of mesosoma, left side; (2) head and anterior half of mesosoma and fore leg, right side; (3) head and antenna, lateral view. (1, 2) Scale bar = 0.2 mm; (3) Scale bar = 0.5 mm.

Figure 6. Palaeocolastes bruesi n. gen. n. sp., holotype, female. (1) Wings; (2) metasoma and ovipositor, lateral view. Scale bars = 0.2 mm.
Diagnosis
This new monotypic genus is similar to Colastes Haliday, Reference Haliday1833 by having the hind wing with recurrent vein present and submedial cell considerably large, fore wing with second radiomedial vein present, and recurrent vein running into the first radiomedial cell, mesoscutum elevated high above pronotum, occipital carina probably present only laterally, and ovipositor evenly curved and without nodes or serrations apically. However, Palaeocolastes n. gen. differs from Colastes by the parallel vein (2CUb) of the fore wing arising almost from the middle of the vein (2CUa) and closing distally the brachial (first subdiscal) cell (versus from its posterior third), and the nervulus (1cu-a) almost interstitial (versus distinctly postfurcal).
Description
Female. Body length 2.8 mm; fore wing length 2.7 mm.
Head
Head not depressed, high and transverse, its maximum height 1.6 times its median length (lateral view). Occiput distinctly concave. Occipital carina present laterally and perhaps absent dorsally. Ocelli small. Head behind eyes roundly narrowed. Frons almost flat. Transverse diameter of eye 3.7 times longer than temple (lateral view). Eye large, without visible emargination opposite antennal sockets, ~1.4 times as high as broad (lateral view). Malar suture perhaps present. Malar space 0.15 times as high as eye, ~0.5 times as high as basal width of mandible. Face height 0.6 times height of eye and 0.9 times width of eyes (lateral view). Clypeus almost flat, with distinct and rather long lower flange. Hypoclypeal depression rather large. Palps invisible.
Antenna
Antenna rather slender, almost filiform, 26-segmented. Scape of antenna wide and rather long, ~2.0 times longer than its maximum width, without apical lobe or basal constriction, its ventral margin (lateral view) not longer than dorsal margin. Basal flagellar segment slender. First flagellar segment subcylindrical, 5.5 times longer than its apical width, 0.9 times as long as second segment. Penultimate segments about 4.0 times longer than their width, almost as long as apical segment. Apical segment acuminate distally.
Mesosoma
Mesosoma rather long, not depressed, its length 2.3 times its height. Neck of prothorax rather long. Pronotum dorsally almost flat, with distinct pronotal carina, mainly rugose dorsally. Mesoscutum rather highly and convex-roundly elevated above pronotum, its median lobe convex, not protruding forward, and without anterolateral corners. Notauli perhaps complete, crenulate. Prescutellar depression (scutellar sulcus) and scutellum almost invisible. Subalar depression shallow and perhaps partly (anteriorly) crenulate. Precoxal sulcus unclear, perhaps absent. Prepectal carina absent. Propodeum areolate-rugose with invisible areas; lateral tubercles absent, weakly and evenly convex (lateral view).
Wings
Fore wing 3.3 times longer than its maximum width. Pterostigma wide, 4.3 times longer than width. Metacarpus (R1a) 1.4 times longer than pterostigma. Radial (marginal) cell not shortened, reaching apex of wing, 2.7 times longer than maximum width. Radial vein (r) arising from basal 0.45 of pterostigma. First (r) and second (3RSa) radial abscissae forming very obtuse angle. Second radial abscissa (3RSa) 2.8 times longer than first abscissa (r), 0.45 times as long as the very weakly and evenly curved third abscissa (3RSb), 1.2 times longer than the almost straight first radiomedial vein (2RS). Both radiomedial veins (2RS and r-m) present. Second radiomedial (submarginal) cell wide and rather long, slightly narrowed toward apex, 2.2 times longer than its maximum width, 1.7 times longer than the wide brachial (first subdiscal) cell. Brachial (first subdiscal) cell straight anteriorly. First medial abscissa ((RS+M)a) slightly sinuate. Recurrent vein (1m-cu) convex, antefurcal and distinctly convergent posteriorly with basal (1-M) vein, 0.7 times as long as first radiomedial vein (2RS), 0.7 times as long as basal vein (1M). Discoidal (first discal) cell rather long, 1.5 times longer than its maximum width, petiolate anteriorly, petiole (1RS) not long. Nervulus (1cu-a) weakly postfurcal. Parallel vein (2CUb) arising from middle of apical margin of brachial (second subdiscal) cell. Brachial (second subdiscal) cell short and wide, closed postero-apically by long, sclerotized and distinctly inclivous brachial vein (2cu-a). Transverse anal veins (1a and 2a) absent. Hind wing 5.5 times longer than its maximum width. Radial vein (RS) arising from basal vein (1r-m) rather far from costal vein (SC+R). Radial (marginal) cell weakly widened distally, without additional transverse vein (r). Medial (basal) cell distinctly widened toward apex, 7.0 times longer than wide, 0.4 times as long as hind wing. Nervellus (cu-a) present, slightly curved. Submedial (subbasal) cell long. First abscissa of mediocubital vein (M+CU) about as long as second abscissa (M). Recurrent vein (m-cu) present, pigmented, interstitial, long, weakly curved toward base of wing.
Legs
Fore femur rather thick and long. Fore tarsus 1.2 times longer than fore tibia. Segments of middle tarsus long and slender. Hind coxa wide and rather long, without basoventral tooth and corner. Fore to hind femora without dorsal protuberances. Hind femur rather thick, ~4.0 times longer than width. Hind tibia widened posteriorly. Hind tibial spurs long and weakly curved. Hind tarsus slender, almost as long as hind tibia, its basitarsus 0.8 times as long as second to fifth segments combined. Second segment of hind tarsus 0.35 times as long as basitarsus, 1.25 times longer than fifth segment (without pretarsus). Claws long, weakly curved, simple.
Metasoma
Metasoma 0.9 times as long as head and mesosoma combined. First tergite sessile, short, and perhaps wide, weakly convex (lateral view), invisible in dorsal view. Only second and at least partly third tergites with distinctly separate laterotergites (epipleura). Suture between second and third tergites present and distinct. Median length of second 1.6 times length of third tergite. Hypopygium medium sized, densely setose. Ovipositor short and weakly evenly curved, apically without nodes and serration. Ovipositor sheath thick, 0.35 times as long as metasoma, about as long as fore femur, 0.4 times as long as mesosoma, ~0.2 times as long as fore wing.
Sculpture and pubescence
Head mainly smooth. Mesosoma mainly smooth. Propodeum mainly reticulate-areolate, the delineated areas invisible. Hind coxa and femur smooth. Metasoma mainly smooth (first tergite invisible dorsally). First tergite perhaps sculptured. Second and following tergites smooth.
Color
Body almost entirely black; metasoma ventrally brown to pale brown partly. Antenna mainly black. Legs almost entirely brown. Ovipositor sheaths black. Fore and hind wings entirely and evenly infuscate. Pterostigma entirely dark brown.
Male
Unknown.
Etymology
This species is named after Professor Charles T. Brues, pioneer of the study of Hymenoptera from Baltic amber.
Remarks
This species represents the first reliable fossil of the cyclostome braconid subfamily Exothecinae from Baltic amber. The Exothecinae, together with its closely related extant subfamilies Opiinae and Alysiinae, probably originated during the early Paleogene because this is the period during which there are the first reliable fossil records of these taxa (Brues, Reference Brues1910; Cockerell, Reference Cockerell1913; Théobald, Reference Théobald1937, Statz, Reference Statz1938; Belokobylskij, Reference Belokobylskij2014).
Subfamily Microtypinae Szépligeti, Reference Szépligeti1908
Remarks
The subfamily Microtypinae includes three described genera, with Microtypus Ratzeburg, Reference Ratzeburg1848 being the largest one (Yu et al., Reference Yu, van Achterberg and Horstmann2016).
Genus Microtypus Ratzeburg, Reference Ratzeburg1848
Type species
Microtypus wesmaelii Ratzeburg, Reference Ratzeburg1848, by monotypy.
Remarks
Nine species of the genus Microtypus Ratzeburg, Reference Ratzeburg1848 were described by Brues (Reference Brues1933) from Baltic amber. However, Brues (Reference Brues1933, p. 73) already mentioned that some of these species “… may go into closely related genera,” perhaps in the subfamily Helconinae (Diospilini). According to the original descriptions and the few illustrations provided, at least M. longicornis Brues, Reference Brues1933; M. terebrator Brues, Reference Brues1933; M. obliquus Brues, Reference Brues1933; M. latipennis Brues, Reference Brues1933; M. leviusculus Brues, Reference Brues1933; and M. longicaudatus Brues, Reference Brues1933 have a four-sided second radiomedial (submarginal) cell, and thus do not belong to Microtypus, but perhaps to Aspicolpus Wesmael, Reference Wesmael1838 or some other related genera from the subfamily Helconinae. According to our revision, only M. brevicornis Brues, Reference Brues1933; M. verticalis Brues, Reference Brues1933; M. triangulifer Brues, Reference Brues1933; and M. brevicornis Brues, Reference Brues1933 actually belong to Microtypus.
Cockerell (Reference Cockerell1921) described Diospiloides hooleyi Cockerell, Reference Cockerell1921 from the Bembridge Marls and placed it within the subfamily Helconinae. However, re-examination of the type of this genus and species has shown that it actually belongs to the genus Microtypus (Belokobylskij, Reference Belokobylskij2014).
Below, we describe a new species of Microtypus, which has the triangular second radiomedial (submarginal) cell of its fore wing distinctly petiolate anteriorly.
Microtypus eocenus Belokobylskij and Zaldívar-Riverón, new species
Figure 7
Holotype
Male, Rovno amber, # JDC 8207 (PIN collection No. 5820/1). Specimen found in western Ukraine (Priabonian stage, 33.9–37.8 Ma).

Figure 7. Microtypus eocenus n. sp., holotype, male. Habitus, lateral view. Scale bar = 1 mm.
Diagnosis
This new species from Rovno amber is similar to M. triangulifer from Baltic amber, but differs from it by having the triangular second radiomedial (submarginal) cell petiolate anteriorly (versus sessile), fore wing distinctly infuscate (versus hyaline), and pterostigma almost black (versus light brown). Microtypus eocenus n. sp. is similar to the extant M. algiricus Szepligeti, Reference Szépligeti1908 (van Achterberg, Reference van Achterberg2010), but distinctly differs from the latter species by its dark body color (versus pale brown or yellow color), 25 segments of antenna (versus 49–52 segments), and body length of 3.9 mm (versus 6.5–7.0 mm).
Description
Male. Body length 3.9 mm; fore wing length 3.1 mm.
Head
Head maximum height with mandibles 1.5 times its maximum median length (lateral view), smooth dorsally and laterally. Occiput distinctly convex. Head behind eyes perhaps strongly narrowed. Transverse diameter of eye ~4.0 times longer than temple (lateral view). Eye large, ~1.6 times as high as broad (lateral view). Malar space very short, ~0.1 times as high as the eye, ~0.25 times as high as basal width of mandible. Face weakly convex, its height 0.75 times height of eye and almost equal to width of eye (lateral view). Clypeus distinctly convex. Mandible wide and short.
Antenna
Antenna distinctly thickened, setiform, with short and dense dark setae, 25-segmented, ~0.9 times as long as body. Scape ~1.5 times longer than its maximum width. First flagellar segment 1.7 times longer than its apical width, about as long as second segment. Penultimate segment 1.7 times longer than their width, 0.4 times as long as first segment, 0.9 times as long as acuminate apical segment.
Mesosoma
Mesosoma short and high, its length 1.5 times its height. Median lobe of mesoscutum highly convex, not protruding forwards, entirely and densely setose, with rather dense punctation, without additional granulation between punctures. Notauli distinct and perhaps complete. Scutellum convex. Mesopleuron mainly smooth and partly with sparse punctation. Precoxal suture (sternaulus) distinct, wide, shallow, strongly oblique, entirely and distinctly areolate-rugose. Posterior mesopleural suture entirely coarsely crenulate. Propodeum (lateral view) strongly and almost linearly oblique from anterior one-fifth to posterior margin, mainly sculptured.
Wings
Fore wing 2.3 times longer than its maximum width, 0.7 times as long as body. Pterostigma wide, 3.6 times longer than width. Metacarpus (R1a) 1.5 times longer than pterostigma; its second abscissa (R1b) rather short. Radial (marginal) cell not shortened, 3.3 times longer than maximum width. Radial vein (r) arising from middle of pterostigma. Second radial abscissa (3RSa + 3RSb) weakly sinuate, 5.2 times longer than first abscissa (r), 4.1 times longer than the straight first radiomedial vein (2RS). Second radiomedial vein (r-m) present, but rather weakly sclerotized. Second radiomedial (submarginal) cell triangular, petiolate anteriorly, almost as long as its maximum width, 0.6 times as long as the wide brachial (first subdiscal) cell. First medial abscissa ((RS+M)a) straight. Brachial (first subdiscal) cell straight anteriorly. Recurrent vein (1m-cu) straight, 0.8 times as long as first radiomedial vein (2RS), 1.4 times longer than second medial abscissa ((RS+M)b). Basal vein (1M) distinctly curved, posteriorly convergent with recurrent vein (1m-cu). Nervulus (1cu-a) interstitial. Discoidal (first discal) cell short, sessile anteriorly, 1.25 times longer than its maximum width. Hind wing 3.8 times longer than its maximum width.
Legs
Hind femur 4.7 times longer than width, densely punctate, and covered entirely with dense and short pale setae. Hind tibia thickened, 6.7 times longer than maximum width; longest tibial spur 0.4 times as long as hind basitarsus. Hind tarsus 0.9 times as long as hind tibia. Second segment of hind tarsus 0.4 times as long as basitarsus, 1.3 times longer than fifth segment (without pretarsus).
Metasoma
Metasoma 1.1 times as long as head and mesosoma combined. First tergite weakly convex (lateral view), not long, ~0.9 times as long as second and third tergites combined, invisible in dorsal view, perhaps sculptured. Second suture present, complete, and distinct. Median length of second tergite about equal to length of third tergite. Tergites behind first one entirely smooth, tergite behind second tergite is almost entirely covered with dense, short, pale setae. Parameres of male genitalia large, wide, subtriangular.
Color
Body (including antenna) entirely black. Tarsi of all legs pale, brown. Fore wing entirely and distinctly evenly infuscate, finer distally. Pterostigma entirely dark brown.
Female
Unknown.
Etymology
This species is named after the geological period dated for Rovno amber.
Remarks
Only five fossil species described from Eocene Baltic amber and the Bembridge Marls impression can be confirmed as belonging to Microtypus Ratzeburg. The fossil genus Microtypus is the morphologically less-derived taxon of the subfamily Microtypinae. The main diagnostic feature of this genus (the developed closed triangular second radiomedial (submarginal) cell of the fore wing) is already present in all Eocene Microtypus species.
Conclusions
Of all known amber and fossil imprint deposits from the Eocene period, Baltic amber is by far the richest source of invertebrate fossils. In particular, thousands of hymenopterans have been recovered from Baltic amber, of which >100 fossil braconid species have been described. This study documents additional new species and one new genus of Braconidae from Baltic amber belonging to the subfamilies Cheloninae, Euphorinae, and Exothecinae. It also brings to light a new species found in Rovno amber from the subfamily Microtypinae, which represents the first braconid species described from this amber deposit.
The new fossil braconid genus described here from the Eocene period, Palaeocolastes n. gen., morphologically resembles the widely distributed, extant exothecine genus Colastes Haliday. This new genus represents the first reliable record of a member of the braconid subfamily Exhotecinae in the fossil fauna. Moreover, the discovery of the second chelonine species of the subgenus Syntaphus Donisthorpe (genus Ascogaster Wesmael) is also of valuable taxonomic significance. The two currently known species of this subgenus are now only known from the Eocene period in Baltic amber and as an imprint fossil in the Bembridge Marls (Insect Limestone). Moreover, the female of Diospilites brevicornis Brues, type species and type genus of the very rare monotypic subfamily Diospilitinae, which is only known from the Eocene period, is illustrated here with digital photographs for the first time, and the variation of some diagnostic features of the species is documented. Further studies of the Eocene fauna from Baltic, and especially Rovno, amber deposits will continue to increase our knowledge of the braconid taxa present during this geological period, and reveal important information of their morphological features and variation.
Acknowledgments
The authors are very thankful to Prof. A. P. Rasnitsyn (Moscow, Russia) for his valuable comments and suggestions during the preparation of this work, and the two reviewers for their useful suggestions for the first version of the manuscript. We thank S. Guzmán for taking some of the pictures included in this work. This work was in part funded by grants provided by the Russian Foundation for Basic Research (project No. 19–04–00027) and the Russian State Research Project No. АААА–А19–119020690101–6 to SAB, and by a grant given by the DGAPA-UNAM (PAPIIT project no. 201119) to AZR.










