Introduction
Catostomid fishes (family Catostomidae) belong to the largest, most diversified, and most widely distributed order of freshwater fishes, the Cypriniformes (carp-like fishes; Nelson, Reference Nelson2006). The cypriniform fishes consist of over 4,000 species, including a large number of commercially important species in Eurasia, many aquarium fishes, and the zebrafish, which is a model organism for bony fishes. Cypriniforms are characterized by toothless oral jaws with a protrusible premaxilla, enlarged ascending premaxillary process, kinethmoid bone, enlarged pharyngeal bone with ankylosed pharyngeal teeth, and three branchiostegal rays. Like other otophysans, they have a well-developed Weberian apparatus that connects the inner ear with the swim bladder as an adaptation for improved hearing.
Catostomids are commonly known as suckers as they have protrusible, fleshy-lipped, subterminal mouths used in food suction. They inhabit a wide range of environments from large rivers, such as the Mississippi (USA) and Yangtze (China), to small tributaries, such as streams along the Rocky Mountains, and from large lakes such as the Great Lakes of North America to small ponds. Their body shapes range from extremely deep to very shallow and change through ontogeny (Nelson and Paetz, Reference Nelson and Paetz1992; Meng et al., Reference Meng, Su and Miao1995). Modern catostomids consist of 13 genera with 72 species. One of 71 North American species, Catostomus catostomus (Forster, Reference Forster1773), extends its range to northeastern Siberia. Only one extant species, Myxocyprinus asiaticus (Bleeker, Reference Bleeker1865), is found solely outside North America, as an endemic form in the Yangtze River of China (Meng et al., Reference Meng, Su and Miao1995; Nelson, Reference Nelson2006).
In contrast to the family’s modern disjunct distribution, catostomid occurrences in the Eocene (56–34 Ma) were abundant in both North America and East Asia. Prior to the Eocene, the oldest catostomids are represented by a disarticulated cleithrum in Paleocene (66–56 Ma) sediments from a single locality in the Paskapoo Formation of Alberta, Canada (Wilson, Reference Wilson1980b). During the Eocene, fossil catostomids reached relatively high taxonomic diversity. Three fossil genera with 10 nominal species from this period have been described from both North America and East Asia (Cope, Reference Cope1872, Reference Cope1874, Reference Cope1875, Reference Cope1893; Wilson, Reference Wilson1977; Grande et al., Reference Grande, Eastman and Cavender1982; Chang et al., Reference Chang, Miao, Chen, Zhou and Chen2001). After the Eocene, catostomids disappeared from the Asian fossil record by the early Oligocene, at the same time becoming common fishes in North America. Many fossil species from western North America have been attributed to modern genera (Smith, Reference Smith1981).
From a coal mine near Osino, Nevada, USA, Cope (Reference Cope1872) reported the first arguably Eocene catostomid species, †Amyzon mentale Cope, Reference Cope1872. In North America, all Eocene catostomids were subsequently assigned to the extinct genus †Amyzon. Cope (Reference Cope1874, Reference Cope1875, Reference Cope1893) reported another four species of †Amyzon, two of which were suggested to be junior synonyms by Bruner (Reference Bruner1991). Drawing on a large collection of specimens, Wilson (Reference Wilson1977) comprehensively redescribed †Amyzon brevipinne Cope, Reference Cope1893 by reexamining the holotype and studying new specimens, and described a new Eocene species, †Amyzon aggregatum Wilson, Reference Wilson1977, from Horsefly, British Columbia, Canada. Later, Grande et al. (Reference Grande, Eastman and Cavender1982) described another species, †Amyzon gosiutense Grande et al., Reference Grande, Eastman and Cavender1982, from the Eocene Green River Formation, USA. In Asia, the first catostomid fossil was recovered from Eocene sediments in Inner Mongolia, China, based on several diagnosable opercles and disarticulated bones (Hussakof, Reference Hussakof1932). One species of †Amyzon, †A. hunanense (Cheng, Reference Cheng1962), and two new genera with new species, †Plesiomyxocyprinus arratiae Liu and Chang, Reference Liu and Chang2009 and †Vasnetzovia artemica Sytchevskaya, Reference Sytchevskaya1986, were reported from southern China, northeastern China, and Siberia, respectively (Sytchevskaya, Reference Sytchevskaya1986; Chang et al, Reference Chang, Miao, Chen, Zhou and Chen2001; Liu and Chang, Reference Liu and Chang2009). From the known Eocene taxa, Wilson (Reference Wilson1977) and Grande et al. (Reference Grande, Eastman and Cavender1982) gave detailed osteological descriptions of three species of †Amyzon, thus improving our knowledge of their morphologies. However, the remaining species are not well understood or taxonomically clarified. One of the well-described species, †A. gosiutense, was concluded to be a junior synonym of †A. aggregatum by Bruner (Reference Bruner1991), whereas some species of †Amyzon described in the nineteenth century lacked diagnosable characters and thus require clarification.
As evident from this short summary of the catostomid fossil record, the Eocene represents a critical time for the evolution and divergence of this group of fishes. To better understand the diversity of early catostomids, recently discovered materials, such as those from the Eocene Kishenehn Formation described here, are taxonomically, systematically, and paleogeographically important. Early catostomid fossil records, together with cyprinid records (family Cyprinidae), represent the oldest known cypriniforms (Chang and Chen, Reference Chang and Chen2008) and thus are also important for understanding the origin and evolution of the order Cypriniformes.
Not only is the taxonomy of the Eocene catostomids unclear, but also the evolutionary relationships of their species have never been studied. In recent works, phylogenetic analyses of modern catostomids have been performed using a variety of data, especially osteology and molecular sequences (Smith, Reference Smith1992; Doosey et al., Reference Doosey, Bart, Saitoh and Miya2010; Chen and Mayden, Reference Chen and Mayden2012). Of those studies, Smith (Reference Smith1992) performed the most comprehensive morphological phylogenetic analysis on the family Catostomidae. He included 62 extant and one recently extinct catostomid species as well as the fossil genus †Amyzon, using 157 morphological, biochemical, developmental, and genetic characters. That analysis suggested that †Amyzon is the sister group to the modern genera Carpiodes and Ictiobus. However, the relationships of the other Eocene genera with the modern genera, and the relationships among the Eocene genera and species, remain poorly known. Our aim here is to improve our understanding of the interrelationships of the species of †Amyzon and the systematic position of the genera †Amyzon and †Plesiomyxocyprinus by performing a series of phylogenetic analyses that include those fossil species with well-preserved specimens based on the characters and data matrix of Smith (Reference Smith1992).
Materials and methods
Methods
The fossil specimens were prepared manually and mechanically. Fossil specimens were scanned for morphometric analysis using an Epson Perfection 3590 scanner and measured using the computer program Image J. Details of structures were photographed using a Nikon DXM 1200C digital camera mounted on a Zeiss Discovery V8 stereo microscope at the University of Alberta and a Nikon SMZ-U mounted on a stereo microscope in the Division of Paleontology, American Museum of Natural History (AMNH).
For the phylogenetic analyses, all characters were coded in Mesquite V 3.03 (Maddison and Maddison, Reference Maddison and Maddison2011) and analyzed in PAUP* 4beta10 for Macintosh computers (Swofford, Reference Swofford2003) and TNT (Goloboff et al., Reference Goloboff, Farris and Nixon2000). In PAUP, all characters were run as unordered and unweighted. Heuristic searches used a ‘furthest’ addition sequence, with no topological constraints enforced, no ancestral taxa identified, and ‘parsimony’ as the optimality criterion. Character changes were optimized onto the resulting trees using Mesquite V3.03 (Maddison and Maddison, Reference Maddison and Maddison2011). In TNT, both traditional heuristic searches and a ‘new technology’ search were used.
The terminology of the anatomical characters follows various significant studies with or without a few modifications: that of the pharyngeal bone and teeth is from Chu (Reference Chu1935); that of the opercular series is from Nelson (Reference Nelson1949); that of the Weberian apparatus is from Nelson (Reference Nelson1948); that of the maxilla is from Miller and Smith (Reference Miller and Smith1981); and that of the caudal skeleton is from Grunbaum et al. (Reference Grünbaum, Coutier and Dumont2003).
Institutional abbreviations
AMNH, American Museum of Natural History, New York, USA; CMN, Canadian Museum of Nature, Ottawa, Canada; GMC, The Geological Museum of China, Beijing, China; IVPP, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, Beijing, China; KU, University of Kansas, Lawrence, USA; ROM, Royal Ontario Museum, Toronto, Canada; UALVP, University of Alberta Laboratory for Vertebrate Paleontology, Edmonton, Canada.
Materials
The majority of the fossil specimens included in this study were collected by one of the authors (MVHW) and his field teams in 1984 and 1992. The Constenius family, John, Leona, and Kurt, also joined the field trip and collected many useful specimens. The family also conducted fieldwork independently and donated their fish specimens to the UALVP.
Fossil and extant fishes used for comparison are listed in Liu et al. (Reference Liu, Chang, Wilson and Murray2015). In addition to those, the holotypes of †Amyzon brevipinne (CMN 6189) and †Amyzon aggregatum (ROM 11019a,b) and the paratype of †Amyzon gosiutense (AMNH FF 10460) were available for comparison. †Amyzon specimens deposited in the ROM and CMN and listed in Wilson (Reference Wilson1974, Reference Wilson1977) were examined by JL. The important specimens of catostomids in the AMNH were also added for comparison, e.g., †Amyzon gosiutense AMNH FF 10401 and †Amyzon commune Cope, Reference Cope1874 AMNH FF 2579 and 8069. Modern comparative fish specimens from the ichthyology collection of AMNH were also used; these are dry skeletons unless otherwise indicated: Carpiodes cyprinus (Lesueur, Reference Lesueur1817) AMNH I-90212; Carpiodes sp. AMNH I-21694; Catostomus catostomus, AMNH I-I-47712, AMNH I-41156 (cleared and stained); Catostomus commersonii (Lacépède, Reference Lacépède1803), AMNH I-55944; Chasmistes brevirostris (Cope, Reference Cope1879), AMNH I- 47030; Deltistes luxatus (Cope, Reference Cope1879), AMNH I-46205; Cycleptus elongatus (Lesueur, Reference Lesueur1817), AMNH I-94810, AMNH I-77906; Erimyzon oblongus (Mitchill, Reference Mitchill1814), AMNH I-88732; Ictiobus cyprinellus (Valenciennes in Cuvier and Valenciennes, Reference Cuvier and Valenciennes1844), AMNH I-56459; Ictiobus bubalus (Rafinesque, Reference Rafinesque1818), AMNH I-88699; Ictiobus meridionalis (Günther, Reference Günther1868), AMNH I-28077; Moxostoma poecilurum (Jordan, Reference Jordan1877), AMNH I-94811; Myxocyprinus asiaticus, AMNH I-22437.
Geology
The fossil specimens of †Amyzon from Montana were collected from oil shales of the Kishenehn Formation. The Kishenehn strata are located in the Kishenehn Basin and the South Fork Basin and are exposed along the cut bank of the Flathead River system, which extends north to the North Fork Flathead River in British Columbia, Canada, and south to the South Fork Flathead River in Montana, USA (Constenius et al., Reference Constenius, Dawson, Pierce, Walter and Wilson1989). The lacustrine Kishenehn Formation has been dated as Eocene and Oligocene (Whipple, Reference Whipple1992). All of the fossils of †Amyzon reported here were collected from the area of Disbrow Creek and Tunnel Creek, small tributaries of the Middle Fork Flathead River, Montana. They were collected from an interval about 20–25m thick (personal communication, Kurt Constenius, 2012) and along the cut bank of the creeks. The sediments from the fossil fish locality belong to the middle part of the Coal Creek Member, consisting of an interbedded sequence of oil shale, marlstone, litharenite, and siltstone, and lesser amounts of lignite, sapropelic coal, tuff, claystone, and mudstone (Whipple, Reference Whipple1992). The age of the middle part of the Coal Creek Member is suggested to be the middle Eocene age of 43.5±4.9 Ma based on fission track analysis of zircon (Whipple, Reference Whipple1992), equivalent to the Lutetian Stage of the Eocene.
Among the vertebrates recovered from Kishenehn Formation, Russell (Reference Russell1954) reported a Kishenehn mammalian fauna from the area of the North Fork Flathead River in British Columbia, whereas Li and Wilson (Reference Li and Wilson1994) studied the bony-tongue fish †Hiodon consteniorum Li and Wilson, Reference Li and Wilson1994 (Osteoglossomorpha: Hiodontidae) from the South Fork strata. The fossils of †Amyzon were mostly collected from the exposures near Tunnel Creek and Disbrow Creek. They are the most abundant vertebrates from the Kishenehn Formation and are preserved as complete, articulated specimens or with only slight disarticulation of the skeleton.
Species of †Amyzon from various Eocene localities are often the most abundant fishes found at a given site (Cope, Reference Cope1874; Wilson, Reference Wilson1977, Reference Wilson1980a, Reference Wilson1984; Grande, Reference Grande1980; Chang et al., Reference Chang, Miao, Chen, Zhou and Chen2001). The specimens of †Amyzon are also dominant in the two Kishenehn sites where they were collected, Tunnel Creek and Disbrow Creek. Most of the specimens represent articulated juveniles, and very few are large adults. This extremely unbalanced combination of juveniles and adults suggests that the new species partitioned its habitat according to size, as do most extant catostomids, possibly because of differences between juveniles and adults in avoidance of predation or food resource utilization. The fish assemblages from these two sites also include small individuals of Hiodon and †Eohiodon (Li and Wilson, Reference Li and Wilson1994; Hilton and Grande, Reference Hilton and Grande2008), along with disarticulated bones of amiids. By comparison with fossil-fish assemblages in British Columbia of a similar age and faunal composition (Wilson, Reference Wilson1980a), the presence of mostly small individuals of †Amyzon and †Eohiodon with disarticulated amiids suggests that the Disbrow and Tunnel creek sites were deposited in a near-shore region of the Eocene lake; however, the nearly uniform excellent articulation and preservation of the small fishes would suggest deeper water. Also relevant to conclusions about the paleoenvironment are the numerous very well-preserved fossil plants and invertebrates (mainly insects) that have been discovered at the same localities (Constenius et al., Reference Constenius, Dawson, Pierce, Walter and Wilson1989). A series of studies on the Kishenehn invertebrates is under way, and some have been published. The Kishenehn Formation in the South Fork area has been claimed as an emerging Konservat Lagerstätte of middle Eocene insects (Huber and Greenwalt, Reference Huber and Greenwalt2011; Harbach and Greenwalt, Reference Harbach and Greenwalt2012; Greenwalt et al., Reference Greenwalt, Goreva, Siljeström, Rose and Harbach2013; Shockley and Greenwalt, Reference Shockley and Greenwalt2013; Greenwalt and Labandeira, Reference Greenwalt and Labandeira2014; Greenwalt and Rust, Reference Greenwalt and Rust2014; Greenwalt et al., Reference Greenwalt, Rose, Siljestrom, Goreva, Constenius and Wingerath2015).
Systematic paleontology
Subdivision Teleostei Müller, Reference Müller1845
Superorder Ostariophysi Sagemehl, Reference Sagemehl1885
Order Cypriniformes Bleeker, Reference Bleeker1860
Family Catostomidae Bonaparte, Reference Bonaparte1840
Genus †Amyzon Cope, Reference Cope1872
Type species
†Amyzon mentale Cope, Reference Cope1872 from the Osino shale, Nevada, USA, by original designation.
Included species
†A. commune Cope, Reference Cope1874, †A. aggregatum Wilson, Reference Wilson1977, †A. gosiutense Grande, Eastman and Cavender, Reference Grande, Eastman and Cavender1982, and †A. hunanense (Cheng, Reference Cheng1962).
Emended diagnosis
Large-sized Eocene catostomid fish with terminal mouth, elongate and emarginate dorsal fin with 19 to 33 principal rays, anal fin with seven to 10 (rarely 11) principal rays originating approximately opposite the posterior end of the dorsal fin base, caudal fin slightly forked with 18 (occasionally 19) principal rays, body deep with ratio of body depth to standard length 0.34 to 0.52 in adults and 0.25 to 0.30 in juveniles and larvae, posteroventral process of dentary moderately elongate, suborbital 2 through 5 thin and deep, sensory canal on preopercle partly superficial and partly embedded, autopterotic ridge intermediate (neither broad nor sharp), predorsal bones 4 to 6 large.
Occurrence
Eocene, North America and East Asia.
†Amyzon kishenehnicum new species

Figure 1 †Amyzon kishenehnicum n. sp., photograph of the holotype specimen UALVP 55260 from the Kishenehn Formation. Scale bar=50 mm.

Figure 2 †Amyzon kishenehnicum n. sp., photographs of juveniles from the Kishenehn Formation and osteological structures. (1) Complete fish in left lateral view UALVP 24137. (2) Otic region of UALVP 24152 showing the otoliths. (3) Weberian apparatus region of UALVP 24137. (4) Gill region of UALVP 24152 showing the pharyngeal tooth and tooth socket. Scale bars=5 mm. Fish anterior of (1) and (3) facing left; of (2) and (4) facing right. cl=cleithrum; L. &. R. ast=left and right asteriscus; lap=lapillus; nc=neural complex; ns=neural spine; op=opercle; par=parietal; pr4=the 4th pleural rib; ptp=posttemporal; ptr=autopterotic; sag=saggita; scl=supracleitrum; scp=scaphium; trp=tripus.
Holotype
UALVP 55260, a complete adult fish preserved on the slab in left lateral view (Fig. 1).
Paratypes
UALVP 24137, 24140, 24147, 24148, 24149, 24152, and 24154, complete and nearly complete juvenile fishes.
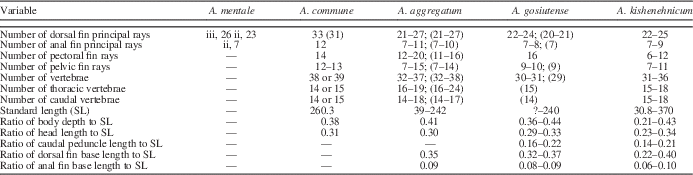
Diagnosis
A species of †Amyzon differing from all other species in having the following combination of characters: body depth/standard length (SL) 0.21–0.42, of which juveniles have 0.21–0.32 and adults 0.36–0.43; vertebrae (exclusive of Weberian apparatus) 31–36 (most 31–32), of which 15–18 (most 16) are precaudal and 15–18 (most 15) are caudal; D 1–4, 22–25; A 1–4,7–9; P 9–12; V 7–11; C 4–7, 9, 9, 4–6; both ethmofrontal and frontoparietal fontanelles present; frontal ridge thick and robust with projections; lateral margin of parietal bow-shaped; second infraorbital (IO2) enlarged and curved composing the ventral orbit; preopercular sensory canal partially enclosed in bone; anteromedial corner of premaxilla angled ninety degrees; dentary process of premaxilla bent anteriorly; fork length of pelvic bone about half total length of pelvic bone; both hypurals two and three (hyp2 and hyp3) fused to the compound centrum.
Age and horizon
43.5±4.9 Ma, middle Eocene (Lutetian), Kishenehn Formation, Coal Creek Member.
Type locality
Tunnel Creek of Middle Fork Flathead River, east of Whitefish, Montana.
Referred specimens
UALVP 23943, 24131–24134, 24138, 24139, 24141–24146, 24150, 24151, 24153, 24155–24199, 24226, 38728–38788, 38792–38807, 38876, 38877, 38881–38920, 38922–38958, 38962–39039, 52373, in total 295 specimens.
Etymology
The specific epithet kishenehnicum is a combination of the name of the Kishenehn Formation name and the Latin suffix–icum (neuter) meaning belonging to or pertaining to.
General appearance
The new species is elongated and fusiform, with a deep and laterally compressed body in adults (Fig. 1), a shallow body in juveniles (Fig. 2.1), and nearly straight ventral outline in most individuals. The body size of our specimens ranges from 3.08 cm (UALVP 24143) to around 40 cm (UALVP 55515 and 55156) in standard length (SL; Fig. 3; Table 1). The ratio of body depth to SL ranges from 0.21 to 0.42 (Table 1), with the juveniles possessing a shallower body with a ratio range of 0.21–0.32, and adults having a deeper body with a ratio range of 0.36–0.43. The head length–to-SL ratio is around 0.30 with the range 0.23 to 0.34 (Table 1). The fish has a terminal to subterminal mouth, and the jaw would have been protrusible according to the shape of the mandibular bones and the presence of the kinethmoid. The dorsal fin is emarginate, and the dorsal fin base is long (around one-third of SL) with over 20 principal rays. The caudal fin is slightly forked in adults and forked in juveniles.

Figure 3 (1) Standard-length frequency distributions and (2) scatter diagram of body depth vs. standard length in †Amyzon kishenehnicum n. sp. from the Kishenehn Formation.
Table 1 Meristic and morphometric data for North American species of †Amyzon, including †A. kishenehnicum n. sp., †A mentale, †A. commune, †A. aggregatum, and †A. gosiutense. Values in parentheses are observed from the specimens listed in the Methods and Materials section of this study. For those not otherwise designated, values for each species were obtained from the original descriptions. Standard length is in millimeters. Number of vertebrae does not include the Weberian apparatus.

Skull roof
Skull-roof bones include the frontal and parietal. As in all catostomids, sensory canals are entirely detached from the roofing bones. Both anterior (ethmofrontal) and posterior (frontoparietal) fontanelles are present in †Amyzon kishenehnicum n. sp.
The frontal (Figs. 4.1, 5.1–5.2) resembles that of most catostomids in that it is, overall, broad anteriorly and narrow posteriorly because of the laterally expanded orbital process of the frontal in the anterior part and the medially notched longitudinal margin (given the presence of the frontoparietal fontanelle) in the posterior part. Both anterior and posterior ends are truncated. Two notches are found along the medial margin of the frontal. One is small, located at the anteromedial corner and open to the ethmoid, indicating there is an ethmofrontal fontanelle. The other one is prominent and longitudinally elongated along the medial margin of the posterior part of the frontal, representing the frontoparietal fontanelle. The posterior medial margin of the frontal surrounding the frontoparietal fontanelle is not parallel to the medial line; it angles closer to the midline more posteriorly. This indicates that the frontoparietal fontanelle is broader anteriorly and narrower posteriorly, resembling an elongated wedge.

Figure 4 Photographs of †Amyzon kishenehnicum n. sp. from the Kishenehn Formation, †Amyzon aggregatum, and †Amyzon gosiutense showing osteological differences. (1–5) Specimens of †Amyzon kishenehnicum n. sp. (1) Skull roof of UALVP 55260 showing the frontal with the concave margin for the anterior fontanelle and posterior fontanelle. (2) Parietal of UALVP 24137. (3) Snout region of UALVP 24137. (4) Pelvic bone of UALVP 24137. (5) Caudal skeleton of UALVP 24154. (6–10) Specimens of †A. aggregatum. (6, 7) Frontal and parietal of UALVP 32931. (8) Snout region of UALVP 31125. (9, 10) Caudal skeleton of UALVP 31958 and 40931. (11–14) Specimens of †A. gosiutense AMNH FF 10460 showing (11) skull roof; (12) snout region; (13) pelvic bone; (14) caudal skeleton. Black scale bars=5 mm; scale photographed with specimens is in millimeters. Fish anterior of (1–5, 8, 9) facing left; of (6, 7) facing up; of (10–14) facing right. afn=anterior fontanelle (ethmofrontal); cc=caudal compound centrum; den=dentary; emd=ethmoid; fr=frontal; frd=frontal ridge; hyp=hypural; kem=kinethmoid; max=maxilla; mxn=neck of maxilla; par=parietal; pfn=posterior fontanelle (frontopariental); phy=parhypural; pls=pleurostyle; pmx=premaxilla; psp=pelvic splint; psr=pelvic strut; ptr=autopterotic; rna=rudimentary arch; son=supraorbital notch of frontal; sop=supraorbital process of frontal; sph=sphenotic.

Figure 5 Reconstructive drawings of †Amyzon kishenehnicum n. sp. from the Kishenehn Formation, †Amyzon aggregatum, and †Amyzon gosiutense. (1) Head region drawing of †Amyzon kishenehnicum n. sp. based on the holotype specimen UALVP 55260. (2–5) reconstructive drawings of †Amyzon kishenehnicum n. sp. (6–10) †Amyzon aggregatum. (11–14) †Amyzon gosiutense. (2, 6, 11) Left frontal. (3, 7, 12) Left premaxilla. (4, 8, 13) Left pelvic bone, (5, 9, 10, 14) Caudal skeleton. Reconstructions of (2–14) are based on the respective images of Figure 4. Fish anterior in all drawings facing left. aa=anguloarticular; afn=anterior fontanelle (ethmofrontal); bh=basihyal; br=branchiostegal; cl=cleithrum; den=dentary; ect=ectopterygoid; emd=ethmoid; ent=endopterygoid; fr=frontal; frd=frontal ridge; hym=hyomandibular; hyp=hypural; io=infraorbital; iop=interopercle; lem=lateral ethmoid; lhh=lower hypohyal; max=maxilla; mpt=metapterygoid; mxn=neck of maxilla; nc=neural complex; ns=neural spine; op=opercle; par=parietal; pfn=posterior fontanelle (frontopariental); pmx=premaxilla; pop=preopercle; psp=pelvic splint; psr=pelvic strut; ptp=posttemporal; ptr=autopterotic; qua=quadrate; soc=supraoccipital; ra=retroarticular; scl=supracleithrum; so=supraorbital; sop=subopercle; sph=sphenotic; sym=sympletic; uhh=upper hypohyal.
Laterally, the frontal bears a supraorbital notch (Fig. 4.6), which houses the supraorbital, and a supraorbital process (Fig. 4.6) that contacts the sphenotic (Fig. 4.1) posteriorly and reaches the orbit with its tip. The supraorbital notch is shallow and small.
Dorsally, the frontal bears a prominent longitudinal ridge extending from near the anterior margin to the posterior end (Figs. 4.1, 5.1). The ridge is ornamented with sturdy and dorsally pointed projections.
The parietal is very small, about the size of the posterior narrow part of the frontal (Fig. 4.2). Laterally, the margin is concave and smooth, which gives the parietal a bow shape in dorsal view, instead of triangular, rectangular, or square as in most catostomids. The bow-shaped parietal is also seen in Myxocyprinus and †A. aggregatum (Fig. 4.7). However, the other closely related species †A. gosiutense possesses a slightly convex lateral margin and thus displays a roughly rectangular shape (Fig. 4.11).
The supraoccipital (Fig. 5) is sutured to the parietal posteriorly, forming the dorsal and posteromedial cranial wall. The supraoccipital crest, extending posteriorly toward the neural complex of the Weberian apparatus, is intermediately developed as in Ictiobus, Catostomus, and Moxostoma, less robust than that in Cycleptus, Myxocyprinus, and Erimyzon, and more developed than that in Carpiodes.
Ethmoid region
Both kinethmoid and ethmoid are present in †A. kishenehnicum but are not completely visible in any specimen. Combining the appearance from several specimens, the kinethmoid is rodlike, narrow, and curved dorsally in the middle. Both anterior and posterior ends are enlarged. The ethmoid is domed anteriorly, with an elongated anterior process. It encloses the ethmofrontal fontanelle posteriorly.
The paired lateral ethmoids, located on either side of the ethmoid, are Q-shaped (Fig. 5.1). The circular part of the lateral ethmoid is not porous as in many catostomids, but instead is concave laterally like a bowl with a hollow in the middle. The ventral process is prominent and posteriorly pointed toward the orbit, resembling the condition in †A. aggregatum. In the other catostomids, the ventral process is pointed anteriorly or ventrally or is reduced.
Orbital region
A supraorbital (Fig. 5.1) is present as in the other species of †Amyzon. It is small and rounded. The first infraorbital (IO1, lacrimal; Fig. 5.1) is large and elliptical. IO2 is also large and semi-lunate but much more elongated and curved than IO1 and crosses the ventral orbit. IO3 is much smaller, and the possible IO4 is reduced with only the associated sensory canal present along the posterior margin of the orbit. The sensory canal on IO1 is detached because of preservation, whereas on IO2 it is completely attached and tube-like, running along the lateral surface and closer to the dorsal edge.
Otic region
The sphenotic (Figs. 4.1, 5.1) is bordered both anteriorly and dorsally by the frontal and posteriorly by the autopterotic, as in other catostomids. The wing-shaped postorbital process, produced from the anterolateral sphenotic and forming the posterodorsal margin of the orbit, is nearly the same size as, but less broad than, the central region of the sphenotic. The ventral end of the postorbital process lacks the extended pointed tip, which is also absent in Carpiodes, Cycleptus, certain species of Ictiobus, and in Catostomus. The surface of the sphenotic is slightly depressed for the attachment of opercular muscles (Weisel, Reference Weisel1960). The sphenotic of †A. kishenehnicum is similar to that of other species of †Amyzon, where known.
The autopterotic (Fig. 2.3) is a main component of the posterolateral cranium, posterior to the sphenotic and ventral to the parietal. As in most catostomids, the autopterotic ridge (Smith, Reference Smith1992), which is the outer surficial part and sutured to the sphenotic and parietal, is small, with a depression formed by ridges at the anterior and posterior margins. A sizeable posteroventral process of the autopterotic extends toward the shoulder girdle. The opening of the lateral temporal fossa, surrounded by the concave lateral margins of the parietal, autopterotic, and epiotic, is large, as in Ictiobus, Cycleptus, and Myxocyprinus. The epiotic is a robust bone situated at the posteroventral corner of the cranium and slightly exposed in lateral view.
The arrangement of the otoliths (Fig. 2.2) is like that of other ostariophysan fishes, i.e., the sagitta is delicate and smaller than the other two otoliths (Adams, Reference Adams1940). The sagitta is anteroposteriorly elongated with both ends pointed (Fig. 2.2). The asteriscus is below the sagitta, rounded, and is the largest of the three. The lapillus is small and rounded, and anterior to the other two.
Opercular series
The opercle (Figs. 1, 5.1) has an opercular arm, a concave dorsal margin, and an auricular process as in typical catostomids (Nelson, Reference Nelson1949). The opercular arm is stout, with striations on the outer surface and a truncated dorsal end. The dorsal concave margin is broad and shallow. The auricular process is well developed, with a pointed dorsal tip. The peripheral striations are moderately developed, neither as deep and numerous as in Ictiobus and Carpiodes, nor totally absent as in some Catostomus, Moxostoma, and Cycleptus. The anterior border of the opercle is straight, and the angle of the inferior (ventral) border is more than 30°, thus resembling Nelson’s ‘OP1’ group (Nelson, Reference Nelson1949).
The preopercle (Fig. 5.1) consists of a vertical and a horizontal arm and resembles that of all catostomids except for Myxocyprinus and Cycleptus, in which the horizontal arm is reduced. In †A. kishenehnicum, the height of the vertical arm nearly equals the length of the horizontal arm, resembling that of other species of †Amyzon and some species of Ictiobus. These two arms form a right angle as in other species of †Amyzon, Erimyzon, and some Carpiodes. The preopercular sensory canal runs along the axis of the preopercle. The sensory canal is in an open groove at both ends of the preopercle, whereas there is a thin layer of bone covering the canal in the middle region, thus partially enclosing the canal. There is no evidence of a prominent ridge, as seen in the primitive cypriniform †Jianghanichthys, in †A. kishenehnicum.
The interopercle (Fig. 5.1) has the general form of that in most catostomids, which is roughly lunate in shape and tapered anteriorly in depth. It is situated at the corner of the opercle, overlapped by the preopercle anteriorly and overlapping the subopercle posteriorly. It resembles Nelson’s ‘IOP1’ (Nelson, Reference Nelson1949). The subopercle is similar to that in other species of †Amyzon in having a nearly straight dorsal margin and more curved ventral margin.
Jaw and suspensorium
As in all catostomids, the mouth gape is formed by the premaxilla, maxilla, and dentary (Figs. 4.3, 5.1). The premaxilla in †A. kishenehnicum is L-shaped. The ascending process is nearly as long as the alveolar process. The ascending process is narrow and tapered toward the dorsal pointed end, whereas the alveolar process is broad and slightly curved. The anteromedial corner of the premaxilla forms a sharp right angle as in †A. gosiutense, unlike the rounded-off corner in †A. aggregatum.
The maxilla of †A. kishenehnicum bears two dorsal processes, a narrow neck, a broad body, and a dentary process, as in most catostomids. The neck is short and narrow (Figs. 4.3, 5.1). The process posterodorsal to the neck is at an obtuse angle. A ridge along the anterior margin of the neck, which is for the insertion of the palatomaxillary ligament (Miller and Smith, Reference Miller and Smith1967), is prominent. The ventral (anterior) keel of the body of the maxilla is round, whereas the dorsal (posterior) keel is expanded posterodorsally as a projection. All the features noted for the maxilla are also visible in †A. aggregatum and †A. gosiutense. However, the robust, knob-like dentary process is bent forward in †A. kishenehnicum, resembling that in †A. aggregatum and unlike the truncated and straight process of †A. gosiutense.
The dentary (Fig. 5.1) consists of a ventrally pointed gnathic ramus, a developed coronoid process, and an elongate posteroventral process. A lateral ridge along the gnathic ramus extends from the anterior point to nearly the root of the ramus.
The anguloarticular (Fig. 5.1) is much shorter and narrower than the posteroventral process of the dentary and is overlapped anteriorly by part of it. Posteriorly, the anguloarticular possesses a large socket to articulate with the quadrate. The retroarticular is a prominent and laminar bone ventral to the anguloarticular. The size of the retroarticular is similar to that in Ictiobus and larger than that in Catostomus. The quadrate, resembling that of most cypriniforms, possesses an anterior condyle that articulates with the anguloarticular, a flat dorsal part, an elongated, slender ventral part, and a wedge-shaped slot in between the dorsal and ventral parts. In †A. kishenehnicum, the condyle is large, corresponding to the sizable socket of the anguloarticular. The flat dorsal part is roughly square, whereas the ventral slender part is rod-like and extends far posteriorly. The wedge-shaped sympletic fits into the slot of the quadrate.
Pharyngeal tooth
A single tooth was found in situ along with several tooth sockets on UALVP 24152 (Fig. 2.4). The tooth is compressed as in all catostomids. The overall triangular shape resembles the teeth of the other species of †Amyzon (Grande et al., Reference Grande, Eastman and Cavender1982; Sytchevskaya, Reference Sytchevskaya1986; Liu et al., Reference Liu, Chang and Wilson2010). However, unlike in the other species of †Amyzon, the tooth is short and lacking a hooked conical tip, which may be because it is immature or has been truncated by abrasive wear.
Paired girdles and fin
The posttemporal is overall a flat, vertically elongate bone attaching the shoulder girdle to the skull as in other catostomids (Figs. 2.3, 5.1). A prominent ridge extends along the midline of the upper half of the posttemporal and merges with the posterior edge of the lower half. The upper half of the bone is narrow with a pointed superior end, whereas the lower half is broad with a round ventral end. An anteromedial process at the midpoint of the bone at the bottom of the upper half indicates the area of contact with the extremely elongate posteroventral process of the autopterotic. The supracleithrum (Figs. 2.3, 5.1) is also a flat bone overlapping with the posttemporal in its upper part and with the cleithrum in its lower part. It is slightly broader than, and about twice as high as, the posttemporal. The cleithrum is spoon-shaped (Figs. 2.3, 5.1). In lateral view, a ridge along the anterior margin is followed by a deep, narrow groove. The postcleithrum attaches to the posterior side of the cleithrum and has a slightly curved ventral limb. There are nine to 12 (usually 12; Table 1) pectoral fin rays with the longest one nearly reaching the insertion of the pelvic fin.
The pelvic bone is bifurcated anteriorly into a rod-like lateral strut and a flat medial splint (Figs. 4.4, 5.4). The strut length, measured from the anterior tip of the strut to the posterior end of the pelvic bone excluding the ischial process and thus representative of the pelvic bone length (PL), is slightly longer than the splint length measured in the same manner. The fork length (PFL), from the anterior point of the strut to the posterior-most point of the fork, is about half of the pelvic bone length (PFL/PL 0.448). The posteromedial ischial process is compressed and tapered to a posterior point. The length of the ischial process (posterior end of splint to posterior-most point of ischial process) compared to PL is about one-half (ratio 0.444). There are seven to 11 (usually seven to nine; Table 1) pelvic fin rays attached to the pelvic bone.
Median fins
The dorsal fin base is long with a length-to-SL ratio of 0.22–0.40 (0.22–0.32 found in juveniles, and 0.40 found in the single adult measured). There are 22 to 25 principal dorsal fin rays (Table 1) preceded by one to four rudimentary rays. The first principal ray is unbranched and leads the fin web, and the rest are shorter and branched. They are all segmented distally. The last two rays are frequently articulated to the same pterygiophore and thus counted as one principal ray. There are 22–25 pterygiophores supporting the fin rays. The proximal radial of the first dorsal pterygiophore is thick and robust, indicating it would have supported a large muscle volume and thus probably strong erector dorsalis and depressor dorsalis muscles to control the dorsal fin.
The ratio of anal fin base length to SL is 0.06 to 0.08 for the juveniles and 0.1 for the single sampled adult specimen (UALVP 55260). There are seven to nine principal anal fin rays (Table 1), of which the count nine only occurred in the adult specimen. The first principal anal fin ray is unbranched, and the rest of the fin rays are branched. They are all segmented distally and preceded by one to four rudimentary rays. The anal fin is supported by seven to 10 pterygiophores. Similar to the condition in the dorsal fin, the last principal anal fin ray usually consists of two small rays sharing one support, and the thick first pterygiophore indicates strong erector analis and depressor analis muscles.
Vertebral column
Exclusive of the Weberian apparatus, there are 31 to 36 (mostly 31 or 32) vertebrae, of which 15 to 18 (mostly 16) are precaudal and 15 to 18 (mostly 15) are caudal (Table 1). Two of the four Weberian ossicles are visible: a small triangular tripus and a thin flake-like scaphium (Fig. 2.3). The Weberian apparatus has an exceptionally robust and long fourth pleural rib (Fig. 2.3) as in all other catostomids (Nelson, Reference Nelson1948; Bird and Hernandez, Reference Bird and Hernandez2007). The fourth pleural rib tapers ventrally. In lateral view, the anterior and posterior edges of this rib are slightly elevated and ridge-like, whereas a deep, vertical groove in the middle extends from the bifurcated root to the ventral end. The shape of the neural complex (Fig. 5.1) is roughly a parallelogram, resembling those in †A. aggregatum and †A. gosiutense. The superior margin is slightly convex. The fourth neural spine nearly reaches the superior margin of the neural complex (Fig. 5.1). There are four to five triangular predorsal bones between the fourth neural spine and the ninth or tenth neural spine anterior to the dorsal fin.
Two series of intermuscular bones, the epineural and epipleural (Patterson and Johnson, Reference Patterson and Johnson1995), parallel the vertebral column. The epineural series, dorsal to the vertebral column, spans the length of the column, whereas the epipleural series is restricted to the caudal region ventral to the vertebral column. Both epineural and epipleural bones are forked proximally.
Caudal skeleton and fin
The caudal fin is slightly forked in the adult and forked in the juveniles, with 18 principal fin rays, all of them segmented, of which nine form each of the upper and lower caudal fin lobes. The dorsal-most and the ventral-most principal fin rays are unbranched, whereas the rest are branched. There are three to six upper procurrent rays and three to five lower ones preceding the caudal fin. The count of the lower procurrent rays is usually equal to or less than that of the upper procurrent rays in the same individual.
The caudal skeleton (Figs. 4.5, 5.5) consists of all the elements in other catostomids (Eastman, Reference Eastman1980). The compound centrum, formed by the union of the first preural and first ural centra, bears the posterodorsally extended pleurostyle that is formed by the fused anterior uroneurals (Eastman, Reference Eastman1980; Grünbaum et al., Reference Grünbaum, Coutier and Dumont2003). There are six hypurals (hyp1 to hyp6) and one parhypural below the pleurostyle, and the hypurals decrease in size dorsally in the series. The parhypural is fused with hyp1 proximally and then articulated to the compound centrum as in almost all other catostomids (Eastman, Reference Eastman1980). The gap between hyp2 and hyp3, also called the caudal diastema, is narrow. Both hyp2 and hyp3 are fused to the compound centrum (Figs. 4.5, 5.5) in all specimens that present a good view of the caudal skeleton (UALVP 24137, 24140, 24146, 24154, and 24157). The rudimentary first preural neural arch is small, whereas the epural is correspondingly long. A free uroneural is posterolateral to the pleurostyle in specimens preserved in both left and right lateral view, suggesting that a pair of free posterior uroneurals was present (UALVP 24139, 24146, 24147, and 24157).
Phylogenetic analysis including fossil catostomids
The systematic relationships of catostomid fishes have been studied for decades; for example, Hubbs (Reference Hubbs1930) reviewed the classification of eastern North America catostomids and provided some characters used in his key, which are applicable to the whole family. Among the valuable systematic studies, external morphology of the brain and lips (Miller and Evans, Reference Miller and Evans1965), morphological characters for the tribe Moxostomatini (Jenkins, Reference Jenkins1970), morphological and biochemical characters in Catostomus (Smith and Koehn, Reference Smith and Koehn1971), genetic information (Ferris and Whitt, Reference Ferris and Whitt1978), and developmental characters (Fuiman, Reference Fuiman1985) have all been used to hypothesize evolutionary intrarelationships of the Catostomidae. Nelson (Reference Nelson1948, Reference Nelson1949), Eastman (Reference Eastman1977, Reference Eastman1980), and Doosey and Bart (Reference Doosey and Bart2011) comprehensively studied certain anatomical features among the members of the family Catostomidae, many of which are systematically valuable and informative. As a result, catostomids are among the best-known freshwater fishes.
However, our understanding of their relationships is still undergoing improvement, with more in-depth study and new technology applied to systematic ichthyology. Smith (Reference Smith1992) combined the non-molecular characters from earlier publications and compiled a series of morphological characters to perform the most comprehensive phylogenetic analysis of catostomid fishes to date. More recently, molecular sequences, including both mitochondrial and nuclear DNA, have been used to hypothesize catostomid systematic relationships (Harris and Mayden, Reference Harris and Mayden2001; Harris et al., Reference Harris, Mayden, Espinosa Perez and Garcia De Leon2002; Doosey et al., Reference Doosey, Bart, Saitoh and Miya2010; Chen and Mayden, Reference Chen and Mayden2012; Unmack et al., Reference Unmack, Dowling, Laitinen, Secor, Mayden, Shiozawa and Smith2014).
Three subfamilies were recognized and in widespread use before the 1990s (Gill, Reference Gill1861; Nelson, Reference Nelson1948; Smith, Reference Smith1992). These are Ictiobinae, consisting of Ictiobus and Carpiodes; Cycleptinae, with the American Cycleptus and East Asian Myxocyprinus; and Catostominae, containing the rest, comprising nine genera and more than 60 species. A fourth subfamily, Myxocyprininae, with only the monotypic Myxocyprinus, was proposed by Miller (Reference Miller1959) and is widely recognized and accepted in recent studies (Miller, Reference Miller1959; Harris and Mayden, Reference Harris and Mayden2001; Nelson, Reference Nelson2006; Doosey et al., Reference Doosey, Bart, Saitoh and Miya2010). The Myxocyprininae were separated from Cycleptinae, which were left also with a single genus, Cycleptus. Unless otherwise designated, Cycleptinae and Myxocyprininae in the following discussion represent the extant members only. The fossil members †Amyzon and †Plesiomyxocyprinus have been suggested to be closely related to Ictiobinae and Myxocyprininae, respectively (Smith, Reference Smith1992; Liu and Chang, Reference Liu and Chang2009). However, Smith’s (Reference Smith1992) cladistic analysis on 63 post-Pleistocene species plus †Amyzon and using Cyprinus and Leptobotia as outgroups was the only one to include †Amyzon in a systematic study.
To explore the systematic position of †A. kishenehnicum within the genus †Amyzon, as well as to further understand the evolutionary relationships among extant and Eocene catostomids, we added data for †Plesiomyxocyprinus to Smith’s (Reference Smith1992) data matrix and replaced his codings for †Amyzon with codings for three species of the genus, i.e., †A. aggregatum, †A. gosiutense, and †A. kishenehnicum. We then subjected the data matrix to a series of phylogenetic analyses.
Our first analysis used Smith’s (Reference Smith1992) original data matrix, with its 64 ingroup taxa and two outgroups coded for 157 characters, of which most were ordered and polarized. His analysis was performed in HENNIG86 (Farris, Reference Farris1989) using the parsimony criterion and generated two equally parsimonious trees of 853 steps, a consistency index (CI) of 0.35, and a retention index (RI) of 0.80. Our reanalysis of Smith’s unchanged complete data matrix used PAUP (Swofford, Reference Swofford2003) using heuristic search algorithms and the parsimony criterion, but with all characters unweighted and unordered. In this repeated analysis, 233 equally parsimonious cladograms were found with tree length 778 steps, CI 0.393, and RI 0.783. A strict consensus of the 233 trees suggests that †Amyzon is sister to modern ictiobines, identical to Smith’s (Reference Smith1992) hypothesis. Ictiobinae are sister to Catostominae, rather than the most basal clade in the preferred tree of Smith (Reference Smith1992). Meanwhile, Cycleptus is the most basal clade of the catostomid family, whereas Myxocyprinus is sister to the rest of the catostomids instead of sister to Cycleptus.
Although the preferred tree of Smith (Reference Smith1992) suggested that Cycleptinae are the sister group to Catostominae, the author noticed that the number of characters supporting such a topology was not overwhelming and that the Ictiobinae might be the sister group to the Catostominae (Smith, Reference Smith1992, p. 797). That the topology is changed by using a different computer program and algorithms is entirely reasonable.
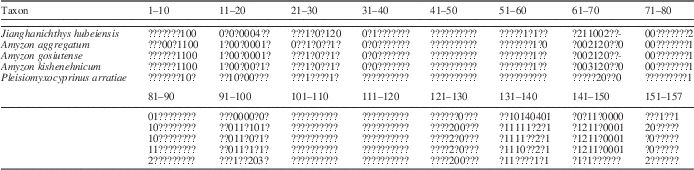
In our second analysis, we coded character states of †Jianghanichthys, †Plesiomyxocyprinus, †Amyzon aggregatum, †Amyzon gosiutense, and †A. kishenehnicum (Table 2) for the characters from Smith’s (Reference Smith1992) data matrix, and we removed the genus †Amyzon from the data matrix as a separate taxon. Our complete data matrix can be found in Morphobank (Project 1277, http://www.morphobank.org/). The genus †Jianghanichthys is a basal cypriniform fish from the early Eocene of China and represents an extinct family of Cypriniformes (Liu et al., Reference Liu, Chang, Wilson and Murray2015). This fish shares a series of symplesiomorphies with both catostomids and cyprinids, and thus is an ideal fossil outgroup member for this study. With the addition of the above taxa, our analyses include three outgroups and 67 ingroup taxa. An analysis in PAUP of the modified data matrix, using the same search algorithm and criteria as we used in our first analysis, generated 1,238 equally parsimonious cladograms with 804 steps, CI 0.381, and RI 0.781. The strict consensus cladogram (Fig. 6) suggests that the genus †Amyzon, rather than the subfamily Ictiobinae, is the most basal clade within the family Catostomidae, whereas †Plesiomyxocyprinus is sister to Myxocyprininae plus Cycleptinae. The Ictiobinae are found to be the sister group of the Catostominae as in the previous analysis.

Figure 6 Strict consensus cladogram of catostomids based on Smith (Reference Smith1992) with the addition of †Amyzon kishenehnicum n. sp. from the Kishenehn Formation, †A. aggregatum, †A. gosiutense, †Plesiomyxocyprinus arratiae, and †Jianghanichthys hubeiensis. Three analyses involving three outgroups and 67 ingroups, performed in PAUP and TNT, and using parsimony criteria generated the same strict consensus cladogram; CI 0.38, RI 0.78.
Table 2 Character states for fossil taxa †Amyzon kishenehnicum n. sp., †A. aggregatum, †A. gosiutense, †Plesiomyxocyprinus arratiae, and †Jianghanichthys hubeiensis. See Smith (Reference Smith1992) for the character description and data matrix of extant taxa. Complete data matrix can be found in Morphobank (Project 1277, http://www.morphobank.org/).

Our third analysis was performed in TNT (Goloboff et al., Reference Goloboff, Farris and Nixon2000) using both traditional search and ‘new technology search’ methods on the same data matrix as the second analysis. Only one taxon, †Jianghanichthys, was designated as an outgroup, rather than three taxa as in the previous analysis. The subanalysis using the traditional search quickly generated 73 equally parsimonious cladograms with 803 steps, CI 0.380, and RI 0.781. Although the matrix had fewer outgroup taxa, these trees are only one step shorter than the equally parsimonious cladograms generated in the PAUP analysis. Nevertheless, the strict consensus cladogram of the TNT analysis is identical to the one from the PAUP analysis (Fig. 6). The other subanalysis, using new technology search, was even faster but only generated five equally parsimonious cladograms with the same length as those from the traditional search. The strict consensus cladogram is also identical to the one from the PAUP analysis (Fig. 6).
All of the analyses with the modified data matrix found †Amyzon to be the most basal clade, possibly because of the inclusion of †Jianghanichthys as an additional outgroup. Within †Amyzon, †A. kishenehnicum is recovered as the sister to †A. aggregatum. Character 97 from the data matrix that united †A. kishenehnicum and †A. aggregatum is the number of post-Weberian vertebrae, of which †A. gosiutense possesses character state 0 with 30–31 vertebrae, whereas the other two species have character state 1 with 32–37 and 31–36 vertebrae, respectively. There are also other osteological characters that support †A. kishenehnicum and †A. aggregatum as sister to each other. The parietal of †A. kishenehnicum and †A. aggregatum is laterally concave and smooth, giving the parietal a bow shape in dorsal view, whereas in †A. gosiutense it is slightly convex laterally and rectangular in dorsal view. Second, but not least, the dentary process of †A. kishenehnicum and †A. aggregatum is knob-like, bent, and directed anteriorly, whereas the knob-like dentary process of †A. gosiutense is straight and downwardly directed.
Remarks on North American †Amyzon
Since Cope (Reference Cope1872) reported the first fossil catostomid species †Amyzon mentale from Osino, Nevada, USA (syntype: USNM V 4074/4075), all Eocene–Oligocene catostomids from North America have been assigned to the extinct genus †Amyzon. Cope reported four additional nominal species of †Amyzon in subsequent studies: †A. commune Cope, Reference Cope1874, †A. fusiforme Cope, Reference Cope1875, †A. pandatum Cope, Reference Cope1875, and †A. brevipinne Cope, Reference Cope1893. Two more Eocene species, †A. aggregatum Wilson, Reference Wilson1977, and †A. gosiutense Grande et al., Reference Grande, Eastman and Cavender1982, were more recently described in greater detail. Drawing on a large collection of specimens, Wilson (Reference Wilson1977) also redescribed †Amyzon brevipinne and emended the specific diagnosis after reexamining the holotype and additional materials. At the species level, this taxon is well established by Wilson (Reference Wilson1977). However, according to our ongoing project, the generic designation of †A. brevipinne needs revision as well.
Bruner (Reference Bruner1991) reviewed the genus †Amyzon from North America and summarized the fossil localities and museum catalogues, as well as presented a bibliography of the genus. He pointed out that †A. pandatum and †A. fusiforme should both be junior synonyms of †A. commune for a number of reasons. First, according to Cope’s original descriptions (Cope, Reference Cope1874, Reference Cope1875), there is no significant morphological difference between †A. commune and †A. pandatum, whereas †A. fusiforme seems to be a juvenile form of †A. commune. Second, the type specimens resemble each other in that the dorsal fin originates posterior to the origin of the pelvic fin, and the end of the dorsal fin base is opposite to or posterior to the insertion of the last anal fin ray. This condition has not been seen in any other species of †Amyzon. Third, all three nominal species were recovered from the Florissant Formation, South Park, Colorado, which improves the possibility that they are the same species. While we expect to undertake further examination of the syntypes of those nominal species, we agree with Bruner (Reference Bruner1991) on the synonymy of †A. pandatum and †A. fusiforme with †A. commune based on the available evidence.
Bruner (Reference Bruner1991) also suggested †A. gosiutense was a synonym of †A. aggregatum based on meristic and metric characters. It is true that the measurements and counts of most morphological structures of these two species overlap, and some characteristics of †A. gosiutense fall within the range seen in †A. aggregatum. However, one reason that the data for †A. gosiutense are within the range of those for †A. aggregatum could be that †A. aggregatum is represented by a much larger number of specimens (Grande et al., Reference Grande, Eastman and Cavender1982; Table 1). Meanwhile, †A. kishenehnicum described here is represented also by a large collection and thus also shows high meristic and morphometric similarity to †A. aggregatum as well as †A. gosiutense (Table 1). However, of even more significance, by comparing the osteology of these three species, we find that they differ from each other in a number of non-meristic osteological characters (Figs. 4, 5).
First, the anterior fontanelle (also known as ethmofrontal, or preepiphysial, fontanelle) is present in †A. kishenehnicum (Figs. 4.1, 5.1, 5.2) and †A. gosiutense (Figs. 4.11, 5.11), but absent in †A. aggregatum (Figs. 4.6, 5.6). All catostomids, including the fossil forms, have at least one fontanelle present. The frontoparietal fontanelle is a notable synapomorphy, distinguishing catostomids from the closely related cyprinids. All catostomid species possess the frontoparietal fontanelle, with the exception of two members of the genus Cycleptus, in which only an ethmofrontal fontanelle is present. Some catostomids, such as Carpiodes, Myxocyprinus, and some species of Ictiobus, have both the ethmofrontal and frontoparietal fontanelles. In the Eocene †Plesiomyxocyprinus, a prominent anteriomedial notch was preserved very well in a disarticulated frontal, clearly showing the presence of an ethmofrontal fontanelle in addition to the frontoparietal fontanelle. In †A. gosiutense, an anteromedial notch on the frontal is also visible in the examined specimen (Figs. 4.11, 5.11; AMNH FF 10460), which suggests an ethmofrontal fontanelle exists. However, there is no anteromedial notch on the frontal of †A. aggregatum (Figs. 4.6, 5.6). The ethmofrontal fontanelle, occasionally present, is restricted in the ethmoid and bordered by the frontal posteriorly (ROM 11041).
Second, the frontal ridge is strong in †A. kishenehnicum (Figs. 4.1, 5.1) and †A. aggregatum, whereas it is weak in †A. gosiutense. The longitudinal ridge of the frontal is a synapomorphy for both fossil and extant catostomids but varies in the length, strength, and ornamental pattern of the ridge. The frontal ridge in most catostomids extends from the middle of the frontal, at a level between where the postorbital processes of the frontal end and the frontoparietal fontanelle begin, to the posterior end of the frontal. However, some catostomids have a longer ridge, which extends nearly to the anterior end of the frontal, such as in †A. kishenehnicum and †A. aggregatum.
In terms of the strength of the ridge, the frontal ridge in some catostomids is weak and slightly raised, but in others it is strong and thick with thick dorsal projections, a thick dorsal flange, or a laminated, laterally extended roofing flange. The dorsal projection and flange of the frontal are common in many modern catostomids, as for example in Ictiobus, Carpiodes, Myxocyprinus, Erimyzon, and Moxostoma, whereas the lateral roofing flange is usually found in Catostomus, Cycleptus, and Chasmistes. In the fossil forms, †A. kishenehnicum and †A. aggregatum present similar frontal ridges, i.e., a strong, thick ridge with projections, whereas †A. gosiutense and †Plesiomyxocyprinus have a weak and slightly raised frontal ridge.
Nevertheless, the supraorbital notch of the frontal is shallow and small in all of †A. kishenehnicum, †A. aggregatum, and †A. gosiutense. In catostomids, the supraorbital notch only exists in those that have a supraorbital bone. For example, the supraorbital bone is absent in Catostomus and Moxostoma, and there is no supraorbital notch; the lateral margin of the anterior part of the frontal forms the dorsal rim of the orbit and is usually truncated. Alternatively, the supraorbital process of the frontal is excluded entirely from the orbit, instead serving as a wedge between the supraorbital and the postorbital process of the sphenotic, for example, in Ictiobus and Carpiodes. The same condition of the lateral margin as in †Amyzon kishenehnicum also appears in the fossil forms †A. aggregatum and †A. gosiutense and in extant representatives Myxocyprinus and Chasmistes. In addition, all of †A. kishenehnicum, †A. aggregatum, and †A. gosiutense possess the truncated posterior margin of the frontal. The posterior margin of the frontal in catostomids is either truncated, oblique, zigzagged, or bears an elongated projection. The truncated margin is common in Myxocyprinus and some species of Catostomus.
Third, the parietal of †A. gosiutense is roughly rectangular instead of bow-shaped as in †A. aggregatum and †A. kishenehnicum (Fig. 4.2, 4.7, 4.11). Most modern catostomids have the roughly rectangular shape of the parietal, whereas the laterally bow-shaped parietal is seen in Myxocyprinus.
Fourth, the anteromedial corner of the premaxilla of †A. gosiutense and †A. kishenehnicum forms a sharp right angle, whereas it is rounded off in †A. aggregatum (Fig. 5.3, 5.7, 5.12). In our sampled extant catostomids, the rounded-off corner of the premaxillae is restricted to Ictiobus, Carpiodes, and Myxocyprinus, whereas the sharp-angled corner of the premaxillae occurs in the rest.
Fifth, the dentary process of the maxilla of †A. gosiutense is straight and truncated resembling that of Cycleptus, whereas the process is bent forward in †A. aggregatum and †A. kishenehnicum, which is a common condition in extant catostomids (Fig. 5.1).
Sixth, the size and arrangement of IO2 and IO3 of †A. kishenehnicum are different from those in †A. aggregatum and †A. gosiutense. In the latter species, IO3 is larger than IO2, and both of them form the ventral margin of the orbit, resembling in this respect the condition in Ictiobus and Moxostoma. By contrast, in †A. kishenehnicum, the elongated IO2 crosses the bottom of the orbit, resembling the condition in catostomids such as Catostomus.
Seventh, the preopercular sensory canal is partially enclosed in the bone in †A. kishenehnicum, whereas it is attached along a ridge on the middle line of the bone in †A. aggregatum and †A. gosiutense. In most Eocene catostomids, such as †Plesiomyxocyprinus, †A. aggregatum (UALVP 33041), and †A. gosiutense, the sensory canal runs along a ridge that is created by thickening anterior or dorsal to the axis in comparison to the thin posterior lamina of the preopercle (Liu and Chang, Reference Liu and Chang2009, fig. 4e–f). The primitive cypriniform †Jianghanichthys also possesses such a ridge, while the sensory canal is along the ridge on the upper half, but enclosed in the lower half.
Eighth, the pelvic bone fork is shallow in †A. aggregatum (UALVP 24147.2), medium depth in †A. kishenehnicum (Figs. 4.4, 5.4), and deep in †A. gosiutense (Figs. 4.13, 5.13). The variation in pelvic fork length of catostomids has been noted by Liu and Chang (Reference Liu and Chang2009). Three categories of catostomids according to PFL can be recognized: (1) fork shallow, PFL:PL equal to or less than 1:3, e.g., Myxocyprinus, Carpiodes, Ictiobus, †Plesiomyxocyprinus, and †A. aggregatum; (2) fork intermediate, PFL:PL around 1:2, e.g., Cycleptus and †Amyzon including †A. kishenehnicum; (3) fork deep, PFL:PL equal to or more than 2:3, e.g., Catostomus, Moxostoma, Erimyzon, Chasmistes, and †A. gosiutense.
Last, the gap between hyp2 and hyp3 (caudal diastema) in †A. gosiutense (Figs. 4.14, 5.14) is consistently and visibly broader than that in †A. aggregatum (Figs. 4.9, 4.10, 5.9, 5.10). The gap is even narrower in †A. kishenehnicum. Moreover, both hyp2 and hyp3 are fused to the compound centrum proximally in †A. kishenehnicum, whereas only hyp2 is found to be fused to the compound centrum in †A. gosiutense. In †A. aggregatum, hyp3 is occasionally fused to the compound centrum in addition to hyp2. This condition with both hypurals fused to the compound centrum has also been found in †Plesiomyxocyprinus (Liu and Chang, Reference Liu and Chang2009), Minytrema, Myxocyprinus, Ictiobus, Carpiodes, Cycleptus, and Erimyzon, whereas the remaining catostomids have only hyp2 fused to the compound centrum.
As evident from the osteological characters, †A. kishenehnicum, †A. aggregatum, and †A. gosiutense are significantly different from each other. Therefore, we support the validity of †A. gosiutense as a distinct species.
Kinethmoid, Weberian apparatus, and pharyngeal teeth of the North American †Amyzon
Compared to their Asian relatives, North American species of †Amyzon are much better known. However, because of the nature of fossil preservation, some key characteristics of †Amyzon have been unknown since the genus was first studied in 1872 (Cope, Reference Cope1872). As more specimens have been discovered to give us a better understanding of these fishes, more features have become available for study. These account for our revised understanding of the evolutionary morphological traits of the family Catostomidae and even the order Cypriniformes.
The presence of the kinethmoid is a unique and universal synapomorphy of cypriniform fishes (Fink and Fink, Reference Fink and Fink1981). The only report of a ‘kinethmoid’ in noncypriniforms is that of a bone articulated with the maxilla distally and with the vomer and mesethmoid proximally in †Chanoides, which has been suggested to be not homologous with that of cypriniforms (Patterson, Reference Patterson1984). The kinethmoid is an endochondral bone connecting the premaxillary ascending processes and the ethmoid complex via ligaments and contributing to the independently evolved protrusible jaw of cypriniforms (Hernandez et al., Reference Hernandez, Bird and Staab2007; Staab and Hernandez, Reference Staab and Hernandez2010; Staab et al., Reference Staab, Holzman, Hernandez and Wainwright2012). The kinethmoid is believed to have originated de novo in the ancestor of the Cypriniformes. However, it is rarely preserved in early cypriniform fossils (Cope, Reference Cope1872, Reference Cope1874, Reference Cope1875, Reference Cope1893; Liu, Reference Liu1957; Wilson, Reference Wilson1977, Reference Wilson1980b; Grande et al., Reference Grande, Eastman and Cavender1982; Sytchevskaya, Reference Sytchevskaya1986; Zhou, Reference Zhou1990; Chang et al., Reference Chang, Miao, Chen, Zhou and Chen2001; Liu and Chang, Reference Liu and Chang2009). The only description of the kinethmoid in a fossil was that of Wilson (Reference Wilson1977) for †A. aggregatum using the term ‘rostral.’ As more specimens are collected and examined, the morphology and diversification of the kinethmoid in †Amyzon, represented by †A. aggregatum, †A. gosiutense, and †A. kishenehnicum, has become available. Overall, the kinethmoid in these three species has a rod-like shape resembling that of modern catostomids and a very low aspect ratio (width/length) that probably correlated with the generation of great suction (Staab et al., Reference Staab, Holzman, Hernandez and Wainwright2012). The kinethmoid differs among species in shape, width, and presence of ridges on the dorsal end (the anterior end when protruded). In †A. aggregatum, the kinethmoid (Fig. 4.8) is long and thin, resembling that of Moxostoma poecilurum. Its length equals that of the ascending process of the premaxilla (Fig. 4.8). Both ends of the kinethmoid are rounded, whereas the middle is narrow. On the dorsal (anterior) end, only a few longitudinal ridges are present. In †A. gosiutense, the kinethmoid is laterally compressed, with rough ridges on the dorsal end (Fig. 4.12), similar to the condition in Ictiobus and Catostomus. Finally, in †A. kishenehnicum, the kinethmoid is present, but in no specimen is the whole element clearly visible. Inferred from the remains on several specimens, it is probably curved, with both ends rounded and weak ridges on the dorsal (anterior) end; a similar shape can be found in Cycleptus. In summary, the kinethmoid of †Amyzon is variable, resembling that of different subfamilies of modern catostomids except Myxocyprininae.
The well-developed Weberian apparatus is another significant synapomorphy of ostariophysans including cypriniforms. The Weberian apparatus comprises four paired Weberian ossicles and associated vertebral elements, modified from the first four or five vertebrae to transmit sound from the swim/gas bladder to the inner ear sensory cells. The Weberian apparatus of †A. aggregatum has been described by Wilson (Reference Wilson1977), whereas that of †A. gosiutense has been described by Grande et al. (Reference Grande, Eastman and Cavender1982). The Weberian apparatus preserved in specimens of both taxa are revisited by authors of this paper. Together with †A. kishenehnicum, the Weberian apparatus of North American †Amyzon resembles that of Ictiobinae in general (Nelson, Reference Nelson1948). It is characterized by a high neural complex with a lateral ridge and higher posterodorsal corner than anterodorsal corner; the neural spine of vertebra 4 nearly as high as the neural complex; a pair of parallel deep pleural ribs (rib 4) pointing ventrally with a very narrow and deep groove along the posterior margin; and a deep transverse plate with two prominent projections (Fig. 2.3). The deep neural complex and deep pleural rib 4 are probably correlated with the deep and compressed body of †Amyzon as discovered by Nelson (Reference Nelson1948). Remarkably, an exceptionally preserved anterior view of a Weberian apparatus of †A. aggregatum shows that pleural rib 4 (fused with rib 2) is tightly sutured to vertebra 2 shortly below the elongated transverse process of vertebra 2 (UALVP 19540), resembling that in modern catostomids. Among modern cypriniform fishes, only in catostomids do the pleural ribs of vertebra 4 and 2 fuse into a robust pleural rib 4 and form a transverse plate. Apparently, this unique feature of catostomids was already present in the Eocene †Amyzon of North America.
A slender pharyngeal bone with one row of 16 or more compressed teeth with a comb-like arrangement is a key synapomorphy of catostomids (Eastman, Reference Eastman1977; Nelson, Reference Nelson2006). Grande et al. (Reference Grande, Eastman and Cavender1982) estimated that †A. gosiutense had 50–60 pharyngeal teeth of conical shape. We have found a complete pharyngeal bone with teeth in posterior (ventral) view that belongs to †A. aggregatum. It bears 32 complete and broken teeth excluding the spaces for missing teeth (UALVP 24217a, ~37 including missing teeth). The teeth become gradually smaller as the pharyngeal bone tapers dorsally, and overall are laterally compressed with an apical projection. More precisely, the first few teeth have obliquely truncated tops probably because of wear; the teeth in the middle part of the bone are compressed with a hooked tip; and the remaining teeth on the dorsal end are very delicate (UALVP 24217 and 32931). According to the description of the pharyngeal teeth of †A. gosiutense, neither the number nor the shape is close to those of †A. aggregatum. One of the Asian Eocene catostomids, †Plesiomyxocyprinus, has a known pharyngeal bone and the teeth are similar in size and shape to those of †A. aggregatum. †Plesiomyxocyprinus has an estimated pharyngeal tooth number of 50+ based on one pharyngeal bone with 40 teeth preserved (IVPP V 12572.2). However, another pharyngeal bone with 14 teeth preserved on its ventral half suggests the total number of teeth was probably about 35, implying high intraspecific variation in the monotypic †Plesiomyxocyprinus (Liu and Chang, Reference Liu and Chang2009, fig. 5). Given this amount of variation, the 50 to 60 pharyngeal teeth suggested for †A. gosiutense vs. about 37 for †A. aggregatum are also reasonable in light of possible interspecific and intraspecific variation (Eastman, Reference Eastman1977) in †Amyzon. In summary, the number of pharyngeal teeth of Eocene catostomids is probably 35–60 and probably exhibits similar intraspecific and interspecific variation to that seen in modern catostomids (Eastman, Reference Eastman1977; Table 1).
Conclusions
In this study, we described a new species, †Amyzon kishenehnicum, founded on 303 specimens from the Eocene Kishenehn Formation, Montana, USA. Drawing on the characters and data matrix of Smith (Reference Smith1992), a series of phylogenetic analyses were performed, suggesting that †A. kishenehnicum is sister to †A. aggregatum, and together with †A. gosiutense form the most basal clade of Catostomidae. Meanwhile, the Asian genus †Plesiomyxocyprinus is closely related to the subfamily Myxocyprininae, supporting the hypothesis that was formulated by Liu and Chang (Reference Liu and Chang2009) without a phylogenetic analysis. We also reviewed the taxonomy of the North American species of †Amyzon by comparing the osteology. The osteological variations demonstrate that †A. gosiutense is a distinct species, although it had been considered to be a junior synonym of †A. aggregatum. Last, we summarized some key characteristics of the North American species of †Amyzon, such as the kinethmoid, Weberian apparatus, and pharyngeal teeth, which are critical to understanding the evolution of the family Catostomidae, as well as the order Cypriniformes.
Acknowledgments
The authors are grateful to the late N.J. Constenius, L. Constenius, and K. Constenius for helping collect specimens in the field and generously donating their own collections to the UALVP. Our thanks go to J. Tseng (AMNH) for manuscript discussion and specimen delivery, A. Lindoe (UALVP) for preparing and collecting specimens, G. Li for field assistance, J. Bruner for collection management (UALVP), X.A. Tseng, P. Tseng, and B. Kruk (UALVP) for obtaining additional specimens. We also thank M. Chang (IVPP), L. Lu (GMC), K. Seymour (ROM), M. Currie (CMN), G. Arratia (KU) and A. Bentley (KU) for collection access, and people at AMNH—J. Maisey, B. Brown, R. Arindell, and A. Gishlick—for specimen access and loans, and J. Conrad for artistic suggestions. J. Maisey and the Division of Paleontology, AMNH provided research space and resources to the first author towards the completion of this study as a visiting researcher in residence. This study is supported by Natural Sciences and Engineering Research Council of Canada Discovery Grants A9180 (to MVHW) and 327448 (to AMM).