Introduction
Upper Ordovician strata, especially shallow-water carbonate rocks, are widespread in northern Laurentia (east-central Alaska and Arctic Canada north of the Ordovician paleoequator), but the brachiopods in these rocks are rather poorly known compared with those reported widely from southern Laurentia (south of the Ordovician paleoequator). In fact, much of our knowledge of Late Ordovician brachiopods of North America came from southern Laurentia, although Richmondian brachiopods are commonly present in northern Laurentia and commonly known as the ‘Arctic fauna’ of paleoequatorial affinity (Nelson, Reference Nelson1959). Within Laurentia, for example, large-shelled pentameroid brachiopods, such as the latest Katian virgianids Proconchidium Sapelnikov in Nikolaev and Sapelnikov, Reference Nikolaev and Sapelnikov1969 and Tcherskidium Nikolaev and Sapelnikov, Reference Nikolaev and Sapelnikov1969 (see Rong et al., Reference Rong, Jones and Nentwich1989), were present only north of the paleoequator, although these forms were abundant in the adjacent tectonic plates (e.g., Siberia and Baltica). Systematic study of these brachiopods has been hampered by two main factors that make fossil collection difficult: the remoteness of potential fossil sites in the Arctic and the common dolomitization of thick carbonate strata. So far, most detailed reports of diverse brachiopod faunas of the Late Ordovician northern Laurentia were from the Mackenzie Mountains, northwestern Canada (Wigington, Reference Wigington1977; Mitchell, Reference Mitchell1978; Jin and Lenz, Reference Jin and Lenz1992) and from the Ogilvie Mountains, Charley River A-1 1:63,360 scale quadrangle, east-central Alaska (Ross and Dutro, Reference Ross and Dutro1966).
In 1992, a spot bulk collection of brachiopods was made from Upper Ordovician strata in an unnamed Cambrian(?)–Ordovician limestone unit (denoted on map as COl) of Brabb (Reference Brabb1970), exposed in several curvilinear bands in the northeastern part of the Black River D-1 1:63,360 scale quadrangle, east-central Alaska (Fig. 1). The fauna reported here occurs as a distinctive east–west trending band of outcrops that bear silicified shells. Acid digestion of the rock yielded fairly abundant brachiopods, with a mixture of normal-sized Late Ordovician shells and micromorphic forms. This band of exposures is surrounded by sedimentary strata of Paleozoic (primarily Silurian and Devonian) age. The main objective of this study is to provide a systematic description of this moderately diverse brachiopod collection and to explore its paleobiogeographic implications.

Figure 1. Locality map of the study area within the Black River D-1 1:63,360 scale quadrangle, east-central Alaska. (1) Google map showing sample locality (66°52′24″N, 141°02′14″W, marked by red drop pin) near the Alaska–Yukon border. (2) Sample locality on detailed topographic map.
Regional geologic setting
The triangular area in east-central Alaska, bounded roughly on the north by the Porcupine River, on the south by the Yukon River, and to the east by the Alaska–Yukon border, is the only portion of Alaska that does not belong to tectonically accreted terranes (Blodgett et al., Reference Blodgett, Rohr and Boucot2002). The Paleozoic rocks of this region appear to represent the westernmost extent of the North American miogeocline from Yukon and the Northwest Territories, and the megafossils of east-central Alaska are very similar to those of northwestern Canada. Nevertheless, this triangular area was divided by Silberling et al. (Reference Silberling, Jones, Monger, Coney, Berg and Plafker1994) into two tectonic elements: (1) the Porcupine terrane, which includes rocks northwest of the Kandik Basin, and (2) North American rocks equivalent to the Nation Arch of the older literature. The Porcupine terrane, considered here merely as a northerly extension of the Nation Arch region, includes a thick succession of Paleozoic rocks exposed in the Black River and southern part of the Coleen quadrangles and extends eastward into Yukon Territory. These rocks were referred to by Morrow and Geldsetzer (Reference Morrow and Geldsetzer1989) as the Porcupine platform, an isolated structurally high region. The Porcupine platform is equivalent to the Yukon stable block of Lenz (Reference Lenz1972). Few papers have been published on the Paleozoic fossil fauna of the Porcupine platform although rich faunas are generally known to be present in its Ordovician–Devonian strata as well as in upper Paleozoic rocks. This platform was the locus of shallow-water carbonate sedimentation during much of the early and middle Paleozoic.
In recent paleogeographic reconstructions, the study area was located in the border zone between Laurentia and subsequently accreted terranes (see summary in Cocks and Torsvik, Reference Cocks and Torsvik2011). The brachiopod collection in this study, albeit of moderate size and diversity, provides a unique opportunity for investigating the characteristics of the brachiopod fauna living ‘on the edge’ of Laurentia during the Late Ordovician, as well as its paleobiogeographic implications. These conclusions are also supported by the limited previous faunal studies in lower and middle Paleozoic strata of the Black River 1:250,000 scale quadrangle. This includes the presence of the early Middle Ordovician gastropod Palliseria (Rohr and Blodgett, Reference Rohr and Blodgett1994), a genus restricted to North America, primarily to the Great Basin and western Canada, although a few occurrences have been noted as well in Oklahoma and the state of Chihuahua in Mexico. Lower Devonian brachiopods described and listed from the Salmontrout Limestone by Lane and Ormiston (Reference Lane and Ormiston1979) are also typical faunal forms of northwestern Laurentia (present-day coordinates) in Yukon Territory and Northwest Territories (Blodgett et al., Reference Blodgett, Rohr and Boucot2002).
Paleobiogeographic implications
In this study, 20 late Katian brachiopod faunas from Laurentia (mostly of Richmondian age) and other paleotropically located tectonic plates or terranes are used for a multivariate analysis using the PAST software package (Hammer et al., Reference Hammer, Harper and Ryan2001). The faunas have been well documented in recent systematic descriptions or taxonomic updates, making them suitable for quantitative analysis. A binary data set is constructed on the basis of the presence or absence of a genus in each of the faunas. The dendrogram (Fig. 2), generated using Simpson similarity coefficient (paired group), identifies a distinct Laurentia faunal province, composed mostly of faunas from epicontinental inland basins, in agreement with the results of previous studies (e.g., Candela, Reference Candela2014; Jin et al., Reference Jin, Sohrabi and Sproat2014). With the caveat of its relatively low diversity (nine genera) from a spot collection with inherent collection bias, the faunule from east-central Alaska notably clusters closely with Laurentia and seems to have a closer biogeographic affinity to Laurentia than does the fauna of Scotland, which was a peri-Laurentia terrane in the Ordovician (Cocks and Torsvik, Reference Cocks and Torsvik2011).

Figure 2. Cluster analysis of the late Katian (Richmondian) brachiopod faunas from Laurentia and other tectonic plates, using the software package PAST (Hammer et al., Reference Hammer, Harper and Ryan2001; Simpson similarity coefficient, paired group). Note that the small brachiopod sample from Alaska shows closest faunal affinity with those from Laurentia. Anticosti Island, Quebec, Vaureal Formation. Cincinnati Arch, Richmondian strata in Ohio, Kentucky, and Indiana. Farewell Terrane, upper Katian strata, western Alaska. Hudson Bay, Churchill River Group. Manitoba, Stony Mountain Formation, southern Manitoba. Mackenzie Mountains, western marginal platform; lower Whittaker Formation. Iowa, upper Maquoketa Group. Texas, Montoya Group, Trans-Pecos Texas. Tennessee, Fernvale Formation. Oklahoma, Unit 3C, Viola Springs Formation. Wyoming, Bighorn Dolomite. Avalonia: England–Wales–southern Ireland. Baltica, Estonia and Lithuania, Pirgu Stage. Boda Limestone, Sweden, Baltica. Gorny Altai, Orlov Horizon. Kazakhstan, upper Chokpar Formation and Dulankara Horizon, Dulankara. Scotland, Girvan district. Sette-Daban, Siberian Platform, Nirunda and Bur horizons. South China, Xiazhen Formation, JYC area. Taimyr, Siberian Platform, Korotkinskaya Formation. (For details, see Supplementary Data.)
Late Ordovician brachiopods of Laurentia differentiated into a distinct pericratonic fauna and an intracratonic fauna. The pericratonic fauna, characterized by the Scoto-Appalachian fauna, thrived from latest Darriwilian to early Katian in plate-margin seas around Laurentia and had a strong paleobiogeographic affinity to Baltica (for summaries, see Jaanusson and Bergström, Reference Jaanusson and Bergström1980; Sohrabi and Jin, Reference Sohrabi and Jin2013; Candela, Reference Candela2014). By the late Katian, however, this fauna became drastically diminished in the pericratonic seas of Laurentia. In the middle–late Katian, much of the Laurentia interior was inundated by marine transgression, and a strongly endemic intracratonic brachiopod fauna became predominant in the epicontinental and inland seas (see Jin et al., Reference Jin, Sohrabi and Sproat2014 for summary). Despite its endemism at the global scale, the intracratonic brachiopod fauna had a high degree of homogeneity in generic composition in the epicontinental seas of Laurentia, with some subtle paleoecological and paleolatitudinal gradients detectable especially at the species level. The large shells of Plaesiomys accidentalis (Okulitch, Reference Okulitch1943), for example, have a distinct trilobed cardinal process and dense aditicules along rib crests, and the species has been interpreted to be a typical taxon in the late Katian equatorial to low-tropical latitudes. Its presence in the brachiopod faunule from east-central Alaska can be regarded as a strong indicator of its biogeographic affinity to Laurentia.
The occurrence of Tcherskidium in the study area was part of a large-shelled virgianid pentameride fauna in northern Laurentia. Tcherskidium and Proconchidium often formed recurrent, thick, and largely monospecific brachiopod shell banks in north-central Alaska, northern Baffin Island, and North Greenland (Rong et al., Reference Rong, Jones and Nentwich1989; Harper et al., Reference Harper, Jin, Rasmussen, Rasmussen and Stouge2007). This type of coquina, usually dominated by congeneric species, was widespread in northern Baltica, pericratonic Siberia, Kolyma, and smaller terranes between Siberia and Laurentia (e.g., Nikolaev, Reference Nikolaev1974; Sapelnikov, Reference Sapelnikov1985; Nikiforova, Reference Nikiforova1989; Modzalevskaya, Reference Modzalevskaya, Nekhorosheva and Sobolevskaya2018). At present, it is poorly understood what paleoecological and/or paleogeographical factors controlled the distribution of large-shelled virgianids and confined them to northern Laurentia. In other tectonic plates, these large virgianids occurred predominantly in the paleoequatorial zone and northward, suggesting that these brachiopods lived exclusively in shallow, warm tropical seas devoid of cool-water upwelling. Southern Laurentia, albeit tropically located during the Late Ordovician, experienced frequent cool-water invasions from Gondwana (e.g., Pope and Reed, Reference Pope and Read1997), where a continental ice sheet was accumulating. By comparison, all the tectonic plates and terranes in the Northern Hemisphere were located between the midtropics and the equator, and there were no landmasses in the high latitudes or the north polar region to accumulate an ice sheet and to generate significant cold currents to invade the northern tropics (Cocks and Torsvik, Reference Cocks and Torsvik2011). If this interpretation holds true, the large-shelled virgianids in the late Katian would have been a sensitive indicator of warm seas.
The highly diverse Late Ordovician brachiopod fauna from western Alaska (Farewell terrane) was reported and interpreted by Rasmussen et al. (Reference Rasmussen, Harper and Blodgett2012) to be of deepwater origin. That Christiania–Leptellina-bearing western Alaskan fauna had a few taxa in common with the latest Darriwilian–earliest Katian Scoto–Appalachian fauna but showed little affinity with the epicontinental intracratonic late Katian brachiopod faunas analyzed in this study.
Materials and methods
This study is based on a spot collection gathered by Blodgett (his locality 92ABd8) on June 12, 1992, from the unnamed Cambrian?–Ordovician limestone (COl) unit of Brabb (Reference Brabb1970). Gastropods from this locality were earlier described by Rohr et al. (Reference Rohr, Blodgett and Frederick2016) and were assigned a New Mexico Musuem of Natural History locality number (NMMNH 10296 in Rohr et al., Reference Rohr, Blodgett and Frederick2016). This locality is situated in the NE1/4 SE1/4 SE1/4 sec. 29, T 24 N, R 31 E, Black River D-1 1:63,360 scale quadrangle of east-central Alaska (66°52′24″N, 141°02′14″W), approximate elevation 3,360 ft. (1,024 m) according to the helicopter altimeter (Fig. 1). Acid digestion of the limestone samples yielded finely silicified fossils, including normal-sized as well as minute juvenile forms of brachiopods. The relatively abundant gastropods included a large-shelled operculum of Maclurites (see Rohr et al., Reference Rohr, Blodgett and Frederick2016). Macluritids are common also in the Middle and Upper Ordovician strata of epicontinental seas in Laurentia. Other silicified fossils that were observed, but not collected, in rubble blocks of limestone include abundant tabulate corals and bulbous stromatoporoids.
Repository and institutional abbreviation
All the brachiopod specimens used in this study are housed in the Geological Survey of Canada (GSC), Ottawa.
Systematic paleontology
Order Orthida Schuchert and Cooper, Reference Schuchert and Cooper1932
Superfamily Orthoidea Woodward, Reference Woodward1852
Family Hesperorthidae Schuchert and Cooper, Reference Schuchert and Cooper1931
Genus Hesperorthis Schuchert and Cooper, Reference Schuchert and Cooper1931
Type species
Orthis tricenaria Conrad, Reference Conrad1843. Decorah Shale (lower Katian), Wisconsin (Rice, Reference Rice1987).
Hesperorthis pyramidalis (Twenhofel, Reference Twenhofel1928)
Figure 3.1–3.12
- Reference Twenhofel1928
Orthis davidsoni var. pyramidalis Twenhofel, p. 174, pl. 15, figs. 4–6.
- Reference Jin and Zhan2008
Hesperorthis pyramidalis (Twenhofel); Jin and Zhan, p. 11, pl. 1, figs. 1–19.
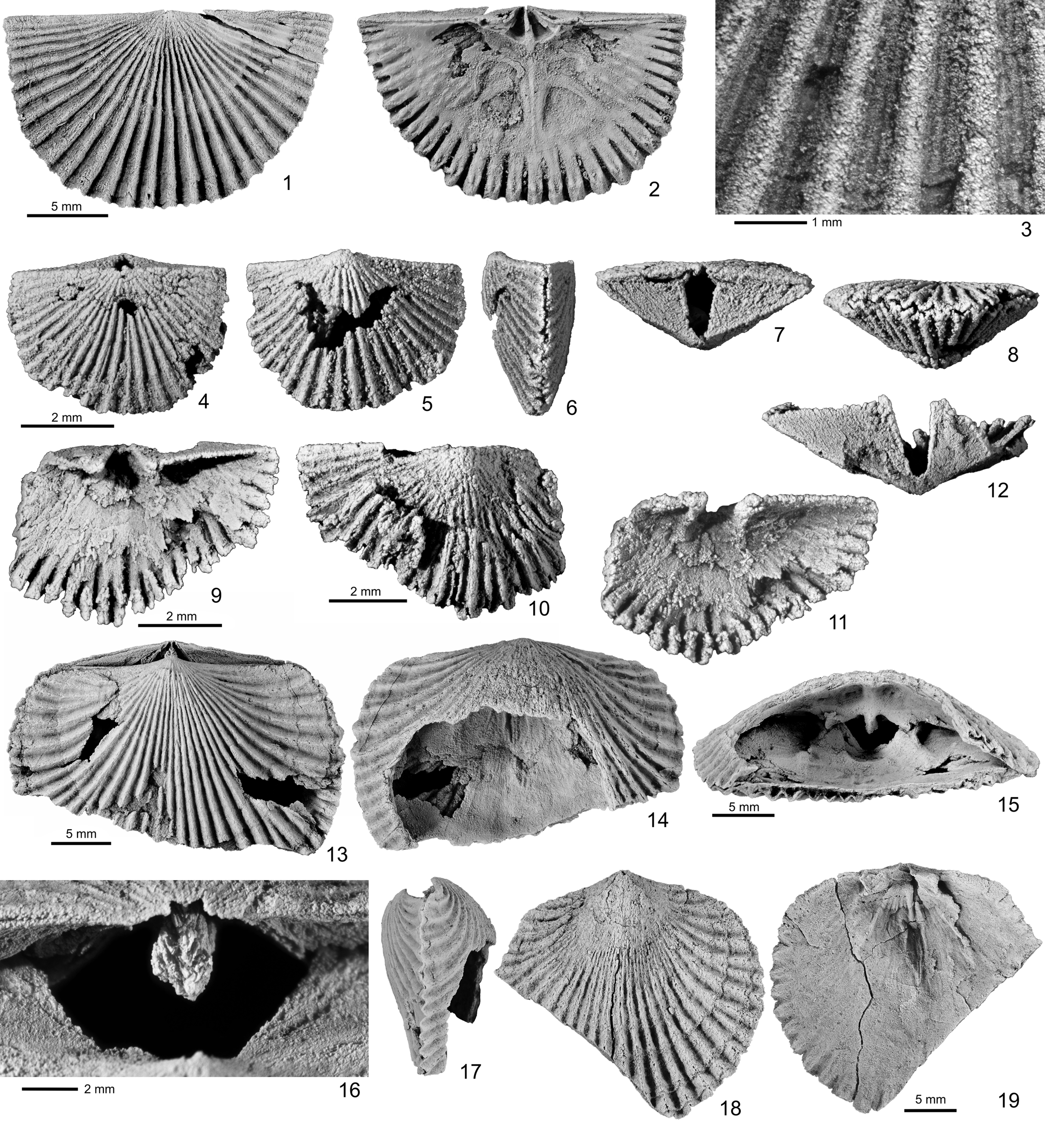
Figure 3. Silicified orthoids from unnamed upper Katian strata, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska. (1–12) Hesperorthis pyramidalis (Twenhofel, Reference Twenhofel1928); (1–3) GSC 131804: (1) exterior and (2) interior of dorsal valve, with (3) local enlargement to show capillae in interspaces; (4–8) GSC 131805: (4) dorsal, (5) ventral, (6) lateral, (7) posterior, and (8) anterior views of small shell, with nearly catacline ventral interarea; (9–12) GSC 131806: (9) interior, (10) exterior, (11) oblique anterior, and (12) posterior views of small ventral valve. (13–19) Plaesiomys occidentalis (Okulitch, Reference Okulitch1943), locality 92ABd8; (13–17) GSC 131807: (13) ventral, (14) dorsal, (15) anterior, (16) posterior, and (17) lateral views of anteriorly damaged shell; (18, 19) GSC 131808: (18) exterior and (19) anterior views of ventral valve.
Types
Holotype YPM 10419, Lousy Cove, Ellis Bay Formation (Twenhofel, Reference Twenhofel1928). Jin and Zhan (Reference Jin and Zhan2008) noted that species was most common in the aulacerid biostrome unit, lower Prinsta Member, basal Ellis Bay Formation, lower Hirnantian.
Description (Alaska material)
Shells represented mostly by immature forms; small conjoined shells having average length of 3.5 mm, width 4.9 mm, and thickness 2.1 mm (see Appendix). A large dorsal valve attaining length of 12.3 mm, width 20.3 mm. Shell outline semicircular, planoconvex to strongly ventribiconvex; greatest shell width attained at hinge line; anterior commissure rectimarginate to slightly sulcate (Fig. 3.1, 3.8). Costae coarse, rounded, simple or with rare bifurcation or asymmetrical intercalation of second-order costellae, reaching a total of 30 per valve; Capillae well developed, better preserved in interspaces than on ribs, normally two or three in each interspace toward anterior margin of shell (Fig. 3.3); growth lines fine but poorly preserved due to silicification.
Ventral exterior
Valve moderately deep, pyramidal, with uniform curvature, lacking fold or sulcus; interarea high, with apical angle of 125°–128°, approaching catacline (Fig. 3.6); surface of interarea mostly planar, becoming slightly incurved only toward beak, bearing fine, longitudinal striae. Delthyrium high, relatively narrow, essentially open, covered only apically by minute pseudodeltidium (Fig. 3.7, 3.12).
Dorsal exterior
Valve flat, without sulcus or fold; interarea very low, attaining approximately one-third of ventral interarea height, anacline. Notothyrium open, and no antigydium observed in available specimens.
Ventral interior
Teeth shaped like small knobs; dental plates robust, high, but confined to umbonal cavity and not extending beyond hinge line (Fig. 3.9, 3.11). Muscle field small, confined to umbonal area, deep posteriorly, not clearly impressed in silicified specimens.
Dorsal interior
Notothyrial cavity not raised above valve floor but bounded anteriorly by raised ridge. Cardinal process simple, blade-like (Fig. 3.2). Brachiophores seemingly short, probably due to incomplete preservation. Dorsal muscle field larger, extending beyond midlength of valve, occupying about one-third of valve width, with low but well-defined myophragm; larger anterior pair of adductor muscle scars separated from smaller posterior pair by transverse ridges (Fig. 3.2).
Materials
Conjoined shells (1); ventral valves (6, mostly incomplete); dorsal valves (13, mostly incomplete).
Remarks
The Alaskan material is assigned to Hesperorthis pyramidalis on the basis of the high, planar ventral interarea and the generally simple, rounded costae with prominent capillae, which are best preserved in the only large specimen available in the collection. In the smaller conjoined shells from Alaska, the ventral-valve apical angle is about 10° greater than that of the adult shells from Anticosti Island, probably because of the lower ventral interarea in immature shells. Some of the thinner costella-like ribs (e.g., Fig. 3.10) were the result of preservation artifact when the crest of a rib was not preserved during silicification, giving the hollow ribs a superficially split appearance. In its type area of Anticosti Island, H. pyramidalis is known only from the lower Hirnantian beds of the Ellis Bay Formation (Jin and Zhan, Reference Jin and Zhan2008). The Alaskan material reported herein extends its range to the upper Katian (Richmondian). Hesperorthis sp. from the upper Katian of the Farewell terrane of Alaska (Rasmussen et al., Reference Rasmussen, Harper and Blodgett2012) has more sparsely spaced costae and strong growth lines, but it does not have any clearly observable capillae as shown by the Alaskan material in this study.
Family Plaesiomyidae Schuchert, Reference Schuchert and von Zittel1913
Subfamily Plaesiomyinae Schuchert, Reference Schuchert and von Zittel1913
Genus Plaesiomys Hall and Clarke, Reference Hall and Clarke1892
Type species
Orthis subquadrata Hall, Reference Hall1847. Richmond Group, Richmondian (upper Katian), Ohio (type specimens and type horizon discussed by Howe, Reference Howe1966).
Remarks
The taxonomic relationship between Plaesiomys and Dinorthis Hall and Clarke, Reference Hall and Clarke1892 has been in a state of change. Whereas Plasiomys was treated as a subgenus of Dinorthis in the classic work of Schuchert and Cooper (Reference Schuchert and Cooper1932), Dinorthis was regarded as a subgenus of Plaesiomys by Williams and Wright (Reference Williams, Wright and Moore1965) in the original Treatise on Invertebrate Paleontology. Williams and Harper (Reference Williams, Harper and Kaesler2000) considered the two as separate genera, as has been the case in most North American literature since the 1960s.
The presence of epipunctae has been confirmed in some key North American plaesiomyids (e.g., Plaesiomys, Retrosirostra Schuchert and Cooper, Reference Schuchert and Cooper1931, and Austinella Foerste, Reference Foerste1909; see Jin et al., Reference Jin, Zhan, Copper and Caldwell2007; Jin and Zhan, Reference Jin and Zhan2008; Jin and Copper, Reference Jin and Copper2010; Sproat and Jin, Reference Sproat and Jin2013). It remains to be determined, however, whether this seemingly diagnostic character for plaesiomyids is present in the type material and type species of Dinorthis. The commonly silicified specimens of Dinorthis pectinella (Emmons, Reference Emmons1842) in North America have made this determination difficult.
In this study, Plaesiomys is treated as an independent genus from Dinorthis, and the recognition of Plaesiomys occidentalis (Okulitch, Reference Okulitch1943) follows the concept of Sproat and Jin (Reference Sproat and Jin2013).
Plaesiomys occidentalis (Okulitch, Reference Okulitch1943)
Figures 3.13–3.19, 4
- Reference Okulitch1943
Pionorthis occidentalis Okulitch, p. 71, pl. 1, figs. 8–10.
- Reference Okulitch1943
Pionorthis cf. P. carletona (Twenhofel, Reference Twenhofel1928); Okulitch, pl. 1, fig. 7.
- Reference Ross1957
Dinorthis (Plaesiomys ?) cf. D. (P.) occidentalis; Ross, pl. 37, figs. 16, 19, 20, 23.
- Reference Ross1957
Dinorthis (Pionorthis ?) cf. D. (P.) occidentalis; Ross, pl. 37, figs. 17, 18, 21, 22.
- Reference Ross1957
Dinorthis (Pionorthis ?) n. sp. Ross, pl. 38, figs. 1, 2, 5, 6.
- 1957
Dinorthis (?) sp. Ross, pl. 38, figs. 3, 4, 7, 8, 11.
- Reference Macomber1970
Plaesiomys (Dinorthis) occidentalis; Macomber, p. 430, pl. 75, figs. 12–15; pl. 76, figs. 1–27.
- Reference Jin, Caldwell and Norford1997
Dinorthis occidentalis; Jin et al., p. 21, pl. 1, figs. 13–16; pl. 2, figs. 1–17.
- Reference Jin and Zhan2001
Dinorthis occidentalis; Jin and Zhan Reference Jin and Zhan2001, p. 17, figs. 6–9; pl. 1, figs. 1–22; pl. 2, figs. 1–13; pl. 3, figs. 1–5; pl. 21, fig. 2.
- Reference Jin, Zhan, Copper and Caldwell2007
Dinorthis occidentalis; Jin et al., p. 678, fig. 10.1–10.5.
- Reference Sproat and Jin2013
Plaesiomys occidentalis; Sproat and Jin, p. 884, fig. 14A–K.
Types
Holotype GSC 2043 and paratype GSC 2043a, by original designation, Gunn Member, Stony Mountain Formation (upper Katian), Stony Mountain, southern Manitoba.
Description (Alaskan material)
Shell subcircular to subrectangular in outline, slightly wider than long. Largest specimen (dorsal valve) attaining length of 28.6 mm, width of 32.7 mm, and valve depth of 9.3 mm (see Appendix). Lateral profile equibiconvex to weakly dorsibiconvex. Hinge line straight, wide, extending for about two-thirds of shell width; cardinal extremities rounded. Ventral valve moderately convex in umbonal area, becoming flattened in peripheral portions. Ventral interarea apsacline, with small, incurved beak; delthyrium open (Figs. 3.15, 3.16, 4.1). Dorsal valve evenly convex except for faint sulcus-like medial depression in some specimens. Dorsal interarea approaching orthocline, with minute, erect beak and open notothyrium (Figs. 3.13, 3.15–3.17, 4.3, 4.4, 4.7, 4.10). Shell costae simple, rounded, of uniform strength from apex to anterior margin. Some costae may bifurcate in anterior part of shell, more common in relatively large shells. Fine concentric growth lines best preserved in anterior portion of shell; coarser growth lamellae sparse, present close to shell margin. Aditicules densely spaced, arranged in single column along crest of each rib (Fig. 4.5, 4.9). Epipunctae present but poorly preserved in silicified shells.
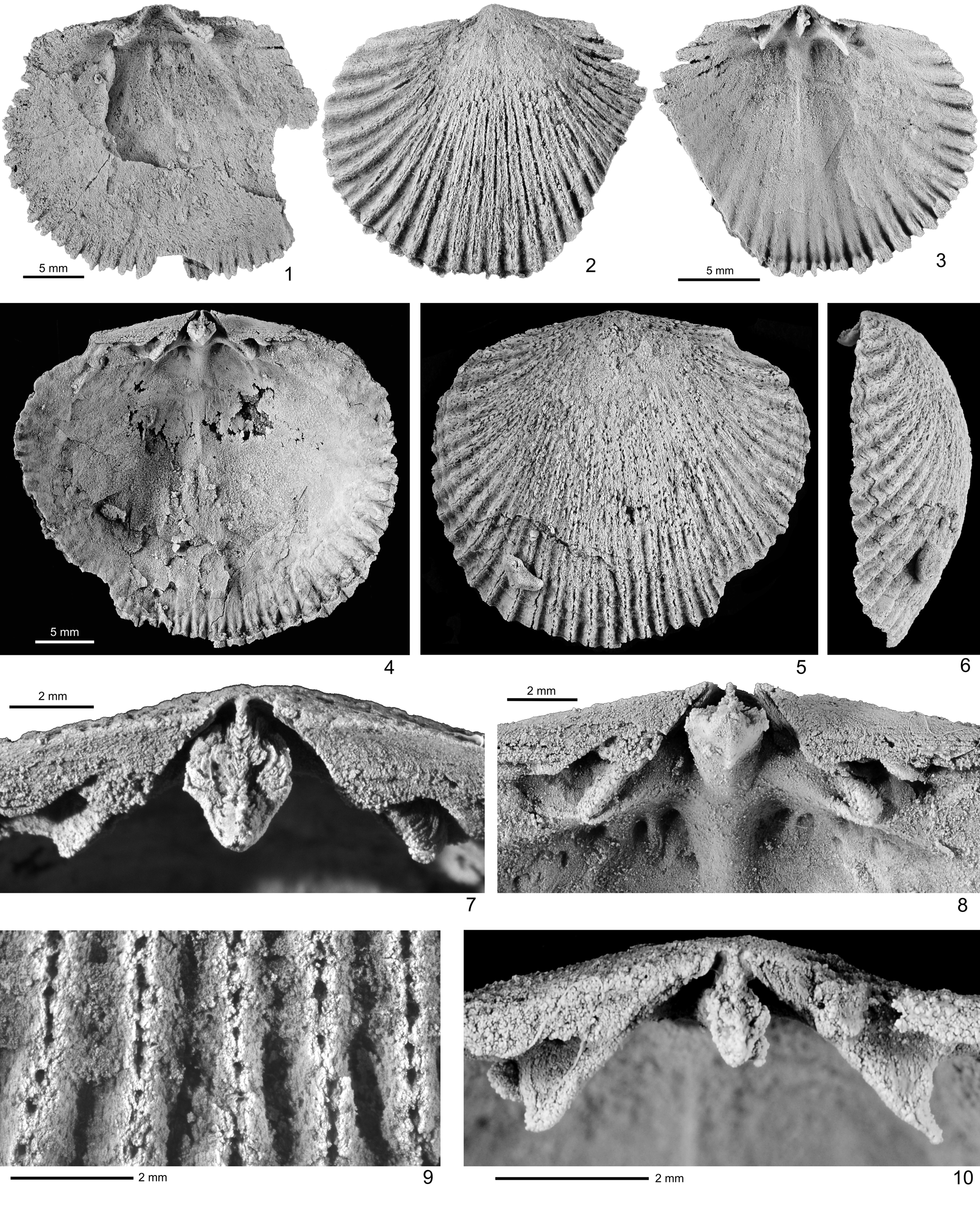
Figure 4. Silicified Plaesiomys occidentalis (Okulitch, Reference Okulitch1943) from unnamed upper Katian strata, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska. (1) GSC 131809, interior of ventral valve, with large muscle field and slender, elongate oval adductor scars enclosed by diductor scars laterally and anteriorly; (2, 3, 10) GSC 131810: (2) exterior and (3) interior of medium-sized dorsal valve and (10) local enlargement of cardinalia; (4–9) GSC 131811: (4) interior, (5) exterior, and (6) lateral views of dorsal valve; (7–9) local enlargement of (7, 8) trilobed cardinal process and (9) dense, single-column aditicules per rib.
Ventral interior
Dental plates low, thickened, extending anteriorly beyond hinge line as plate-like posterolateral bounding ridges of muscle field. Lateral cavities may be filled by secondary shell thickening. Muscle field large, subrectangular in outline (Figs. 3.19, 4.1), occupying slightly over one-third of valve width and approximately one-half of shell length; adductor muscle scars relatively slender, elongate oval, more deeply impressed than diductor muscle scars, bearing thin medial and lateral ridges. Diductor muscle scars wider anteriorly, enclosing adductor scars anteriorly, but anterior margin poorly delimited. Marginal crenulations strong but confined to a band of 2–3 mm (Figs. 3.19, 4.1, 4.3, 4.4).
Dorsal interior
Notothyrial platform clearly thickened and raised above valve floor, delimited by raised anterior edge. Dental sockets deep, open anteriorly. Cardinal process robust, distally swollen to develop trifid myophore (Fig. 4.4, 4.7, 4.8, 4.10) in large shells, bearing chevron-patterned crenulations on top, and leaving only narrow gap on each side of notothyrial cavity. Brachiophores short, strong, diverging from each other at about 83°, flanking notothyrial platform anteriorly, extending into short processes distally. Adductor muscle field quadrilobate (Fig. 4.3, 4.4); medial ridge (myophragm) thick posteriorly, tapering toward anterior margin of muscle field; transverse ridge (myophragm) faint; posterior margin of each posterior adductor scar bearing two short longitudinal ridges (Fig. 4.8); anterior margin of muscle field poorly defined. Marginal crenulations similar to those of ventral valve.
Materials
Conjoined shells (two, incomplete); ventral valves (three); dorsal valves (two); fragments (six).
Remarks
The maximum shell size in the Alaskan material is nearly identical to that of P. occidentalis described by Jin and Zhan (Reference Jin and Zhan2001), especially in their well-developed and closely spaced aditicules and the inflated, trifid myophore of the cardinal process. These characters, together with relatively rare bifurcation of ribs, are characteristic of warm-water species of Plaesiomys in contrast to the cool-water species (typified by more intense rib bifurcation, more widely spaced aditicules, and bilobed cardinal process) found in higher tropics of the Late Ordovician, such as Anticosti Island and the Cincinnati Arch region (see Sproat and Jin, Reference Sproat and Jin2013 for a detailed discussion).
Order Strophomenida Öpik, Reference Öpik1934
Superfamily Plectambonitoidea Jones, Reference Jones1928
Family Sowerbyellidae Öpik, Reference Öpik1930
Genus Eoplectodonta Kozlowski, Reference Kozlowski1929
Type species
Sowerbyella precursor Jones, Reference Jones1928, p. 437; lower Llandovery, Wales.
Eoplectodonta sp.
Figure 5.9, 5.10
Remarks
Only one ventral valve is present in the collection. It lacks well-preserved surface ornaments because of either taphonomic abrasion or incomplete silicification. The valve has a preserved length of 16.3 mm and width 20.8 mm (Fig. 5.9). It is assigned to Eoplectodonta on the basis of its strong convexity, an arched pseudodeltidium, groove-and-ridge-type denticles on the denticular plates in the medial portions of the hinge line (Fig. 5.10), and relatively large, rounded, bilobed muscle field. Unfortunately, no dorsal valve is available for study, which makes it reasonable to treat this taxon under open nomenclature.

Figure 5. Silicified strophomenoids from unnamed upper Katian strata, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska, unless noted otherwise. (1–8) Holtedahlina sp. (1–4) GSC 131812: (1) exterior, (2) lateral, (3) mirror-imaged interior view of half dorsal valve, and (4) local enlargement of external surface to show rounded multicostellae. (5–8) Manitoba Museum of Natural History specimen MMMN I-3283: (5) dorsal, (6) ventral, (7) lateral, and (8) anterior views of calcareous (nonsilicified) shell from lower Penitentiary Member, Stony Mountain Formation, Mariash Quarry, southern Manitoba, for comparison with specimen from Alaska. (9, 10) Eoplectodonta sp. GSC 131813: (9) exterior and (10) interior of exfoliated ventral valve. (11, 12) Leptaena sp. GSC 131814: (11) exterior and (12) interior views of dorsal valve.
Superfamily Strophomenoidea King, Reference King1846
Family Strophomenidae King, Reference King1846
Subfamily Strophomeninae King, Reference King1846
Genus Holtedahlina Foerste, Reference Foerste1924
Type species
Leptaena sulcata de Verneuil, Reference de Verneuil1848. Waynesville Formation (Richmondian), Indiana, USA.
Holtedahlina sp.
Figure 5.1–5.8
Remarks
Only one incomplete dorsal valve is in the collection for study. It is 17.7 mm long, with an estimated width of 28.0 mm (by mirror imaging of the preserved portion; Fig. 5.1, 5.3) and a depth of 5.3 mm. It is assigned to Holtedahlina under open nomenclature on the basis of its moderate convexity, relatively strong multicostellae with a rounded crest, and strong, imbricated growth lamellae (Fig. 5.4). The shell size, shape, and surface ornaments closely resemble those of a well-preserved Holtedahlina shell from the Stony Mountain Formation (lower Penitentiary Member, upper Katian) of southern Manitoba (Fig. 5.5–5.8). The internal structures are similar to those of Strophomena, with posteriorly directed, bilobed cardinal process, recurved socket ridges (Fig. 5.2, 5.3). One long, strong side septum is preserved. Vascula genitalia well impressed in the posterolateral portions of the valve as anterolaterally splaying grooves and ridges (Fig. 5.3).
Subfamily Leptaeninae Hall and Clarke, 1894
Genus Leptaena Dalman, Reference Dalman1828
Remarks
Only one poorly preserved dorsal valve is available for study. It is a relatively large specimen in the collection, 19.4 mm long and 28.3 mm wide (Fig. 5.11). It has distinctly differentiated disk and geniculated trail as characteristic of Leptaena, although concentric rugae have been obscured partly by silicification of the shell and rock matrix. Internally, the two lobes of the cardinal process are clearly discrete and ventrally directed, and the rounded adductor muscle field is delimited by a ridge, also typical of Leptaena (Fig. 5.12). Unfortunately, further morphological details are lacking for identification to a specific level.
Order Pentamerida Schuchert and Cooper, Reference Schuchert and Cooper1931
Suborder Pentameridina Schuchert and Cooper, Reference Schuchert and Cooper1931
Superfamily Pentameroidea M'Coy, Reference M'Coy1844
Family Virgianidae Boucot and Amsden, Reference Boucot and Amsden1963
Genus Brevilamnulella Amsden, Reference Amsden1974
Type species
Brevilamnulella thebesensis Amsden, Reference Amsden1974.
Brevilamnulella minuta new species
Figure 6
Types
Holotype, GSC 131815 (Fig. 6.1–6.5), upper Katian strata from “unnamed Cambrian?-Ordovician limestone unit” (Brabb, Reference Brabb1970), locality 92ABd8 (see Materials and methods for details), Black River D-1 1:63,360 scale quadrangle, east-central Alaska. Figured paratypes, GSC 131816–131821 (Fig. 6.6–6.20), same strata and locality.

Figure 6. Silicified Brevilamnulella minuta n. sp. from unnamed upper Katian strata, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska. (1–5) GSC 131815, holotype: (1) dorsal, (2) ventral, (3) lateral, (4) posterior, and (5) anterior views. (6–10) GSC 131816, paratype: (6) dorsal, (7) ventral, (8) lateral, (9) posterior, and (10) anterior views of minute, weakly elongate shell. (11, 12) GSC 131817, paratype: (11) interior and (12) tilted anterior views of dorsal valve, showing basolaterally inclined inner hinge plates. (13) GSC 131818, paratype, interior of dorsal valve, with crus well preserved. (14–16) GSC 131819, paratype: (14) interior, (15) anterior, and (16) lateral views of posterior part of ventral valve, showing relatively strong spondylium and median septum. (17, 18) GSC 131820, paratype: (17) interior and (18) anterior views of incomplete ventral valve. (19, 20) GSC 131821: (19) anterior and (20) interior views of small dorsal valve.
Diagnosis
Minute, subrhomboidal, equibiconvex shells, with low ventral beak and incipient ventral interarea. Spondylium rather large relative to shell size, broad V-shaped, supported by robust median septum. Inner hinge plate forming flange at junction with crural base; outer hinge plates basolaterally inclined; crura rod-like.
Description
Shell small, subrhomboidal in outline, with tapering posterior and maximum width located in anterior half of shell, equibiconvex in lateral view; largest complete shell in collection 5.3 mm long, 5.5 mm wide, and 2.6 mm thick (see Appendix). Hinge line very short; anterior margin rectimarginate. Ventral valve moderately and evenly convex, with low, suberect beak; ventral interarea minuscule, moderately apsacline (Fig. 6.3, 6.4, 6.8, 6.9); delthyrium open. Dorsal umbo may be as high as ventral umbo; dorsal interarea also incipient, open, nearly orthocline; delthyrium open. Shell surface smooth, without ribbing, fold, or sulcus.
Ventral interior
Spondylium large relative to shell size, broadly V-shaped, attaining maximum length of 1.5 mm, opening width of 1.5 mm, and bottom width of 0.6 mm in relatively large specimens (Fig. 6.14–6.18); median septum strong, slightly longer than spondylium along its junction with valve floor but receding dorsally and thus not supporting distal portion of spondylium.
Dorsal interior
Cardinalia relatively small, confined largely to posterior 1 mm of valve. Inner hinge plates triangular in outline, deflected ventrolaterally from each other at about 90° (Fig. 6.11–6.13, 6.19, 6.20). Outer hinge plates lower and smaller than inner hinge plates, basolaterally tilted from each other at about 55° (Fig. 6.12, 6.19). Crural base forming flange at junction with inner and outer hinge plates, extending into short, rod-like crura distally (Fig. 6.13, 6.20).
Etymology
From the Latin minutus, denoting the tiny shells of the new species.
Materials
Twelve conjoined shells (mostly minute); ventral valves (172, predominantly posterior portions with spondylium); dorsal valves (57, mostly posterior portions with cardinalia).
Remarks
The Alaskan shells are assigned to Brevilamnulella on the basis of its small, smooth shells and proportionally small cardinalia. In its minute, posteriorly tapering shell, the new species can be distinguished easily from all other known species of Brevilamnulella. All the conjoined shells and incomplete valves in the fairly large sample are similar in size to, or smaller than, the specimens figured in this study. B. minuta n. sp. shows some similarity to immature shells of the type species B. thebesensis and Viridita lenticularis Jin and Copper, Reference Jin and Copper2000 (see Jin and Copper, Reference Jin and Copper2010). Brevilamnulella thebesensis, however, is not known to have a posteriorly tapered shell during early ontogeny, nor does it have the basolaterally tilted inner hinge plates seen in the new species. The basolaterally inclined inner hinge plates are similar to the configuration of those in Clorinda (see Jin et al., Reference Jin, Caldwell and Norford1993; Jin and Copper, Reference Jin and Copper2000), but the Alaskan shells do not have the inflated ventral umbo or the clearly defined dorsal fold and ventral sulcus typical of Clorinda. This may be interpreted as a morphoplastic character in the late Katian Brevilamnulella, which have been interpreted as the ancestor to the early Silurian virgianid, stricklandiid, and clorindid lineages (Jin and Copper, Reference Jin and Copper2010; Rasmussen et al., Reference Rasmussen, Ebbestad and Harper2010). Similarly, Brevilamnulella kjerulfi (Kiaer, Reference Kiaer1902) reported by Rasmussen et al. (Reference Rasmussen, Ebbestad and Harper2010, p. 142, fig. 6) from the uppermost Katian strata (later revised to be from the upper Hirnantian by Kröger et al., Reference Kröger, Ebbestad, Lehnert, Ullmann, Korte, Frei and Rasmussen2015) of the Boda Limestone at the Osmundsberget Quarry, Sweden, may also have irregularly developed, basolaterally directly inner hinge plates.
Genus Tcherskidium Nikolaev and Sapelnikov, Reference Nikolaev and Sapelnikov1969
Type species
Conchidium (?) unicum Nikolaev, Reference Nikolaev and Balashov1968 (p. 47, pl. 65, figs. 1–3). Iryuda Formation (upper part), Iryuda Horizon, upper Katian, Omulevsk Mountains, northeast Siberia.
Tcherskidium tenuicostatus new species
Figure 7
Types
Holotype, GSC 131829 (Fig. 7.1, 7.2); figured paratypes, GSC 131830–131834 (Fig. 7.3–7.8); upper Katian strata of unnamed stratigraphic unit, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska.

Figure 7. Tcherskidium tenuicostatus n. sp. from unnamed upper Katian strata, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska. (1, 2) Holotype GSC 131829: (1) exterior and (2) interior view of ventral valve, naturally split along side wall of long median septum and spondylium. (3) Paratype, GSC 131830, transverse section of dorsal valve, showing inner and outer hinge plates and their smooth junction with crural base. (4, 5) Paratype, GSC 131831, transverse section of ventral valve showing strongly thickened shell wall and median septum and V-shaped spondylium with double lamellar-layered floor. (6) Paratype, GSC 131832, exterior of ventral valve showing fine simple costae. (7) Paratype, GSC 131833, interior of ventral valve showing part of spondylium and long median septum. (8) Paratype, GSC 131834, ventral valve split along median septum and one side of spondylium (with dark outline).
Diagnosis
Large, strongly ventribiconvex, very finely costate shells of Tcherskidium, with strong inner and outer hinge plates extending to level of the hinge line.
Description
Shell large, elongate oval, strong ventribiconvex, with maximum (preserved) length 50 mm, width 40 mm, and depth 25 mm of ventral valve (Fig. 7.1, 7.2); greatest width located around anterior third of shell; hinge line considerably shorter than maximum width of shell. Ventral valve evenly and strongly convex, lacking fold or sulcus, two to three times as deep as dorsal valve; with extremely thickened shell wall (5–10 mm) in umbonal part, resulting in complete fill of umbonal cavity in some large shells; ventral umbo rounded, inflated, and strongly arched, with strong, incurved beak; pseudodeltidium not observed. Dorsal valve much smaller and less convex than ventral valve, without fold or sulcus. Costae very fine, simple, with rounded crest and narrow interspace, without noticeable intercalation or bifurcation, averaging 14 per 10 mm in anterior part of shell. Growth lines poorly developed or preserved.
Spondylium long, deep, attaining roughly one-half depth and slightly over one-half length of ventral valve; V-shaped in cross section, but floor of spondylium consisting of narrow U-shaped basal lamellar layer, middle granular thickening, and V-shaped capping lamellar layer (Fig. 7.2, 7.4, 7.5). Median septum long, extending near anterior margin of valve; extremely thickened in posterior half, similar to posterior shell wall (Fig. 7.5). Teeth and socket structures weak (or poorly preserved). Inner and outer hinge plates much thinner and shorter than internal structures of ventral valve. Inner plates discrete but inclined slightly in basomedial direction (Fig. 7.3); outer hinge plates higher but shorter than inner hinge plates, receding before reaching level of hinge line. Crural base rod-like in cross section, fused smoothly with inner and outer hinge plates without forming flanges. Muscle scars not available for observation in studied specimens.
Etymology
From the Latin tenuis (fine, small) and costatus (ribbed), referring to the unusually fine shell ribs.
Materials
In addition to the figured holotype and paratypes, there are about 30 broken pieces of shells, predominantly of ventral valves, in the collection, all from the same locality 92ABd8.
Remarks
In Alaska, three forms of Tcherskidium have been reported from both accretionary terranes and the western margin of Laurentia. The material used to establish Tcherskidium tenuicostatus n. sp. was mentioned (without illustration) by Blodgett et al. (Reference Blodgett, Rohr and Boucot2002, p. 275). Another similar species with fine ribbing, a new Tcherskidium species of Blodgett et al. (Reference Blodgett, Rohr, Harris and Rong1988, fig. 4A–D, Reference Blodgett, Rohr and Boucot2002, fig. 3.1–3.5), was illustrated but not formally described from the Shublik Mountains, Mt. Michelson Quadrangle (part of the North Slope subterrane of the Arctic Alaska terrane). This yet unnamed new species is present also in Lone Mountain, McGrath Quadrangle, western central Alaska, belonging to the Nixon Fork subterrane of the Farewell terrane (Blodgett et al., Reference Blodgett, Rohr and Boucot2002, p. 275). These two previously unnamed species from Alaska occur in a shallow-water, inner carbonate platform facies rich in gastropods. The type species, Tcherskidium unicum (Nikolaev, Reference Nikolaev and Balashov1968), occurs commonly in Siberia (Kolyma, Taymyr, Novosibirsk Island; see summary by Sapelnikov, Reference Sapelnikov1985; Modzalevskaya, Reference Modzalevskaya, Nekhorosheva and Sobolevskaya2018), but has been recognized by Blodgett et al. (Reference Blodgett, Rohr and Boucot2002, p. 282) from the Baird Mountains Quadrangle of the northwestern Brooks Range.
Tcherskidium unicum is characterized by a large shell with high and strongly arched ventral umbo and relatively coarse ribs (usually 8–10 costae per 10 mm; e.g., Nikiforova, Reference Nikiforova1989; Modzalevskaya, Reference Modzalevskaya, Nekhorosheva and Sobolevskaya2018). The two species erected by Nikolaev (Reference Nikolaev1974), T. kovechovi and T. tchukoticum, were regarded as junior synonyms of T. unicum by Oradovskaya (Reference Oradovskaya and Sokolov1983) on the basis of her large collections from the Omulevsk and Chukotsk mountains because they are indistinguishable from the type species, especially in external morphology. The internal structures, however, suggest notable differences (Nikolaev, Reference Nikolaev1974): T. kovechovi has a much shorter median septum that recedes well short of the distal end of the spondylium and lacks inner hinge plates, whereas T. tchukoticum has better-developed inner and outer hinge plates than the other two Siberian species. At present, we suggest retaining the three Siberian species of Tcherskidium until a large number of specimens are studied in detail to determine the range of variations in internal structures.
Tcherskidium tenuicostata n. sp. can be easily distinguished from the three congeneric species from Siberia by its finer ribbing (average 14 costellae per 10 mm compared to 8–10 per 10 mm) and notably stronger inner and outer hinge plates that extend anteriorly to the level of the hinge line. In T. unicum, the inner hinge plates are extremely low and tend to disappear before reaching the level of the hinge line (Nikolaev, Reference Nikolaev1974, p. 67, fig. 1; Sapelnikov, Reference Sapelnikov1985, p. 40, fig. 19). In the ventral valve, the spondylial floor with double lamellar layers (Fig. 7.4) is observed in both T. tenuicostatus and T. unicum (compare with Sapelnikov, Reference Sapelnikov1985, p. 40, fig. 19).
Tcherskidium? sp. reported by Nikolaev (Reference Nikolaev1974) from the Selennyakh Range, northeast Siberia, questionably assigned to the Upper Ordovician, has well-developed inner and outer hinge plates as in T. tenuisostata, but the Siberian form differs in having a median septum that is much shorter than the spondylium, as in T. kovechovi.
Tcherskidium? ulkuntasensis Rukavishnikova and Sapelnikov, Reference Rukavishnikova and Sapelnikov1973 from the Holorhynchus bed of Chu-Ili Range of southern Kazakhstan has a notably smaller shell with less-numerous costae, quite unlike the typical Tcherskidium.
Order Rhynchonellida Kuhn, Reference Kuhn1949
Superfamily Rhynchonelloidea Gray, Reference Gray1848
Family Rhynchotrematidae Schuchert, Reference Schuchert and von Zittel1913
Genus Rhynchotrema Hall, Reference Hall1860
Type species
Atrypa increbescens Hall, Reference Hall1847. Trenton Limestone, early Katian, New York.
Rhynchotrema iowense Wang, Reference Wang1949
Figure 81–8.10
- Reference Wang1949
Rhynchotrema iowense Wang, p. 12, pl. 4C, figs. 1–9.
- Reference Howe1967
Rhynchotrema iowense; Howe, p. 858, pl. 105, figs. 5, 7–9, 11.
- Reference Macomber1970
Rhynchotrema iowense; Macomber, p. 444, pl. 80, figs. 33–47.
- Reference Jin and Lenz1992
Rhynchotrema iowense; Jin and Lenz, p. 142, pl. 3, figs. 13–22.
- Reference Jin and Zhan2001
Rhynchotrema iowense; Jin and Zhan, p. 46, pl. 15, figs. 1–19; text-fig. 22.
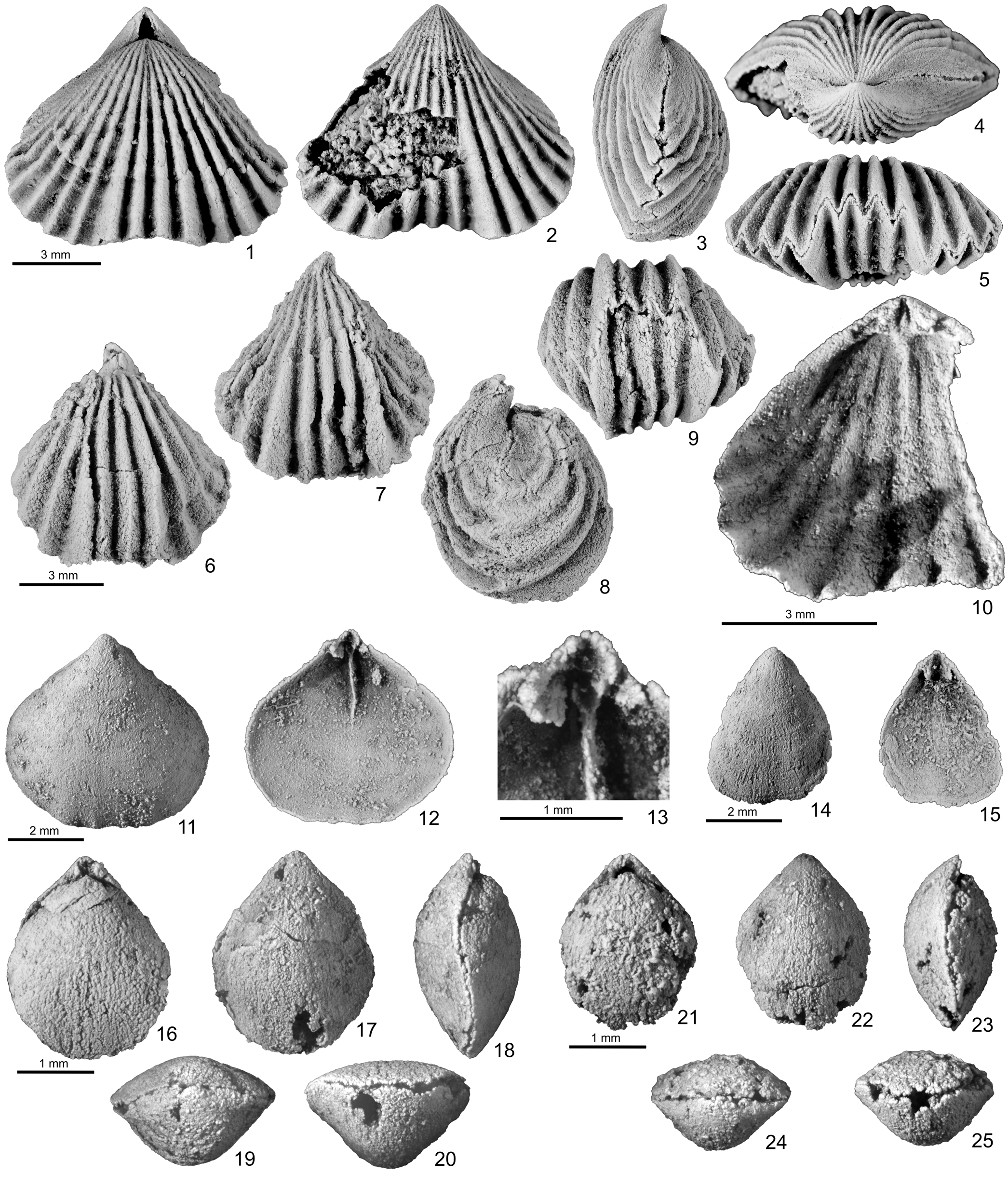
Figure 8. Silicified brachiopods from unnamed upper Katian strata, locality 92ABd8, Black River D-1 1:63,360 scale quadrangle, east-central Alaska. (1–10) Rhynchotrema iowense Wang, Reference Wang1949. (1–5) GSC 131822: (1) dorsal, (2) ventral, (3) lateral, (4) posterior, and (5) anterior views; (6–9) GSC 131823: (6) dorsal, (7) ventral, (8) lateral, and (9) posterior views of relatively small shell; (10) GSC 131824, interior of dorsal valve showing septalium with blade-like cardinal process. (11–25) Whitfieldella sp. (11–13) GSC 131825: (11) exterior, (12) interior, and (13) local enlargement of dorsal valve, showing small septalium and prominent median septum; (14, 15) GSC 131826: (14) exterior and (15) interior of small ventral valve showing discrete dental plates; (16–20) GSC 131827: (16) dorsal, (17) ventral, (18) lateral, (19) posterior, and (20) anterior views of very small shell; (21–25) GSC 131828: (21) dorsal, (22) ventral, (23) lateral, (24) posterior, and (25) anterior views of very minute shell (slightly over 1 mm wide).
Types
Holotype by original designation of Wang (Reference Wang1949), Brainard Shale, Richmondian (late Katian), Iowa.
Materials
Conjoined shells (four); ventral valves (three, incomplete); dorsal valves (five, incomplete).
Remarks
The Alaskan shells are assignable to Rhynchotrema on the basis of its relatively consistent development of four simple costae on the dorsal fold, three costae in the ventral sulcus, and a well-developed septalium with a blade-like cardinal process (Fig. 8.10). They are most similar to shells of R. iowense from the upper Katian Red River Formation or equivalent strata of Williston Basin in Wyoming (Macomber, Reference Macomber1970) and southern Manitoba (Jin and Zhan, Reference Jin and Zhan2001), especially in its relatively strong and sparse costae. The Alaskan shells, however, are predominantly very small in size, possibly representing their early ontogenetic stage. This may explain why some of them tend to be slightly longer than wide (see Appendix), as is typical of most rhynchonellide shells (see Jin, Reference Jin1989 for examples).
Order Athyrida Boucot, Johnson, and Staton, Reference Boucot, Johnson and Staton1964
Superfamily Meristelloidea Waagen, Reference Waagen1883
Family Meristellidae Waagen, Reference Waagen1883
Subfamily Whitfieldellinae Alvarez, Rong, and Boucot, Reference Alvarez, Rong and Boucot1998
Genus Whitfieldella Hall and Clarke, Reference Hall and Clarke1893
Type species
Atrypa nitida Hall, Reference Hall1843. Waldron Shale, lower Silurian, Wenlock, Indiana.
Remarks
The ordinal spelling of ‘Athyrida’ follows Copper and Jin (Reference Copper and Jin2017), who traced the nomenclatural history of this taxonomic name.
Whitfieldella sp.
Figure 8.11–8.25
Materials
Conjoined shells (35, minute); ventral valves (eight); dorsal valves (seven).
Remarks
The Alaskan shells are very small in size, with the largest available specimen (a dorsal valve) measuring 5.4 mm long, 5.6 mm wide (Fig. 8.11). Other conjoined shells are typically minute, moderately elongate, equibiconvex, less than 3 mm long (see Appendix). The lateral commissure is sinusoidal, and the anterior commissure is faintly sulciplicate (Fig. 8.11). Despite its small size, one ventral valve shows a weak, anteriorly developed medial furrow, typical of Whitfieldella (Fig. 8.14). Internal structures are also characteristic of the genus in its well-defined, subparallel dental plates, long median septum extending near the midlength of dorsal valve, and a small septalium (Fig. 8.12, 8.13). Unfortunately, the spiralium and jugum are not preserved.
Acknowledgments
Blodgett thanks the Alaska Division of Geological and Geophysical Survey for providing him helicopter logistical support to collect the fossil brachiopod fauna described here. T. Shcherbanenko of the Novosibirsk State University kindly provided us with digital copies of some Russian literature used for this study. Funding for the systematic study is provided by a Discovery Grant to Jin from the Natural Sciences and Engineering Research Council of Canada.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.31zcrjdh2.
Appendix: Measurements of shell dimensions (mm)
Plaesiomys occidentalis (Okulitch, 1943). Asterisk (*) denotes measurement of actually preserved portion.

Plaesiomys occidentalis (Okulitch, Reference Okulitch1943). Asterisk (*) denotes measurement of actually preserved portion.

Brevilamnulella minuta n. sp.

Rhynchotrema iowense Wang, Reference Wang1949.

Whitfieldella sp.











