Introduction
Conulariids, an extinct (Ediacaran–Triassic) group of marine invertebrates now generally classified as scyphozoan cnidarians (e.g., Jerre, Reference Jerre1994; Hughes et al., Reference Hughes, Gunderson and Weedon2000; Van Iten et al., Reference Van Iten, Leme, Simões, Marques and Collins2006b; John et al., Reference John, Hughes, Galaviz, Gunderson and Meyer2010; Robson and Young, Reference Robson and Young2013; Van Iten et al. Reference Van Iten, Burkey, Leme and Marques2014a, Reference Van Iten, Marques, Leme, Pacheco and Simõesb), produced a finely lamellar, apatite-bearing periderm that in some species (e.g., Metaconularia papillata Hall, Reference Hall1847) attained lengths in excess of 500 mm, potentially making conulariids some of the largest cnidarian polyps that ever lived. Nearly all previous investigators have interpreted the conulariid periderm as an ectodermal derivative that grew by accretion of whole lamellae to its inner surface (centripetal accretion) and/or by extension of the periderm along its apertural margin (e.g., Barrande, Reference Barrande1867; Bouček and Ulrich, Reference Bouček and Ulrich1929; Kozlowski, Reference Kozlowski1968; Bischoff, Reference Bischoff1978; Feldmann and Babcock, Reference Feldmann and Babcock1986; Babcock and Feldmann, Reference Babcock and Feldmann1986a, Reference Babcock and Feldmannc; Van Iten, Reference Van Iten1991a, Reference Van Itenb, Reference Van Iten1992a, Reference Van Itenb; Jerre, Reference Jerre1994; Brood, Reference Brood1995; Van Iten et al., Reference Van Iten, Vhylasová, Zhu and Yi2005, Reference Van Iten, Leme and Simões2006a; John et al., Reference John, Hughes, Galaviz, Gunderson and Meyer2010). Furthermore, some authors (e.g., Moore and Harrington, Reference Moore and Harrington1956b; Babcock and Feldman, Reference Babcock and Feldmann1986a, Reference Babcock and Feldmannc) have concluded that in addition to calcium phosphate, the conulariid periderm also contained substantial amounts of organic matter. This conclusion is based in part on the observation that certain relatively thin-walled specimens exhibit highly regular inward folding (plicated closure) of the apertural region (e.g., Moore and Harrington, Reference Moore and Harrington1956b; Van Iten et al., Reference Van Iten, Moussa and Yahaya2008; Sendino et al., Reference Sendino, Zàgoršek and Vhylasová2011; Fig. 1). Such occurrences suggest that the periderm of living conulariids possessed a high degree of flexibility, a characteristic that is difficult to reconcile with the alternative hypothesis that it consisted predominantly or exclusively of rigid mineral matter. As demonstrated by scanning electron imaging and x-ray diffraction analysis, the mineral component of the conulariid periderm consists of microcrystalline carbonate apatite. Finally, comparisons of conulariids with the chitinous periderm of the polypoid stage of coronate scyphozoans have led some authors (e.g., Chapman, Reference Chapman1966; Chapman and Werner, Reference Chapman and Werner1972; Werner, Reference Werner1966a, Reference Wernerb, Reference Werner1967; Van Iten, Reference Van Iten1992a) to argue further that the conulariid and coronate periderms are mutually homologous.

Figure 1 Archaeoconularia slateri (Reed, Reference Reed1933) (Upper Ordovician, Katian; Farden Member, South Threave Formation, near Girvan, southwestern Scotland). Apertural end of the holotype (GLAHM S9710), showing plicated closure of the aperture with smooth bending of the low midline carina (revealed through exfoliation of shallower lamellae originally covering it) at the base of the apertural lappets (arrow).
This long history of previous research notwithstanding, certain basic problems concerning the structure and growth of the conulariid periderm remain poorly resolved (Leme et al., Reference Leme, Simões, Rodrigues, Van Iten and Marques2008). In addition to the question of the original organic content of the periderm, these include the manner in which conulariids produced such gross anatomical structures as transverse ribs and internal carinae and the nature of extensional growth, of which there seems to be no direct record in the form of growth lines (Babcock and Feldmann, Reference Babcock and Feldmann1986a, Reference Babcock and Feldmannc; Van Iten, Reference Van Iten1992a). The transverse ribs traditionally have been characterized as sites of gradual thickening of individual lamellae, but they have also been interpreted as sites of a discrete, nonlaminate internal rod (Babcock and Feldmann, Reference Babcock and Feldmann1986a, Reference Babcock and Feldmannc; Feldmann and Babcock, Reference Feldmann and Babcock1986). Although the latter hypothesis has since been falsified (see John et al., Reference John, Hughes, Galaviz, Gunderson and Meyer2010 and references cited therein), the traditional interpretation still suffers from a lack of clarity regarding the number, composition, and scale of the lamellae involved in thickening. Internal carinae at the corners and midlines of the conulariid periderm likewise have received varied interpretations of formation and function. Thought by some authors to have been embedded within a gastric septum (midlines) and to have served as attachment sites for a longitudinal retractor muscle (e.g., Kiderlen, Reference Kiderlen1937; Moore and Harrington, Reference Moore and Harrington1956a; Chapman, Reference Chapman1966; Werner, Reference Werner1966a, Reference Wernerb, Reference Werner1967; Van Iten, Reference Van Iten1991b, Reference Van Iten1992b; Van Iten et al., Reference Van Iten, Fitzke and Cox1996, Reference Van Iten, Moussa and Yahaya2008), these structures were formed through localized inflection and thickening of fine lamellae. Again, however, detailed information on the number and character of the lamellae actually involved in the formation of internal carinae has been lacking.
The immediate purpose of the present study was to determine the presence or absence of organic matter, either as an organic matrix incorporated in mineralized lamellae or as discrete lamellae between mineralized layers, and to describe and interpret in greater detail the microstructure and growth of the transverse ribs and internal carinae. The ultimate goal of this investigation was to provide additional data for use in making anatomical comparisons of conulariids and other taxa, including coronate scyphozoans.
General morphology
The steeply pyramidal conulariid periderm usually exhibits four sides or faces but in rare cases has three, five, or six of these features (e.g., Babcock et al., Reference Babcock, Feldmann and Wilson1987). The wide end of the periderm is termed the aperture, and the opposite end, if preserved, tapers into a near point or to a nonornamented apical wall, or schott (Fig. 2.1), produced when the periderm was severed (Van Iten, Reference Van Iten1991a). The aperture on certain specimens exhibits lappets that evidently were flexible in life, capable of folding inward to close the periderm (e.g., Moore and Harrington, Reference Moore and Harrington1956a, Reference Moore and Harringtonb; Van Iten et al., Reference Van Iten, Moussa and Yahaya2008; Sendino et al., Reference Sendino, Zàgoršek and Vhylasová2011; Fig. 1).

Figure 2 Principal gross anatomical features of the conulariid periderm. (1) Partial flattened periderm showing the external surface of two faces, with a magnified view of external features in the inset. Images courtesy of the American Museum of Natural History (AMNH FI 33018 SYN [left image] and AMNH FI 25043 HOLO [inset]); (2) transverse section through part of an inflated periderm illustrating the laminar structure along with a corner sulcus and carina and a midline carina. E=external; I=internal.
In many conulariids, including most species of Conularia Miller in Sowerby, Reference Sowerby1821 and Paraconularia Sinclair, Reference Sinclair1940, the faces exhibit regular transverse ridges (ribs) that arch toward the aperture and bear a single row of more or less regularly spaced swellings, called nodes (e.g., Moore and Harrington, Reference Moore and Harrington1956a; Babcock and Feldmann, Reference Babcock and Feldmann1986c; Van Iten, Reference Van Iten1992a). The trough-like area between two adjacent transverse ribs is called the interspace. Some conulariids (e.g., all species of Metaconularia Foerste, Reference Foerste1928) lack transverse ribs and exhibit instead adaperturally arching rows of closely spaced nodes. The midline is a longitudinal feature on the face, sometimes simply the midpoint of the arching ribs or node rows, but sometimes more prominent; in species bearing transverse ribs, for example, the midline may be an actual break in the continuity of the ribs and may even mark where the ribs end and become offset. The corner groove, or sulcus, is a longitudinal furrow that runs the entire length of the periderm. Again in many species, the midlines and/or corner sulci are sites of inwardly projecting carinae (e.g., Barrande, Reference Barrande1867; Bischoff, Reference Bischoff1978; Van Iten, Reference Van Iten1991a, Reference Van Iten1992a, Reference Van Itenb; Jerre, Reference Jerre1994; Van Iten et al., Reference Van Iten, Fitzke and Cox1996; Fig. 2.2). The midline carinae follow more or less the same longitudinal path as the midline on the exterior test surface.
Materials and methods
Sample selection
Transmitted light and scanning electron (SEM) imaging were performed on partial specimens (apex and apertural margin missing) of four North American species: Conularia splendida Billings, Reference Billings1866 (Upper Ordovician, lower Maquoketa Formation, Iowa), C. trentonensis Hall, Reference Hall1847 (Upper Ordovician, Trenton Group, New York State), Paraconularia missouriensis (Swallow, Reference Swallow1860) (Lower Mississippian, Fort Payne Formation, Tennessee), and Paraconularia sp. (Lower Mississippian, Fort Payne Formation, Tennessee). Conularia and Paraconularia are two of the most common and widespread conulariid genera of the early–middle Paleozoic and the late Paleozoic, respectively (e.g., Babcock and Feldman, Reference Babcock and Feldmann1986b, Reference Babcock and Feldmannc; Van Iten et al., Reference Van Iten, Moussa and Yahaya2008; Lucas, Reference Lucas2012). Samples examined here came from the middle and apertural regions of the periderm, well above the former apex. In addition to possessing node-bearing transverse ribs, the conulariids also exhibit an internal carina at the midlines and/or corners. All of our samples are housed in the paleontological collections of the Cincinnati Museum Center, Department of Invertebrate Paleontology (CMC 71408-71416).
Using low magnification reflected light microscopy, we also examined 10 specimens of Archaeoconularia slateri (Reed, Reference Reed1933) (Upper Ordovician, Scotland) exhibiting plicated closure. This and other species showing plicated closure (Van Iten et al., Reference Van Iten, Moussa and Yahaya2008) have been featured in previous discussions (e.g., Moore and Harrington, Reference Moore and Harrington1956b) of the composition and original mechanical properties of the conulariid periderm.
Sample preparation
North American specimens were embedded in clear epoxy and then cut with a low-speed serial section saw (blade lubricated with a 50-50 mixture of deionized water and ethylene glycol) to produce thick slices oriented either parallel (longitudinal sections) or perpendicular (transverse sections) to the long axis of the periderm. Slices prepared for SEM imaging were mounted on aluminum stubs and then ground, polished (with 0.3 µm aluminum oxide powder), and cleaned ultrasonically in deionized water. In addition, the composition of some of these samples was determined using an electron microscope equipped with an energy dispersive elemental analyzer. This work was performed mainly to determine whether the conulariids had undergone diagenetic modification including silicification. To ascertain the distribution of organic material within the periderm, the mounted slices were etched and then subjected to critical point drying, a technique that preserves acid-resistant organic matter in its normal relationship to (formerly) mineralized skeletal material. Following the procedures of Clark (Reference Clark1980), the mounts were first soaked in deionized water for 60 seconds and then etched in dilute (0.1 N) HCl for 15 to 60 seconds. In general, this procedure should dissolve calcium phosphate, leaving behind organic matrix and acid-resistant mineralization. The stubs were then removed from the acid, immersed sequentially in two deionized water baths for a minimum of 60 seconds each, and then immersed in a series of ethanol solutions of 50%, 75%, 95%, and 100% (respectively), again for 60 seconds. Transfer of mounts from one solution to another was done while maintaining a fluid meniscus on the periderm samples, thus preventing soft structures from collapsing under surface tension.
Critical point drying also was conducted following the steps presented by Clark (Reference Clark1980). First, the sample chamber was filled with 100% ethanol. Next, the sealed chamber with specimen mounts was purged with liquid CO2 for four minutes and then soaked with CO2 for another four minutes. This pair of steps was repeated four times. For the fourth and final purging/soaking, a container of water was heated to 45°C. After the CO2 source was shut off, the chamber was immersed in the warm water to reach critical point at approximately 1,400 psi. Critical point was maintained for at least 10 minutes to ensure sample equilibration. Following this step, the CO2 was bled off gradually, allowing at least one minute for each 300 PSI pressure drop to prevent uneven pressure changes within fractures and to minimize turbulence along sample surfaces.
From each conulariid specimen, we also extracted samples that were etched with HCl but dried in air. This material was examined using SEM to determine whether surface features revealed by critical point drying were weak and flexible and thus composed of organic matter. More specifically, wet, soft organic material exposed by etching of the mineral component will suffer collapse (see Clark, Reference Clark1980, pl. 1, figs. b, c, Reference Clark1999b, fig 6.) under the pressure of surface tension as water evaporates in air. Following drying, all specimen mounts were stored in a low-humidity environment to prevent deterioration of the organic component. Specimen mounts for SEM imaging and energy dispersive elemental analysis (EDS) were coated with gold palladium. EDS analysis was conducted using a Hitachi model S-3500N scanning electron microscope at an accelerating voltage of 20 Kv. Low voltage (3.5 Kv) secondary electron imaging was conducted using a Hitachi model S-3000N instrument to minimize beam penetration and consequent washing out of images.
Repositories and institutional abbreviations
Examined specimens of A. slateri are housed in the paleontological collections of the Hunterian Museum in Glasgow, Scotland (GLAHM) and the British Geological Survey in Edinburgh, Scotland.
Results
Preservation
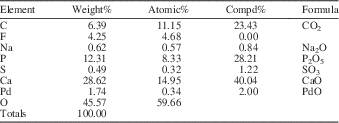
EDS analysis of selected periderm samples produced strong peaks for carbon (C), calcium (Ca), oxygen (O), and phosphorus (P), a weaker peak for fluorine (F), but no peaks for silicon (Si), indicating that silicification of original carbonate apatite was not a factor in their preservation (Appendix). In addition, transmitted light microscopy revealed a very fine crystal structure accompanied by nonrandom sweeping polarization (Fig. 5.4–5.5). Together, the fine crystal structure and nonrandom polarization indicate that our samples have not undergone gross recrystallization as this process would have replaced original crystalline material with larger, nonoriented crystals.
Microlamellae
Examined conulariids are constructed of extremely thin, more or less distinct lamellae (Figs. 3, 4, 5.6), here termed microlamellae, that range from approximately 0.5 to 3 µm thick and are either parallel are concordant with the external and internal surfaces of the periderm (Figs. 4.5, 5.1, 5.2). No other microscopic features that could be interpreted as variations in incremental growth (i.e., growth lines) were observed (see also Van Iten, Reference Van Iten1992a). On etched samples, in places where the microlamellae are most distinct (Figs. 3.1–3.4, 3.6–3.8, 5.1, 5.2, 5.5), protruding microlamellae that are largely undissolved alternate with shallow gaps of similar width and geometry. In areas where this microstructure is less clear (e.g., Figs. 3.3 (the far left side), 4.3), the distinction between protruding microlamellae and intervening gaps is reduced or lost, owing to either increase or decrease in the ratio of void space to undigested periderm within individual microlamellae. Examination of air-dried comparison samples (Fig. 3.5, 3.6) shows that the protruding microlamellae have collapsed to form gelatinous-looking mats, demonstrating (along with their resistance to acid etching) the soft organic nature of these layers, which largely escaped acid digestion. The narrow, variably developed gaps between them correspond to microlamellae or portions thereof that were mostly phosphatic, or organic-poor, and thus were largely dissolved during etching. Extending across the gaps are very slender strands of organic matter that, like the organic-rich microlamellae, resisted etching (Fig. 3.7, 3.8). Judging from the relative proportions of acid-resistant material and void space in SEM photomicrographs (e.g., Fig. 5.6), organic matter accounted for more than 50% of the total volume of the periderm. The high relative abundance of this organic matter, and its appearance as an interconnected meshwork (microlamellae plus cross-connecting strands or fibers), in varying proportions relative to the dissolved mineral component, allows us to refer to it as ‘organic matrix.’ This is the term applied to the organic component found in the shells and skeletons of many calcium carbonate biomineralizers, including preservation in their fossils (Mann, Reference Mann2001).

Figure 3 Scanning electron photomicrographs (secondary electron mode) of microlamellae in samples of conulariid periderm prepared using HCl etching and either critical point or air drying. (1–4) Samples prepared using critical point drying: (1) CMC 71416 (Paraconularia missouriensis; interspace, longitudinal section); (2) CMC 71411 (Conularia splendida; interspace, transverse section); (3) CMC 71413 (Paraconularia sp.; interspace, longitudinal section); (4) CMC 71408 (C. trentonensis; transverse rib, longitudinal section). (5, 6) air-dried comparison samples: (5) sample complimentary to CMC 71413 (Fig. 3.3); (6) sample complimentary to CMC 71408 (Fig. 3.4). (7, 8) Details of microlamellae, showing extremely slender strands of organic matrix (arrows) crossing the narrow gaps (organic-poor microlamellae) left by acid etching: (7) CMC 71413; (8) CMC 71416.

Figure 4 Scanning electron photomicrographs (secondary electron mode) of acid-etched and critical point dried samples of Paraconularia. (1–4) CMC 71413 (Paraconularia sp.): (1) transverse section through an interspace; (2) longitudinal section through an interspace; (3) detail of a transverse section through a corner carina, showing gradual thickening (vertical white bars on either side of the arrows) of an organic-rich microlamina (between the arrows) toward the axial plane of the carina; (4) gradual thickening of the same microlamina, from the opposite side of the corner carina. (5) Longitudinal section through a transverse rib of CMC 71416 (P. missouriensis).

Figure 5 Thin-section preparations of conulariid periderm samples showing well-developed macrolamellae. (1, 2) Transverse sections viewed in plane polarized light: (1) CMC 71412 (Paraconularia sp.; specimen flattened, with two corner groove carinae nearly touching each other); (2) different specimen (CMC 71413) of the same species, with one corner groove carina visible. (3–5) Thin sections of CMC 71413 viewed in slightly cross-polarized light: (3) longitudinal section through two transverse ribs, showing that the upper two macrolamellae appear relatively dark in the interspaces but bright where they thicken within the ribs (hachures in the accompanying line drawing indicate relatively dark macrolamellae or portions thereof); (4) transverse section through a single midline carina; (5) transverse section through an internal corner carina. (6) SEM image (secondary electron mode) of an etched and critical point dried preparation corresponding to Fig. 5.5. E=external; I=internal.
In addition to variation in distinctness/organic matter content, microlamellae exhibit regular variation in thickness. Within the interspaces, both organic-rich and organic-poor microlamellae exhibit a uniform thickness that generally ranges from about 0.5 to 1.5 µm (Figs. 3.1, 3.3, 3.7, 3.8, 4.1, 4.2). Within the fold-like transverse ribs and internal carinae, organic-rich and organic-poor microlamellae widen gradually toward the axial plane of these structures, reaching a maximum thickness that is approximately two to three times their thickness within the interspaces (e.g., Fig. 4.3, 4.4). This difference is close to the difference in total thickness between the interspaces and the ribs/carinae and suggests further that all or most of the microlamellae passing through the latter structures undergo thickening. Thus, variation in the total thickness of the periderm can be accounted for solely by variation in the thickness of individual microlamellae; there is no need (or evidence) for insertion or intercalation of additional lamellae or other structures such as elongate rods. Within the core of both ribs and carinae, the distinction between organic-rich and organic-poor microlamellae may be reduced (Fig. 4.3, 4.4). In our material this appears to have been caused by an increase in the amount of organic matter in microlamellae that, in the interspaces, underwent dissolution and thus were organic poor in these areas. However, in a nonetched polished section through a single transverse rib illustrated by Van Iten (Reference Van Iten1992a, pl. 1, fig. 5), reduced distinctness of microlamellae within this structure appears to have been caused by an increase in the amount of mineral matter in microlamellae that were organic rich in areas flanking the rib (see discussion of lamellar microstructure in the following).
Macrolamellae
Macrolamellae (Figs. 4.5, 5) are relatively thick (approximately 5–75 µm) layers, commonly variable in color and/or degree of transparency, that are visible at low magnifications in transmitted light and probably correspond to the lamellae described in earlier, pre-SEM investigations. Comparisons of transmitted light and corresponding SEM images suggest that macrolamellae consist of bundles of microlamellae of similar composition (organic matter/mineral matter ratio) and, possibly, growth history (see discussion that follows). For example, in CMC 71412 (Paraconularia sp.; Fig. 5.1), relatively dark macrolamellae (transmitted light) alternate with macrolamellae that are bright. In addition, the macrolamellae are approximately equal in thickness and are continuous in both transverse and longitudinal sections. In another specimen of Paraconularia sp. (CMC 71413; Fig. 5.2–5.5), the macrolamellae vary in thickness and show less continuity than in CMC 71412, especially the dark macrolamellae within the transverse ribs (see also Van Iten, Reference Van Iten1992a, text-fig. 2). The same macrolamellae are visible in corresponding SEM images (e.g., Fig. 5.6), in which the macrolamellae differ from each other in the distinctness of their component microlamellae. In macrolamellae where the microstructure is relatively indistinct, there is either a predominance of organic material over (dissolved) apatite, or vice versa. However, there does not seem to be a close correspondence between this ratio and the appearance (bright versus dark) of the macrolamellae in transmitted light. Finally, and again in CMC 71413, dark macrolamellae within the interspaces fan out and disappear within the core of some of the transverse ribs, with macrolamellae on one side of the core reappearing and tapering back together on its opposite side (Fig. 5.3; see also Van Iten, Reference Van Iten1992a, pl. 1, fig. 5). The bright macrolamellae, by contrast, appear to thicken.
Discussion
Organic matrix
Several previous investigators (e.g., Moore and Harrington, Reference Moore and Harrington1956b; Babcock and Feldmann, Reference Babcock and Feldmann1986a, Reference Babcock and Feldmannc) have stated that the conulariid periderm is composed of calcium phosphate and organic material. Moore and Harrington (Reference Moore and Harrington1956b), for example, stated that the periderm contains chitin and chitinophosphate, while Babcock and Feldmann (Reference Babcock and Feldmann1986a, Reference Babcock and Feldmannc) stated that it consists of fine phosphatic lamellae alternating with lamellae composed of protein. Relying primarily on high magnification SEM imaging of acid-etched material, we have found that organic material is present in conulariids as a matrix in combination with soluble mineral matter. Organic matrices in shells and skeletons in general are thought to have served as templates for biomineralization (Mann, Reference Mann2001) as well as adding tensile and elastic strength to the mineral component, thus forming a strong biocomposite material. In conulariids the organic matrix also distinguishes the lamellae by enriching some with organic matter and leaving others more mineralized. This effect can be seen at the smallest scale in the microlamellae, the fundamental structural unit of the conulariid periderm (Van Iten, Reference Van Iten1992a).
The presence of organic matrix in our conulariid samples contradicts the notion, once widely accepted (Cuif et al., Reference Cuif, Dauphin and Sorauf2011), that this material generally should not survive fossilization or last for hundreds of millions of years except under unusual circumstances such as entombment in asphalt. Moreover, previous studies (Weiner et al., Reference Weiner, Lowenstam and Hood1976; Clark, Reference Clark1980, Reference Clark1993, Reference Clark1999a; Gupta et al., Reference Gupta, Michels, Briggs, Evershed and Pancost2006) across phyla have yielded results, consisting either of chemical data or microstructural characteristics, that collectively suggest that preservation of original skeletal organic material may be fairly widespread in the fossil record. Such material has been detected, for example, in dinosaur bones (Schweitzer et al., Reference Schweitzer, Wittmeyer, Horner and Toporski2005; Bertazzo et al., Reference Bertazzo, Maidment, Kallepitis, Fearn, Stevens and Xie2015), and moreover, Bertazzo et al. (Reference Bertazzo, Maidment, Kallepitis, Fearn, Stevens and Xie2015) noted that the preservational quality of the dinosaur specimens in their study was not exceptional. Similarly, Clark (Reference Clark1999b) concluded that geological age was not an important factor in organic matrix preservation in general. It appears then that preserved organics may be substantially more common than previously thought, with this material being sufficiently resistant to survive not only in weathered and fragmented dinosaur bones, but also in mollusk fossils dating back to the Pennsylvanian (Clark, Reference Clark1999a) and organic matrix fragments of other taxa from the early Cambrian (Clark, Reference Clark2009, Reference Clark2012).
Lamellar microstructure
In describing the structure of conulariid skeletons, previous investigators (e.g., Barrande, Reference Barrande1867; Moore and Harrington, Reference Moore and Harrington1956b; Babcock and Feldmann, Reference Babcock and Feldmann1986c) have applied the term ‘lamella’ to layer structures that range in scale from microscopic to plainly visible to the naked eye. These differences have been attributed to original variation between species (Brood, Reference Brood1995), but the results of our study suggest that they may actually reflect different concepts of what constitutes a single lamella, a situation we should expect to see given the advances in imaging technology that have occurred during the period in question. Thus, the earliest research (Barrande, Reference Barrande1867; Bouček and Ulrich, Reference Bouček and Ulrich1929; Moore and Harrington, Reference Moore and Harrington1956b) and some later work (Kozlowski, Reference Kozlowski1968; Bischoff, Reference Bischoff1978; Brood, Reference Brood1995) were undertaken using transmitted light microscopy at relatively low magnifications. Our results, obtained using high magnification SEM imaging, suggest that most earlier investigations dealt with characteristics of macrolamellae.
Later workers (Van Iten, Reference Van Iten1991a, Reference Van Itenb, Reference Van Iten1992a, Reference Van Itenb, Reference Van Iten, Leme, Simões, Marques and Collins2006b; Jerre, Reference Jerre1994; John et al., Reference John, Hughes, Galaviz, Gunderson and Meyer2010) also relied mainly on scanning electron microscopy, but their reports still may describe a combination of what we term macrolamellar and microlamellar characteristics. In addition, most previously conducted SEM imaging was performed on polished sections. Without etching it is difficult to discern the thinnest microlamellae (see for example Jerre, Reference Jerre1994, fig. 3), though extremely thin lamellae have been detected in other phosphatic skeletons using backscattered electron imaging (e.g., Muscente and Xiao, Reference Muscente and Xiao2015). Macrolamellae, by contrast, can be distinguished even on polished sections (Fig. 5.6). The alternating ‘dense’ and ‘vacuity-bearing’ microlamellae observed in polished sections by Van Iten (Reference Van Iten1992a) probably were accentuated by the organic-rich microlamellae being preferentially removed by the polishing agent, owing to their being softer than the organic-poor (more mineralized) lamellar structures. Thus, the dense microlamellae probably represent more mineralized portions of the test, and the vacuity-bearing microlamellae represent the more organic-rich portions. Consequently, results obtained using unetched polished sections are opposite to those obtained using etched sections: in etched sections the dense lamellar structures are organic- rich while vacuity-bearing lamellar structures are more mineralized, the original calcium phosphate having been digested.
Growth
As stated in the introduction, previous authors have posited that growth of conulariids involved accretion of whole lamellae to the inner surface of the periderm (centripetal accretion) as well as extension or lengthening of the periderm by addition of new material along its apertural margin (marginal growth). However, neither we nor previous investigators have detected evidence of marginal growth in conulariids in the form of growth lines inclined to the external or internal surface of the periderm (see for example Raup and Stanley, Reference Raup and Stanley1978, fig. 3–1). Still, based on analysis of macroscopic patterns of deviation from normal ornamentation in cleft and scalloped ‘injuries’ (Babcock et al., Reference Babcock, Feldmann and Wilson1987), Van Iten (Reference Van Iten1992a) proposed that marginal growth of an outermost, nonmineralized layer (or set of layers), not preserved in conulariid fossils, was followed by thickening of the periderm through accretion of apatitic lamellae (the alternating organic-poor and organic-rich microlamellae here observed) to its inner surface. Under this hypothesis, then, cleft and scalloped injuries are nonpenetrative, surface features, produced by deposition of microlamellae on the inner surface of damaged or malformed portions of the former nonmineralized external layer. In conulariids, any external growth lines, such as transverse ribs, may well have been formed as structural elements having no particular relationship to the individual’s growth other than the occasional need for space (as with the varices of some gastropods). We might further infer that any ornamentation on the exterior of the periderm originally was related to folds in the ectoderm as the initial microlamella was produced and as new microlamellae were added to the periderm during growth. Thus it would be in the interior of the periderm that the most meaningful growth lines would be found, in the alternations of the organic-rich and organic-poor microlamellae. These, properly termed ‘internal growth lines’ to avoid confusion with ‘external growth lines’ (see Clark, Reference Clark1974, fig. 1), would more closely reflect both growth rates and the influence of environmental changes on the organism. Any features on the interior of the periderm, particularly carinae, that are not parallel to the exterior surface must have formed through localized inflection and/or thickening of new microlamellae at those sites. Although at present we are unable to determine numerical growth rates for conulariids, if we make the plausible assumption that accreted microlamellae were produced at the rate of one per day, then because the interspaces of specimens here examined are as much as 0.6 mm thick (e.g., Fig. 5.1), our oldest specimens lived for well over one year, an estimate that agrees with observed life spans of up to two or three years for modern scyphozoan polyps (e.g., Arai, Reference Arai2009).
Van Iten (Reference Van Iten1992a, p. 363) described the growth of transverse ribs and internal carinae as involving “gradual, symmetrical thickening of individual lamellae.” Results of the present study corroborate this hypothesis as the observed degree of thickening of individual microlamellae is sufficient to account for the observed differences in thickness between transverse ribs/internal carinae and the interspaces. Still, some elaboration on the model of Van Iten (Reference Van Iten1992a) is required in order to better understand the growth of the conulariid periderm. For example, inspection of Figure 4.5, which includes a single transverse rib, suggests that there may be two macrolamellar-scale zones (Zones I and II) that differed from each other in the relative amounts of mineral and organic matter production. In Zone I it appears that accentuated production of apatite was the main contributor to peridermal thickening as there is more void space per unit volume in this region than in Zone II. In the latter zone, the lower volumetric density of void space suggests that increased production of organic matrix was the main contributor to peridermal thickening. According to Clark (Reference Clark1999a), similar patterns in other taxa may reflect more or less continuous production of organic matter accompanied by slowing or even cessation of mineral production. In turn, this kind of variation may have been caused by regular, periodic variation in the ambient environment or by random environmental stresses (Clark Reference Clark1974, Reference Clark1999a). Among the conulariid samples here examined, the specimen shown in Figure 5.1 (Paraconularia sp.), which exhibits multiple, alternating dark and bright macrolamellae of more or less uniform thickness, may record the strongest evidence of periodic variation in the ambient paleoenvironment.
Conulariid affinities
As mentioned earlier in this paper, several previous investigators have argued that the conulariid periderm is homologous to the chitinous periderm of polypoid coronate scyphozoans. Specific points of similarity include (1) periderm composed of extremely thin, mutually parallel or concordant microlamellae; (2) microlamellae produced through centripetal accretion and, possibly, extensional growth along the apertural margin; (3) inflection and/or thickening of microlamellae to produce seriated or continuous internal carinae at the interradii and/or perradii; (4) damage to the periderm, including punctures and severance, repaired by deposition of smooth (nonornamented) microlamellae (Van Iten, Reference Van Iten1992a). The results of the present study add a fifth similarity to this list, namely the presence in both coronates and conulariids of extremely slender organic pillars that cross-connect the chitinous (coronates) or organic-rich (conulariids) microlamellae (Fig. 3.7, 3.8). Using transmission electron microscopy, Chapman and Werner (Reference Chapman and Werner1972) demonstrated that the extremely thin (12 nm) coronate lamellae are separated by lamella-like gaps bridged by minute slender pillars. These pillars are similar in shape and arrangement to the processes here documented in conulariids, though as discussed, the gaps between the organic-rich microlamellae in conulariids are artifacts of the dissolution of skeletal apatite. Nevertheless, it would be interesting to see whether a similar microstructure is present in other taxa possessing lamellar skeletons and that have been allied with conulariids, including for example the extinct genus Sphenothallus Hall, Reference Hall1847 (e.g., Moore and Harrington, Reference Moore and Harrington1956a; Van Iten et al., Reference Van Iten, Cox and Mapes1992; Vinn and Kirsimäe, Reference Vinn and Kirsimäe2014; Muscente and Xiao, Reference Muscente and Xiao2015).
To be sure, a number of noncnidarian invertebrate taxa, including for example certain groups of brachiopods, produce or produced an apatite-based exoskeleton exhibiting a lamellar microstructure defined by alternating apatitic and organic microlamellae (Williams, Reference Williams1997). Furthermore, and like conulariids, the valves of organo-phosphatic brachiopods exhibit thread-like pillars of hollow apatite, postulated to have formerly contained organic matter cross-connecting the organic lamellae (Williams, Reference Williams1997). Thus, the additional microstructural similarity pointed out here between conulariids and coronate scyphopolyps may not be unique to these two groups, though it does not detract from hypotheses of homology between their periderms. Moreover, uniquely shared macroscopic similarities between these two taxa (e.g., Van Iten et al., Reference Van Iten, Leme, Simões, Marques and Collins2006b) make it unlikely that conulariids were more closely related to brachiopods or other noncnidarian groups than they were to coronate scyphozoans. In short, while we have clarified certain basic aspects of the structure and composition of the conulariid periderm, our results may not in fact shed much additional light on the question of the phylogenetic affinities of this group.
Mechanical properties
The central discovery of this investigation, namely that the conulariid periderm consists of submicron-thick, alternating organic-rich and organic-poor microlamellae, may bear on the behavior of this structure under directed stress. Similar structures, termed ‘composite materials,’ have one component with good compressional strength (in conulariids, the apatite mineral) and another with good tensile and elastic strength (in conulariids, the organic matrix), giving the entire structure exceptional resistance to stresses of all kinds (Jackson et al., Reference Jackson, Vincent and Turner1988; Mann, Reference Mann2001). Composite biominerals in general have evolved in nearly all phyla, with varying proportions of mineral and organic components. Mollusk shells, for example, are approximately 95% calcium carbonate, reflecting their need for compressive strength, but the 5% of organic matrix still serves to dramatically increase their elasticity and tensile strength, making the shells as much as 3,000 times as resistant to fracture as the pure mineral. The effect is present under tensile stresses as well; under wet conditions (as in life), mollusk nacre—mother of pearl—can be 10 times as strong as the pure mineral.
Although mollusk shells are flexible in a structural sense, their relatively great thickness and very high ratios of mineral to organic components preclude the type of flexibility we might expect from the thin, organic-rich conulariid periderm. There is a wide range of flexibility in the shells of crustaceans, but they also show a wide range of mineralization, in terms of both time (state of ecdysis) and place (carapace vs. joints). Although vertebrate bones show similar variation in their mineral-to-organic ratios, they do have the same mineral (apatite) as conulariids. Bones have been studied extensively, and there are some data on relationships between mineral-to-organic ratios and flexibility. Mann (Reference Mann2001), for example, presents evidence that flexibility (the inverse of Young’s modulus) varies directly with relative organic content.
As documented by previous investigators, certain relatively thin-walled conulariids show evidence of original flexibility, having crumpled or folded under directed shear or compressional stress rather than breaking (e.g., Moore and Harrington, Reference Moore and Harrington1956b, fig. 24.1) or showing triangular or lobate facial lappets folded neatly inward along rectilinear, smoothly rounded bands of flexure (e.g., Moore and Harrington, Reference Moore and Harrington1956b, fig. 24.2, 24.3; Kowalski, Reference Kowalski1935, pl. 7, figs. 1, 2; Van Iten et al., Reference Van Iten, Moussa and Yahaya2008, fig. 1.5). Some species, including Archaeoconularia slateri, exhibit plicated closure (Moore and Harrington, Reference Moore and Harrington1956a), in which the apertural end has been completely covered by highly regular, inward bending of the periderm below the apertural ends of the corners, again along smoothly curved bands of flexure. The midlines of A. slateri, a species lacking transverse ribs, are sites of a low internal carina, the thickest part of the periderm. However, even in specimens showing plicated closure, the carina, rather than being broken, is smoothly curved as it crosses the base of the lappet (Fig. 1). Although plicated closure in this species has been documented by previous investigators (e.g., Reed, Reference Reed1933, fig. 2a, c), until now no one had noted that this folding also involved internal carinae, which at first glance might be thought to have been relatively rigid structures incapable of undergoing such high strain without breaking. Our discovery of the presence of organic-rich microlamellae in other conulariids helps to make sense of these previous gross morphological observations. Evidently the internal midline carina of A. slateri had a high enough organic content to permit repeated elastic bending and closure of the apertural portion of this structure in life, presumably with reopening of the aperture involving relaxation of longitudinal retractor muscles and elastic rebound of the periderm along the lines of flexure.
Summary and conclusions
-
1. Together with transmitted light and SEM observations of previous investigators, our results demonstrate that conulariids exhibit little variation in basic peridermal microstructure. All specimens of Conularia and Paraconularia here examined consist of extremely thin (approximately 0.5 to 3 µm), variably distinct, mutually parallel or concordant microlamellae that are alternately organic rich and organic poor. Organic-rich microlamellae are cross connected by slender fibers of the same material, and together with the microlamellae they constituted an organic matrix, which presumably served as a template for the production of skeletal apatite. Microlamellae in relatively thick specimens may be grouped in coarse (approximately 5 to 75 µm) macrolamellae, which vary in the relative proportions of original organic and mineral matter.
-
2. Distinguishing between microlamellae and macrolamellae helps to clarify apparent discrepancies between previous characterizations of conulariid microstructure, with earlier investigators having relied either on light microscopy or SEM. Microlamellae are readily discerned in etched specimens prepared for secondary electron imaging, while macrolamellae are best observed using transmitted light (plane polarized or nonpolarized), in which organic-rich macrolamellae are relatively dark in color.
-
3. The observed thickening of individual microlamellae in transverse ribs and internal carinae generally supports Van Iten’s (Reference Van Iten1992a) previous interpretations of the formation of these gross anatomical structures. The additional information documented here illuminates how this occurred, namely through deposition of additional mineral matter or through thickening of organic-rich microlamellae, depending possibly on environmental stresses.
-
4. Etched specimens that have been processed by critical point drying preferentially display the organic microlamellae and cross-connecting organic fibers left by dissolution of calcium phosphate in the organic-poor (mineralized) microlamellae.
-
5. Finally, the organic-rich biocomposite forming the conulariid periderm appears to have given this structure a high degree of flexibility, enabling relatively thin periderms to crumple or fold (rather than break) under directed shear or compressional stress and to undergo highly regular, presumably repeated inward folding (plicated closure with elastic rebound) of the apertural region.
Acknowledgments
This study is based mainly on a master’s thesis in geology completed by RCF at Kansas State University, with advisement by the two junior authors and with overview by A. Archer, S. Datta, and K. Miller. RCF thanks D. Frederick and J. Deibert (Department of Geology, Austin Peay State University, Tennessee) for permission to use their department’s SEM and thin section equipment, and S. Greenwood for assistance in the field. Use of the scanning electron microscope at Hanover College (Indiana) was supported by a grant to HVI from the Hanover College Faculty Development Committee. HVI also thanks J. Liston and M.T. Dean for permission to borrow specimens of Archaeoconularia slateri in the paleontological collections of the Hunterian Museum, Glasgow (JL) and the British Geological Survey, Edinburgh (MTD). Finally, constructive comments by N.C. Hughes (University of California Riverside) and U. Balthasar (University of Glasgow, Scotland) improved our manuscript substantially.
Appendix
Results of energy dispersive spectroscopy (EDS) analysis of a portion (secondary electron image) of one of the conulariid samples (CMC 71413) examined in the present study.